Abstract
Immune-mediated adverse drug reactions (IADRs) represent a significant problem in clinical practice and drug development. Studies of the underlying mechanisms of IADRs have been hampered by the lack of animal models. Halothane causes severe allergic hepatitis with clinical features consistent with an IADR. Our ultimate goal is to develop a mouse model of halothane hepatitis. Evidence suggests that adaptive immune responses targeting liver protein adducts of the reactive metabolite (TFA) play an important role in the pathogenesis. The present study demonstrated that the combination of an anti-CD40 antibody and a Toll-like receptor (TLR) agonist served as a potent adjuvant in generating TFA-specific T cell responses in mice. Both CD4+ and CD8+ subsets of T cells were activated and the TFA-specific responses were detected not only in the spleen but also in the liver of mice immunized with mouse serum albumin adducts of TFA (TFA-MSA) plus the combined CD40/TLR agonist. Whereas all three TLR agonists examined were effective in eliciting TFA-specific immune responses in BALB/cByJ mice, only polyI:C was effective in DBA/1 mice and none of the TLR agonists could aid the generation of TFA-specific T cells in C57BL/6J mice. This result, combined with our previous finding that BALB/cByJ mice were the most susceptible to halothane-induced acute liver injury, provides the basis for employing this strain in future studies. Collectively, our data demonstrated the successful completion of a crucial first step in the development of a murine model of halothane hepatitis.
Keywords: Anti-CD40 antibody, CpG DNA, Drug, LPS, PolyI:C, TLR agonist
1. Introduction
Immune-mediated adverse drug reactions (IADRs), including allergic hepatitis, lupus, cutaneous reactions, and blood dyscrasias, account for approximately 6–10% of all adverse drug reactions. Although IADRs are often referred to as rare (afflicting 1/100 to 1/100,000 patients), their unpredictable and serious nature makes them a significant problem in clinical practice, public health and drug development. Our current knowledge on the underlying mechanisms of IADRs is limited due to the dearth of animal models that bear similar clinical characteristics as those in humans. The present report describes the critical first step toward developing a murine model of halothane hepatitis.
Halothane is known to cause mild liver injury with a transient elevation of serum aminotransferase in approximately 20% of patients treated with this inhalation anesthetic (Wright et al., 1975). A much more severe form of liver injury (halothane hepatitis), which can lead to liver failure, develops in a small proportion of patients (1 in 35,000) (Ray and Drummond, 1991). Ample evidence suggests that halothane hepatitis is caused by an immune reaction against endogenous proteins that have been covalently modified by the trifluoroacetyl chloride (TFA) metabolite of halothane (Kenna et al., 1988;Pohl, 1990). Sera from patients afflicted with halothane hepatitis contain specific antibodies recognizing TFA-modified liver proteins (Vergani et al., 1980;Satoh et al., 1989). TFA-protein adducts have also been detected in liver biopsies obtained from halothane hepatitis patients (Pohl et al., 1989). Therefore, the generation TFA-specific immune response is critical in the initiation of halothane hepatitis in humans, and we believe, a key step in the development of animal models.
It has been established that co-administration of antigens with adjuvants, which stimulate the innate immune cells, profoundly impacts the magnitude and nature of the ensuing adaptive immune responses. Toll-like receptor (TLR) agonists, as a class of adjuvant, have been intensively studied in recent years. TLRs are a family of innate immune receptors that recognize various bacterial- and viral-derived molecular structures (agonists), such as lipopolysaccharide (LPS), ssRNA, dsRNA, flagellin, and unmethlated CpG sequences (Akira, 2003). Antigen presenting cells (APCs), such as dendritic cells, macrophages, and B cells, express high levels of TLRs. Stimulation of APCs through TLRs activates these cells to produce pro-inflammatory cytokines, up-regulate co-stimulatory molecule expression, and migrate into T cell areas of lymphoid tissues (Pulendran, 2004), all characteristics of a potent APC. Numerous studies have demonstrated the use of TLR agonists to elicit antigen-specific T cell responses (Rhee et al., 2002;Lore et al., 2003;Heit et al., 2004;Wille-Reece et al., 2006). CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily, is expressed on APCs and plays an essential role in triggering cellular immune responses mediated by CD8+ T cells. It has recently been reported that immunization of mice with a protein antigen in combination of both an anti-CD40 antibody (Ab) and a TLR agonist (CD40/TLR) induces potent CD8+ T cell activation and expansion (Ahonen et al., 2004).
In the present study, we adopted the combined CD40/TLR agonists approach and demonstrated that immunization of mice with mouse serum albumin adducts of TFA (TFA-MSA) in the presence of the combined agonists could elicit CD4+ and CD8+ T cell responses systemically as well as locally within the liver. Moreover, the efficacies of 3 TLR agonists and the susceptibilities of 3 strains of mice were compared. This proof-of-principle study provides a stepping stone for further effort in the development of a model of immune-mediated halothane-induced hepatitis.
