Abstract
Background
Ventricular premature complexes (PVCs) on a 2-minute electrocardiogram (ECG) are a common, largely asymptomatic finding, associated with increased risk of coronary heart disease (CHD) and death. They may reflect atherosclerosis or other pathogenic pathways that predispose to arrhythmias and stroke.
Methods/Results
We conducted a prospective evaluation of the Atherosclerosis Risk In Communities Study cohort (n=14,783) of middle aged men and women to assess whether the presence of PVCs at study baseline (1987-89) influenced the risk of incident stroke through 31st December 2004. PVCs were seen in 6.1% of the participants at baseline, and 729 (4.9%) had incident stroke. The unadjusted cumulative proportion of incident stroke in individuals with any PVC was 6.6% compared to 4.1% in those without PVC. The unadjusted hazard ratio (HR) of incident stroke in individuals with any PVC compared to those without any PVCs was 1.71 (95% Confidence Interval (CI) 1.33, 2.20).
Among individuals without hypertension and diabetes at baseline, PVCs were independently associated with incident stroke (HR: 1.72 (1.14, 2.59)). Among those with either diabetes or hypertension the presence of any PVCs did not increase the risk of stroke. The association was stronger for non-carotid embolic stroke than for thrombotic stroke and its magnitude increased with higher frequency of PVCs.
Conclusions
Frequent PVCs are associated with risk of incident stroke in participants free of hypertension and diabetes. This suggests that PVCs may contribute to atrio-ventricular remodeling or may be risk marker for incident stroke, particularly embolic stroke.
Keywords: Stroke, Risk factors, Arrhythmia, Ventricular premature complexes, Atrial Fibrillation
Background
Premature Ventricular Complexes (PVCs) are mostly asymptomatic irregular heart rhythms commonly seen on electrocardiograms (ECGs) of the middle aged and elderly 1, 2. Several lines of research suggest that PVCs may be a marker of higher sub-clinical atherosclerotic burden or higher arrythmogenic potential 3-5 or both, thus potentially linked to stroke.
Changes in heart rate, blood pressure, and stroke volume during and after PVCs, a consequence of changes in ventricular filling, ventricular contractility, and baroreflex activity are well known 6. Frequent PVCs are associated with impaired ventricular relaxation 7, and have the potential to remodel the heart 8, 9. In addition to their putative arrythmogenic potential such adverse remodeling may increase the risk of atrial fibrillation, potentially increasing the risk of clot formation and embolization.
In contrast to the established association of atrial fibrillation with incident stroke the relationship of ventricular rhythm abnormalities with stroke has not been much studied 10. No published study has examined whether the association between PVCs and stroke differ by stroke subtype (embolic vs. thrombotic), as this may point towards different mechanisms.
In this study we examined an association between PVCs and incident stroke in middle aged men and women from four US communities. We also explored whether such an association differs in sub-groups with and without the major risk factors for stroke and we considered stroke sub-types.
Methods
Study population
The ARIC Study enrolled 15,792 subjects ages 45-64 years in four U.S. communities using area-probability sampling: Forsyth County, NC; city of Jackson, MS; seven northwestern suburbs of Minneapolis, MN; and Washington County, MD at visit 1 (1987-89). Black residents were over-sampled in Forsyth County, NC, while enrollment at the Jackson, MS site was restricted to black residents. A complete description of the ARIC communities and of the design has been published 11. The response rate was 75% for home interviews and among those who interviewed at home 86-88% came for clinic visit for communities other than Jackson (response = 63%). Whites who responded to initial home interviews but not clinic visit had poorer socio-economic status, and poor health than non-responders, with little difference among women and blacks 12. The cohort participated in four examinations including baseline visit, and also annual telephone interviews. Baseline examinations of the cohort were conducted from 1987 to 1989 to collect information about socioeconomic indicators, medical history, family history, cardiovascular risk factors, serum chemistries, ECGs, and medication use. Over 90% of the surviving cohort members responded to the recent annual telephone interviews.