2. Material and methods
2.1. Materials
Incomplete Freund’s adjuvant (IFA), S-Ethyl trifluorothioacetate, Percoll, heparin and LPS were purchased from Sigma-Aldrich (St. Louis, MO). The following reagents were purchased from various venders: MSA (MP Biomedicals, Solon, OH); Complete Freund’s adjuvant containing 4mg/ml of M. Tuberculosis H-37 RA (CFA, Chondrex, Inc. Redmond, WA); Concholepas concholepas hemocyanin (CCH; EMD Chemicals; Inc., Gibbstown, NJ), Polyinosinic-polycytidylic acid (polyI:C) and [methyl-3H] thymidine (GE Healthcare Bio-Sciences Corp., Piscataway, NJ); Rat anti-mouse CD40 Ab (FGK4.5; Bio X Cell, West Lebanon, NH); Mouse IFN-γ ELISA kit (R&D Systems, Inc., Minneapolis, MN). Nonmethylated, phosphorothioate-modified CpG ODN 1826 (TCCATGACGTTCCTGACGTT) was synthesized by Integrated DNA Technologies (Coralville, IA).
2.2. Animals
Female BALB/cByJ, C57BL/6J and DBA/1 mice (7–10 week of age) were purchased from the Jackson Laboratory and kept in the Center for Laboratory Animal Care at the University of Colorado Denver (UCD) for one week before treatments. All animal experiments were performed in accordance with guidelines from the UCD Institutional Animal Care and Use Committee (IACUC).
2.3. Synthesis of TFA-protein adducts
TFA-protein adducts, including TFA-MSA and TFA-CCH were synthesized by the reaction of S-Ethyl trifluorothioacetate with the respective proteins (Goldberger and Anfinsen, 1962;Goldberger, 1967). S-Ethyl trifluorothioacetate (600 µl) was added to the protein solution (100 mg in 10 ml of water). The reaction mixture was maintained at pH between 9.9 and 10 by the addition of 1N NaOH. After 30 min of mixing at room temperature, the pH of reaction mixture was adjusted to pH 7 by adding 0.1 M sodium acetate. Non-reacted S-Ethyl trifluorothioacetate and the by-product of the reaction were removed by subjecting the reaction mixture to de-salting columns. The extent of TFA-modification of the protein was determined by a quantitative measurement of ε-amino groups of lysine residues using trinitrobenzene sulfonic acid (Sigma).
2.4. Immunization of mice with TFA-MSA and CFA
Female BALB/cByJ mice were subcutaneously (s.c.) injected at the base of the tail with TFA-MSA (50 µg) or MSA in PBS emulsified with an equal volume of CFA (50 µl). After one week, the mice were immunized with the same amount of protein emulsified with IFA. One week after the last immunization, mice were sacrificed, and the inguinal and peri-aortic lymph nodes (LN) were isolated and disrupted to yield single cell suspension. The cells were cultured (5 × 105 cells/well) in 96-well plates for 4 days in the presence of 100 µg /ml of various antigens. [3 H] thymidine (0.5 µCi/well) was included during the last 16 h of culturing, and T cell proliferation was measured by [3 H] thymidine incorporation. In addition, the splenocytes were isolated and cultured (10 × 106 cells/well) in 24-well plates for 48 h in the presence of 50 µg /ml of various antigens. The supernatants were collected to measure IFN-γ production by ELISA following the manufacture’s instructions (R&D Systems, Inc.)
2.5. Immunization of mice with TFA-MSA and the combined CD40/TLR agonist
Female BALB/cByJ, DBA/1 and C57BL/6J mice were injected intraperitoneally (i.p.) with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab and 100 µg of 1 of the 3 TLR agonists. One week later, the splenocytes and liver mononuclear cells (LMNC) were isolated and cultured as described above. CD4+ T cells or CD8+ T cells were further purified using T cell enrichment kit from STEMCELL Technologies (Vancouver, BC, Canada) according to the manufacture’s instructions.
2.6. LMNC isolation and ex vivo re-stimulation
LMNC were isolated from mice immunized with TFA-MSA in conjugation with various adjuvant as described previously (You et al., 2006). In brief, the liver was perfused in situ with Hank’s balanced salt solution (HBSS) for 5 min and disrupted in HBSS containing 0.5% of FBS and 0.6% of citrate-dextrose (ACD-A, Sigma). Single cell suspensions were filtered through a 100 µm cell strainer, and centrifuged at 300 g for 5 min. The pellet was resuspended in 15 ml of 35% Percoll containing 50 U/ml of heparin and centrifuged at 500 g for 12 min. The resulting pellet was collected and resuspended in 1 ml of red blood cell lysing buffer (Sigma). After 5 min, the cells were washed in HBSS containg 0.6% ACD-A and 0.5% BSA. LMNC (5 × 105 cells/well) were cultured in 96-well plates in the presence of 50 µg /ml of various antigens for 48 h. The supernatant was collected and measured for IFN-γ production by ELISA.
2.7. Statistical Analysis
Data are presented as mean ± SEM. Two-tailed Student’s t-test was used to compare two groups. Comparisons among multiple groups were performed using one-way analysis of variance (ANOVA) with a post-hoc test of significance between individual groups. Differences were considered significant when P<0.05.