For the present analysis, those with missing values for the 12-lead ECG (n = 191) and the missing 3-lead 2-minute rhythm strip or on pacemakers (n = 74) were excluded. Also, participants with the following cardiac rhythm disturbances were excluded: Wolf-Parkinson- White syndrome (n = 6), atrial fibrillation/flutter (n = 35), wandering atrial pacemaker (n = 6), and supra-ventricular tachycardia (n = 5), and not sinus rhythm (n = 67) were excluded. Participants with missing or invalid values for key covariates were excluded (n = 619). Also, participants with missing information (n=211) or prevalent history (n=105) of stroke were excluded. Finally, we excluded 48 participants who didn't identify themselves as either white or black. Some observations met more than one exclusion criteria. The remaining 14,783 (93.6% of original) cohort participants were included in this study analyses.
In a subset analysis involving adjustment for LVH using Cornell voltage, another 1,009 participants with ineligible values for Cornell voltage were excluded (n= 665 participants with QRS ≥120 ms, suggesting bundle branch block; n = 369 with missing or zero value for Cornell voltage).
Premature Ventricular Complex
A supine 12-lead ECG at rest was obtained using the MAC PC10 personal cardiogram (Marquette Electronics, Milwaukee, Wisconsin) and a 2-minute 3-lead (V1, II, and V5) rhythm strip. Participants were requested to fast and to refrain from smoking and consuming caffeinated beverages before the examination. Electrocardiographic data processing, monitoring, and quality control have been described elsewhere 13. Rhythm strips were classified 3 times by independent trained coders for total supra-ventricular, ventricular complexes and ventricular runs, bigeminy, trigeminy, and multiform complexes. Coding for PVCs was done before this study's hypothesis was formulated and before stroke outcomes were ascertained. Adjudication of disagreements was performed by the ECG center principal investigator or coding supervisor. PVCs and heart rate were determined from the rhythm strip.
The primary exposure was presence of any PVCs on the 2-minute rhythm ECG. PVCs seen on a 2 -minute ECG rhythm strip are highly correlated with high frequency PVCs seen on 24 hour recordings 14. Further PVCs were classified by the frequency of their occurrence in 2 minute rhythm strip i.e., single PVC; two-three, and four or more.
Other covariates
Covariates known to be associated with PVCs, and other atherosclerotic and embolic events including incident stroke events, were considered during analyses1. These are presented in Table 1, and were ascertained during the cohort baseline interviews and examinations. A parsimonious set of potential covariates for the relation of PVCs to incident stroke was chosen based on a strong physiologic relation to PVCs (e.g., electrolytes, sinus rate) or known association with stroke (e.g., age, prevalent CHD, hypertension, current smoking, diabetes, ventricular hypertrophy, educational attainment, gender, ethnicity). Measures of alcohol consumption, obesity, and obstructive lung disease were not included as potential confounders due to the absence of a strong physiologic link to prevalent PVCs 15.
Table 1. Characteristics of participants by presence of premature ventricular complexes at study baseline, and follow up stroke: The Atherosclerosis Risk In Communities Study.
| Characteristics* | Baseline PVCs† | Stroke during follow up | ||
|---|---|---|---|---|
| Yes (n= 899) | No (n= 13884) | Yes (n= 729) | No (n= 14054) | |
| Age (in yrs) | 56.2 (5.2) | 54.0 (5.7) | 56.3 (5.6) | 54.0 (5.7) |
| Women | 46.9 | 56.0 | 48.3 | 55.9 |
| African American | 29.6 | 25.8 | 43.3 | 25.2 |
| Education (no high school) | 29.9 | 36.3 | 26.3 | 36.4 |
| Education (high school, no college) | 40.9 | 41.0 | 35.1 | 41.3 |
| Prevalent CHD | 12.7 | 4.1 | 10.2 | 4.3 |
| Hypertension | 44.9 | 33.6 | 59.9 | 33.0 |
| Diabetes | 13.0 | 9.2 | 24.0 | 8.7 |
| Current smoking | 26.8 | 25.8 | 35.5 | 25.3 |
| Systolic blood pressure (mm of Hg) | 124.8 (19.2) | 120.8 (18.7) | 131.9 (22.6) | 120.5 (18.3) |
| Low-density lipoprotein cholesterol (mg/dL) | 137.6 (38.9) | 137.6 (39.3) | 143.6 (40.2) | 137.3 (39.2) |
| High-density lipoprotein cholesterol (mg/dL) | 49.9 (16.6) | 51.8 (17.1) | 48.8 (16.2) | 51.9 (17.1) |
| Body mass index (kg/mˆ2) | 28.4 (5.3) | 27.6 (5.3) | 28.8 (5.5) | 27.6 (5.3) |
| Heart rate (beats/min) | 68.0 (11.0) | 66.6 (10.2) | 67.7 (11.3) | 66.6 (10.2) |
| Cornell voltage‡ (mV) | 1.29 (0.56) | 1.20 (0.53) | 1.44 (0.67) | 1.19 (5.2) |
| Serum potassium (Meq/L) | 4.4 (0.5) | 4.4 (0.5) | 4.4 (0.5) | 4.4 (0.5) |
| Serum magnesium (mmol/L) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) | 1.6 (0.2) |
| ACEI | 4.7 | 3.0 | 6.0 | 2.9 |
| β blocker medication use | 12.5 | 10.2 | 18.7 | 9.9 |
| Calcium channel blocker use | 5.7 | 3.2 | 5.9 | 3.2 |
| Other antihypertension drugs | 8.2 | 5.4 | 9.3 | 5.4 |
| Digoxin | 3.6 | 1.2 | 3.0 | 1.2 |
| Anti-arrhythmic | 2.9 | 0.5 | 1.1 | 0.7 |
| Overall incident stroke§ | 7.3 | 4.8 | - | - |
| Thrombotic stroke§ | 4.2 | 3.4 | - | - |
| Embolic stroke§ | 2.0 | 0.8 | - | - |
| Death§ | 31.2 | 16.1 | 46.6 | 16.7 |
| Stroke§ or death§ | 34.0 | 18.8 | - | - |
| Average follow up duration (years) | 13.8 (4.4) | 15.1 (3.3) | 9.4 (4.3) | 15.3 (3.0) |
Characteristics expressed as percentage or means with standard deviation of participants in respective columns
PVC (premature ventricular complexes) coded by investigators using 2-minute (3-lead ECG strips) at baseline
Cornell voltage was calculated in those with QRS <120 ms, with non-missing values, and not having prevalent CHD
Incident stroke and death from study enrollment (1987-89) through 31st December 2004 expressed as cumulative percentage
Standardized and validated methods were used to collect information on covariates. Detailed information about of the characterization of prevalent CHD 16, hypertension (Manual 11), diabetes (Manual 10), educational attainment, medication intake history 17, and Cornell voltage estimated left ventricular (LV) mass 18 is presented in the ARIC Study Protocol available at the study's website. Similarly, the definition of these covariates can be found in the variable dictionaries for visit 1 at the study's website.
Study outcomes
To identify incident stroke, cohort participants were followed over time through annual telephone interviews, triennial field center examinations, surveillance of the ARIC community hospitals for all cohort members hospitalizations, and the review of death certificates, physician questionnaires, coroner/medical examiner reports, and informant interviews. Hospital reports were reviewed for evidence of acute stroke if the discharge diagnosis included a cerebrovascular disease code (International Classification of Diseases, 9th revision, code 430 to 438), if a cerebrovascular procedure was mentioned in the summary, or if the computed tomography or magnetic resonance report showed evidence of cerebrovascular disease. Medical records for potential stroke events were forwarded to a single nurse abstractor at a central ARIC office who abstracted each record for number, type, and severity of neurological deficits and supporting angiographic, CT, MRI, spinal tap, or autopsy evidence. ARIC adapted National Survey of Stroke criteria for its stroke definition 19. A computerized algorithm and physician reviewer independently confirmed the diagnosis of stroke, with disagreements adjudicated by a second physician reviewer. Stroke cases were further classified as definite versus probable, and into further subtypes as embolic versus thrombotic stroke 20. Participants were followed from enrollment through December 31, 2004. Baseline ECG has no role in ascertainment of stroke.
Stroke occurs mostly in the elderly, and the chances of death due to other common causes will remove many high risk individuals from the risk sets. This has the potential to bias the results in those with high risk of death due to other risk factors. Thus a combined end-point consisting of incident stroke or death occurring before incidence stroke was analyzed additionally.
To study whether the observed association may be mediated by incident atrial fibrillation, we tested if PVCs are associated with incident atrial fibrillation occurring prior to incident stroke. AF was defined as the presence of ICD-9 code 427.31 in the hospital discharge codes. Patients with a diagnosis of atrial flutter (ICD-9 code 427.32) not developing AF during subsequent follow-up were not considered cases. We excluded AF diagnoses occurring simultaneously with heart revascularization surgery (ICD-9 code 36.X) or other cardiac surgery involving the heart valves or septa (ICD-9 code 35.X), without evidence of AF in subsequent hospitalizations or study exams, or subsequent to an incident stroke 21.
Statistical methods
Descriptive statistical analyses were done by presence or absence of any PVCs at study baseline including information on important covariates and incident stroke. Rate of stroke by race, gender and PVC categories were estimated assuming constant rate over time and Poisson distribution. Cox regression models were used to estimate the difference in log hazards contrasting presence of any PVC with no PVC. Finally, multivariate Cox regression model including interaction terms between main exposure and race, gender, and traditional risk factors for stroke, after adjusting for other potential confounders associated with prevalent PVCs and possibly stroke were fitted.
The above interaction terms would suggest if there is any difference in the association between PVC and stroke in the strata of race, gender or important risk factors for stroke i.e., prevalent CHD disease, hypertension, diabetes and current smoking status after adjusting for other potential confounders and demographic characteristics. Additionally, adjustment for left ventricular mass using Cornell voltage was done in a subset analysis as this required further exclusion of study participants. AF occurring after baseline visit was not included in regression models because it was considered as a potential intermediary in our hypothetical pathway linking PVC to stroke.
The proportional hazard assumption was evaluated both using graphical methods (ln-ln S (t) graphs) and statistical tests involving continuous time interaction terms (Cox tests). The log-linearity assumption of ordinal variables was examined, and as a result education categories were considered as nominal following violation of log-linearity.
To test if the studied association differed by race, gender, and other major risk factors for stroke, a product term between each of these and exposure variable was included. This product term if statistically significant indicates heterogeneity in the effect estimate and suggests that results be presented stratified by such variable(s).
All statistical computations were performed using SAS software version 9.1.3 (SAS Inc., Cary, North Carolina) and STATA/IC version 10 (StataCorp, College Station, Texas). A p value of < 0.05 for a two-sided null hypothesis was considered statistically significant for main covariates. As is customary for tests of interaction in epidemiologic studies, a p value of <0.2 was considered statistically significant.
Results
PVCs on the baseline 2-minute rhythm strip were identified in 899 (6.1%) of the 14,783 of the study participants. The prevalence of 30 PVCs/hr, ≥ 60 PVCs/hr, and complex PVCs were 2.4%, 2.9%, and 0.8%, respectively. The differences in the characteristics of individuals with any PVCs vs. no PVC are shown in Table 1.
Overall, 729 (4.9%) of the 14,783 participants had incident stroke. The cumulative proportion of incident stroke in individuals with any PVC was 7.3% compared to 4.8% in those without PVC. There were no differences in rate ratio for incident stroke when comparing the presence vs. absence of PVC across four races, gender groups.
Those with PVCs had a higher rate of incident stroke (Figure 1), and combined end-point than individuals without any PVCs – hazard ratio of 1.71 (95% Confidence Interval (CI) 1.33, 2.20), and 1.70 (1.51, 1.92), respectively. The estimates after adjusting for age, gender, and race for incident stroke and combined end-point were 1.4 (1.08, 1.80), and 1.62 (1.44, 1.83), respectively.
Figure 1.
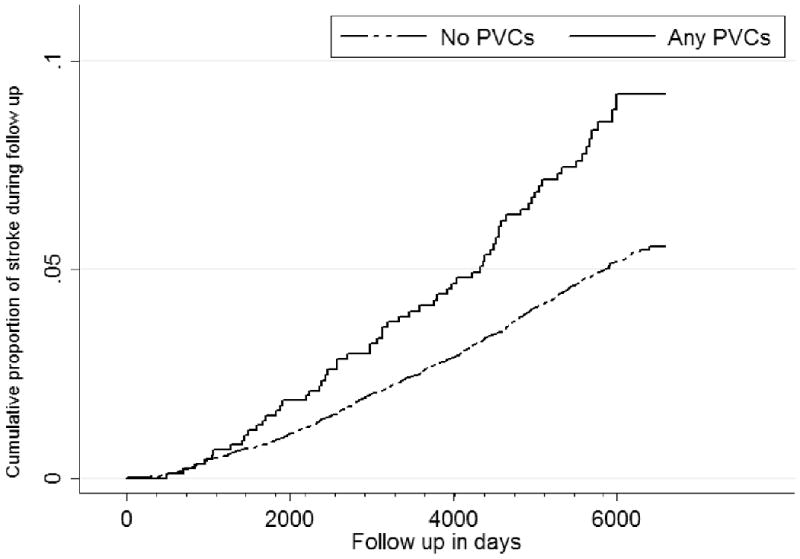
Cumulative proportion with stroke among any PVC (solid line, top, n = 899 at start of follow up) vs. no PVC (dotted line, bottom, n = 13884 at start of follow up) from the Atherosclerosis Risk In Communities Study (1987-89 through 2004). PVCs = Premature Ventricular Complexes
Among individuals without diabetes and hypertension at baseline the hazard ratio of incident stroke was 1.72 (95% CI 1.14, 2.59) for individuals with any PVC vs. those without any PVCs, after adjusting for potential confounders (Table 2). The above hazard ratio contrasting presence of any PVCs with no PVCs was lower in those with hypertension and diabetes (Table 2, and Table 3). When examining the relationship with definite stroke in the model presented in table 2, the hazards ratio contrasting those with any PVCs vs. no PVCs was 1.67 (0.92, 3.01). The estimates did not change appreciably after adjustment for Cornell voltage. Further, the estimates did not change appreciably after adjusting for baseline intake of medications such as beta blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, anti-arrhythmic drugs, and Digoxin.
Table 2.
Multivariate adjusted Cox regression model for stroke*: The Atherosclerosis Risk In Communities Study
| Variables | Hazard rate ratios † | 95% Confidence Intervals | Pr > ChiSq | |
|---|---|---|---|---|
| Any PVC‡ | 1.72 | 1.14 | 2.59 | 0.01 |
| Age at intake (per SD) | 1.41 | 1.30 | 1.53 | <.0001 |
| HDL cholesterol (per SD) | 0.94 | 0.86 | 1.03 | 0.19 |
| LDL cholesterol (per SD) | 1.08 | 1.00 | 1.16 | 0.04 |
| Potassium (per SD) | 1.05 | 0.97 | 1.13 | 0.27 |
| Magnesium (per SD) | 0.93 | 0.87 | 1.01 | 0.08 |
| Heart rate (per SD) | 1.10 | 1.03 | 1.18 | 0.01 |
| Race (black vs. white) | 1.77 | 1.49 | 2.11 | <.0001 |
| Gender (Male vs. Female) | 1.38 | 1.17 | 1.63 | 0.00 |
| Education (less than high school vs. college) | 1.43 | 1.18 | 1.74 | 0.00 |
| Education (high school vs. college | 1.09 | 0.90 | 1.33 | 0.36 |
| Prevalent coronary heart disease | 1.89 | 1.47 | 2.43 | <.0001 |
| Hypertension | 2.21 | 1.86 | 2.62 | <.0001 |
| Current cigarette smoker | 1.89 | 1.62 | 2.22 | <.0001 |
| Diabetes | 2.15 | 1.76 | 2.62 | <.0001 |
| PVCs × Hypertension | 0.69 | 0.40 | 1.18 | 0.17 |
| PVCs × Diabetes | 0.56 | 0.29 | 1.09 | 0.09 |
Incident stroke cases from study enrollment (1987-89) through 31st December 2004
Hazard ratio for stroke per standard deviation increment or for test category as compared to referent; however estimates for PVC are for those without diabetes and hypertension and similarly estimates for hypertension and diabetes are for subgroup without PVCs
PVC (premature ventricular complexes) coded by investigators using 2-minute (3-lead ECG strips) at baseline
Table 3.
Hazard ratio for stroke* contrasting any PVC† vs. no PVC by subgroup of traditional risk factors: The Atherosclerosis Risk In Communities Study
| Subgroup | Hazard ratio (95% CI)‡ | % of cohort | ||
|---|---|---|---|---|
| Diabetes | 0.73 | 0.4 | 1.32 | 9 |
| Hypertension | 1.00 | 0.72 | 1.41 | 34 |
| No diabetes and no hypertension | 1.72 | 1.14 | 2.59 | 62 |
| None of the four traditional risk factors§ | 2.09 | 1.21 | 3.58 | 44 |
| Complete study sample | 1.20 | 0.91 | 1.52 | 100 |
Incident stroke cases from study enrollment (1987-89) through 31st December 2004
PVC (premature ventricular complexes) coded by investigators using 2-minute (3-lead ECG strips) at baseline
Estimates from models adjusted for age, gender, race, education, resting heart rate, LDL-cholesterol, HDL-cholesterol, serum K+, and serum Mg++; models were additionally adjusted for hypertension, diabetes, prevalent CHD, and smoking whenever appropriate
No diabetes, hypertension, smoking history, or CHD at study baseline
Among individuals with no major risk factors for stroke (CHD, hypertension, current smoking, and diabetes at baseline; 46.3% of study participants), the hazard ratio of incident stroke was 2.09 (95% CI 1.21, 3.58) for individuals with any PVC vs. those without any PVCs, after adjusting for potential confounders (Table 3).
In the model presented in table 2, the hazard ratio of incident stroke among those with single PVCs/ 2-min, 2-3 PVCs/2-min, and ≥ 4 PVCs compared to no PVCs were 1.55 (95% CI 0.92, 2.62), 1.51 (95% CI 0.82, 3.62), and 2.06 (95% CI, 1.24, 3.42), respectively, without appreciable change in other estimates.
For embolic stroke of non-carotid origin, the hazard ratio comparing any PVCs vs. no PVCs was 3.48 (1.74, 6.95) among non-hypertensive, and 1.21 (0.58, 2.53) among hypertensive after adjusting for all the potential confounders shown in table 2. The above estimates remained similar when using definite embolic stroke. In contrast, for stroke classified as thrombotic, the hazard ratio comparing any PVCs vs. no PVCs was 1.13 (0.78, 1.65) among non-diabetics, and 0.47 (0.21, 1.67) among diabetics. On considering definite thrombotic stroke as the outcome the above hazard ratio was 1.03 (0.62, 1.72) among non-diabetics, and 0.35 (0.11, 1.11) among diabetics.
Lastly, the multivariable hazards of incident atrial fibrillation among those with any PVCs adjusted for age, gender, race, smoking status, heart rate, serum magnesium, serum potassium, prevalent CHD, diabetes, hypertension, LDL lipids, and HDL lipids was 1.56 (95% CI: 1.30, 1.87) times higher than those with no PVCs. Figure 2 shows the cumulative proportion of follow up AF and stroke events among those with PVCs.
Figure 2.
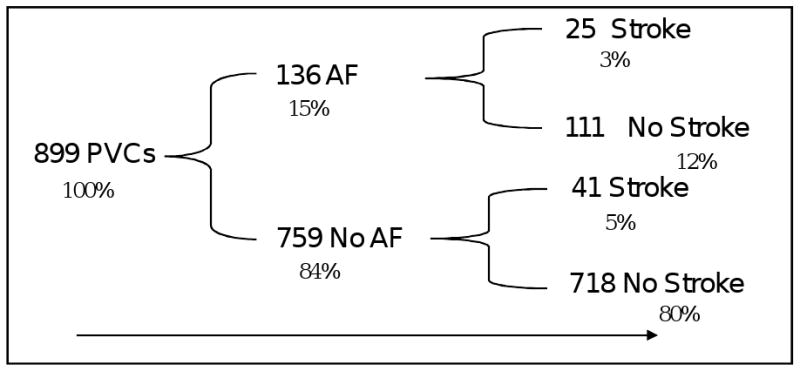
Sketch showing the number (%) of the Atherosclerosis Risk In Community (ARIC) study participants having at least single premature ventricular complex (PVCs) on a 2-minute rhythm strip at study baseline (1987-1989), and follow up atrial fibrillation and stroke among them. Four participants with PVCs had missing information on incident atrial fibrillation and they didn't have stroke. AF was diagnosed during follow up duration using ICD discharge codes and ECG information from three yearly field center visits.
Discussion
In this large community-based cohort of white and black men and women in four locales of the United States we find that PVCs are associated with incident stroke. Further, this relationship is statistically significant and stronger in the sub-group without traditional risk factors for stroke, such as diabetes or hypertension. Our results considering stroke sub-type point towards a stronger association of PVCs with non-carotid artery embolic stroke than with thrombotic stroke (potentially caused by atherosclerosis in the cerebral circulation).
An earlier study has reported a similar but statistically non-significant association 10. Despite the strength of 24-hour Holter recording to monitor premature complexes this study was constrained by a small sample size, yielding only 58 incident stroke events and it included only men, mostly of Caucasian origin 10. The large size of the ARIC cohort and the inclusion of women and African American examinees, as well as the sizeable number of incident stroke events classified as embolic and thrombotic allows us to explore this relationship with greater statistical power, and by stroke sub-type.
The relationship between atrial fibrillation and embolic stroke is well established. Increased left atrial size reflecting cardiac remodeling has been shown to be a strong predictor of atrial fibrillation22. Given the stronger association of PVCs with embolic than with thrombotic stroke observed in our study,, it is plausible that PVCs are associated with increased risk of atrial fibrillation. Similar to our findings, other studies have shown higher risk of new onset atrial fibrillation among those with PVCs 23. Its interesting to note that despite the possibility of some misclassification of stroke sub-type into as cardioembolic 20, the estimates for definite and overall cardioembolic stroke were similar. If this finding is replicated in future studies, individuals with high frequency PVCs could monitored for AF as part of regular clinical encounters and promotion by promoting self-monitoring of heart rhythm in such individuals. A proactive management of other risk factors of stroke could also be considered in individuals with high frequency PVCs.
The strengths of our study include its large sample size, the length and completeness of its follow-up, and its population base that provides for inference to the general population. Since 2-minute rhythm strips are highly specific but less sensitive than 24-hour ambulatory ECG recordings the strength of the associations with PVCs reported here could be underestimated, however. An alternate interpretation of the lack of association between PVCs and stroke among hypertensive individuals in the influence of medications that can suppress PVCs. This has the potential to induce a bias resulting in failure of detecting a true association (type 2 error) in ways that are not amenable to quantification in our data. As an additional note of caution, stroke occurs mostly among elderly and death due to other atherosclerotic processes also has the potential to induce a similar bias. Although a modification of the effect estimate by various established risk factors for stroke was studied, the statistical power to detect such interaction(s) was relatively limited. Also, a criterion p value of 0.2 for interaction test may result in false positive statistical interaction (type 1 error); however, the difference in the size of the estimates supports such interaction. Lastly, time-varying co-morbid conditions were not considered in these analyses as the main exposure itself varies over the course of follow up and we lack information on such intra-individual variability.
These results, if replicated in other future studies have potential clinical implications . PVCs may well turn out to be important risk markers for stroke, particularly embolic stroke, especially in the absence of established risk factors for stroke. In parallel, these results suggest that PVCs seen on a short rhythm strip may point to more than an atherosclerotic phenomenon, such as an indication of an increased tendency towards formation of thrombi or embolization through cardiac remodeling, and possibly atrial fibrillation and other serious arrhythmias.
In conclusion, presence of high frequency of PVCs in an ECG may help in stratifying individuals who are otherwise considered low-risk for stroke due to the absence of important risk factors. The observed findings possibly point to PVCs as markers of a pathological process beyond atherosclerosis.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Thanks to Emily O' Brien and Randi Foraker for their valuable comments on early draft.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Footnotes
Conflicts of Interest: None of the author has any conflicts of interest to declare.
Contributor Information
Sunil K. Agarwal, University of North Carolina at Chapel Hill, NC.
Gerardo Heiss, University of North Carolina at Chapel Hill, NC.
Pentti M. Rautaharju, Wake Forest University School of Medicine, Winston-Salem, NC.
Eyal Shahar, University of Arizona, Tucson, AZ.
Mark W. Massing, University of North Carolina at Chapel Hill, NC.
Ross J. Simpson, Jr., University of North Carolina at Chapel Hill, NC.
References
- 1.Simpson RJ, Jr, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of african american and white men and women: The atherosclerosis risk in communities (aric) study. Am Heart J. 2002;143:535–540. doi: 10.1067/mhj.2002.120298. [DOI] [PubMed] [Google Scholar]
- 2.Rautaharju P, Rautaharju F. Investigative electrocardiography in epidemiological studies and clinical trials. London: Springer; 2007. [Google Scholar]
- 3.Carrim ZI, Khan AA. Mean frequency of premature ventricular complexes as predictor of malignant ventricular arrhythmias. Mt Sinai J Med. 2005;72:374–380. [PubMed] [Google Scholar]
- 4.Meyerfeldt U, Schirdewan A, Wiedemann M, Schutt H, Zimmerman F, Luft FC, Dietz R. The mode of onset of ventricular tachycardia. A patient-specific phenomenon. Eur Heart J. 1997;18:1956–1965. doi: 10.1093/oxfordjournals.eurheartj.a015206. [DOI] [PubMed] [Google Scholar]
- 5.Myerburg RJ. Sudden cardiac death: Epidemiology, causes, and mechanisms. Cardiology. 1987;74(Suppl 2):2–9. doi: 10.1159/000174281. [DOI] [PubMed] [Google Scholar]
- 6.Malik M, Camm AJ. Dynamic electrocardiography. Elmsford, N.Y: Blackwell Futura; 2004. [Google Scholar]
- 7.Topaloglu S, Aras D, Cagli K, Yildiz A, Cagirci G, Cay S, Gunel EN, Baser K, Baysal E, Boyaci A, Korkmaz S. Evaluation of left ventricular diastolic functions in patients with frequent premature ventricular contractions from right ventricular outflow tract. Heart Vessels. 2007;22:328–334. doi: 10.1007/s00380-007-0978-9. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Blom NA, Yu Y, Ma P, Wang Y, Han X, Swenne CA, van der Wall EE. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: An echocardiographic evaluation. Int J Cardiovasc Imaging. 2003;19:295–299. doi: 10.1023/a:1025418531853. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 10.Engstrom G, Hedblad B, Juul-Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: Increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. doi: 10.1161/01.str.31.12.2925. [DOI] [PubMed] [Google Scholar]
- 11.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The atherosclerosis risk in communities (aric) study investigators. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 13.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial population. Atherosclerosis risk in communities (aric) study investigators. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 14.Evenson KR, Welch VL, Cascio WE, Simpson RJ., Jr Validation of a short rhythm strip compared to ambulatory ecg monitoring for ventricular ectopy. J Clin Epidemiol. 2000;53:491–497. doi: 10.1016/s0895-4356(99)00190-0. [DOI] [PubMed] [Google Scholar]
- 15.Massing MW, Simpson RJ, Jr, Rautaharju PM, Schreiner PJ, Crow R, Heiss G. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the atherosclerosis risk in communities cohort) Am J Cardiol. 2006;98:1609–1612. doi: 10.1016/j.amjcard.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 17.Shahar E, Folsom AR, Romm FJ, Bisgard KM, Metcalf PA, Crum L, McGovern PG, Hutchinson RG, Heiss G. Patterns of aspirin use in middle-aged adults: The atherosclerosis risk in communities (aric) study. Am Heart J. 1996;131:915–922. doi: 10.1016/s0002-8703(96)90173-8. [DOI] [PubMed] [Google Scholar]
- 18.Simpson RJ, Jr, Cascio WE, Crow RS, Schreiner PJ, Rautaharju PM, Heiss G. Association of ventricular premature complexes with electrocardiographic-estimated left ventricular mass in a population of african-american and white men and women (the atherosclerosis risk in communities. Am J Cardiol. 2001;87:49–53. doi: 10.1016/s0002-9149(00)01271-6. [DOI] [PubMed] [Google Scholar]
- 19.The national survey of stroke. National institute of neurological and communicative disorders and stroke. Stroke. 1981;12:I1–91. [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the atherosclerosis risk in communities (aric) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and african-americans: The atherosclerosis risk in communities (aric) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Tanabe N, Makiyama Y, Chopra SS, Okura Y, Suzuki H, Matsui K, Watanabe T, Kurashina Y, Aizawa Y. St-segment abnormalities and premature complexes are predictors of new-onset atrial fibrillation: The niigata preventive medicine study. Am Heart J. 2006;152:731–735. doi: 10.1016/j.ahj.2006.05.032. [DOI] [PubMed] [Google Scholar]