3. Results
3.1. Evaluation of the effectiveness of CFA as an adjuvant in generating TFA-specific T cell responses
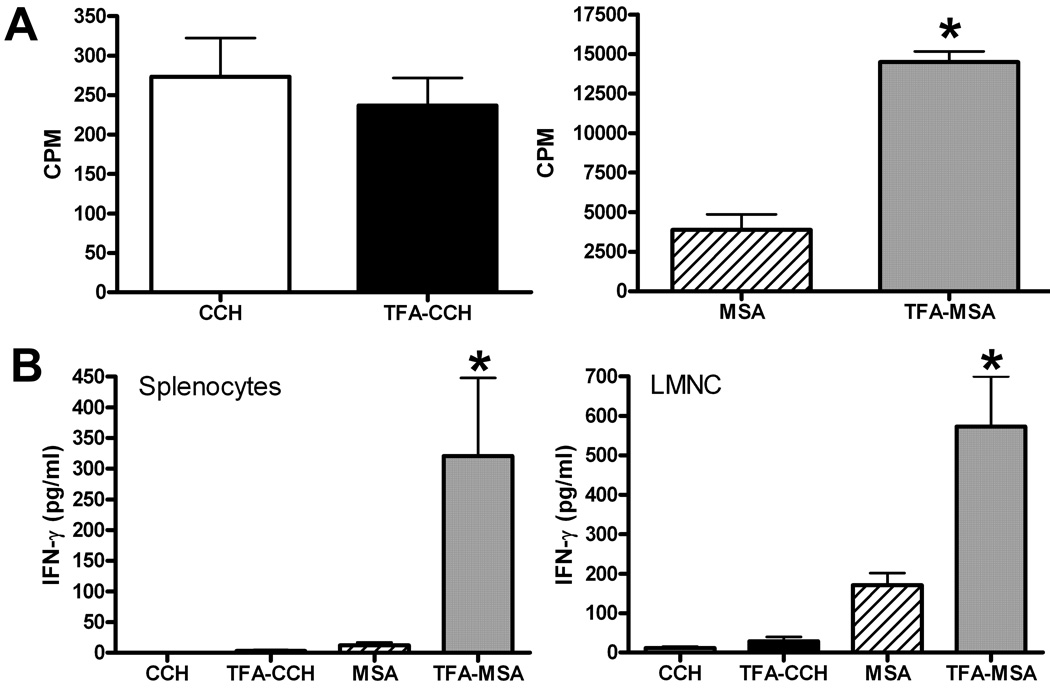
CFA has been the mainstay of immunological adjuvant in research for decades. It has been successfully applied in the establishment of mouse model of rheumatoid arthritis (RA) (Courtenay et al., 1980) and experimental autoimmune encephalomyelitis (EAE) (Fritz et al., 1983). In order to investigate whether CFA can effectively facilitate the induction of TFA-specific immune responses, female BALB/cByJ mice were immunized with TFA-MSA in conjunction with CFA, followed by a booster immunization with TFA-MSA plus IFA one week later. Seven days after the second immunization, the liver, spleen and lymph nodes (inguinal and peri-aortic) were removed. TFA-specific T cell response was determined by measuring T cell proliferation and IFN-γ production in response to ex vivo re-stimulation with TFA-CCH, CCH, TFA-MSA or MSA. Control mice were immunized with MSA in combination with CFA and IFA, and cells isolated from these mice did not respond to ex vivo re-stimulation with any antigens (data not shown). In contrast, lymph node cells isolated from TFA-MSA/CFA-immunized mice exhibited a stronger proliferation response when re-stimulated by TFA-MSA than native MSA (Fig. 1A). Similarly, splenocytes and LMNC isolated from these mice produced much higher amounts of IFN-γ when stimulated by TFA-MSA than by MSA (Fig. 1B). However, the cells isolated from various tissues of TFA-MSA/CFA-immunized mice did not respond to ex vivo re-stimulation by TFA-CCH, suggesting that the immune response generated by using CFA as an adjuvant was dependent on the carrier protein.
Figure 1. Measurement of TFA-specific immune responses in BALB/cByJ mice immunized by TFA-MSA in conjunction with CFA.
Female BALB/cByJ mice were injected s.c. at the base of the tail with the emulsion of TFA-MSA (50 µg protein/mouse) and CFA. One week later, the mice were boosted with TFA-MSA and IFA. Seven days after the last immunization, mice were sacrificed and the LN cells, splenocytes and LMNC were isolated. A. LN cells (5 × 105 /well in 96-well plate) were stimulated with 100 µg/ml of TFA-CCH, CCH, TFA-MSA or MSA for 4 days. T cell proliferation was measured by [3 H]-thymidine incorporation during the last 16 h of incubation. The measurements were carried out in triplicates and the results represent mean ± SEM of 6 mice per group. *P<0.05 compared with MSA stimulation. B. Splenocytes (10 × 106/well in 24-well plate) and LMNC (5 × 105 /well in 96-well plate) were re-stimulated ex vivo with 50µg/ml of TFA-CCH, CCH, TFA-MSA or MSA for 48 h. The supernatants were collected to measure IFN-γ production by ELISA. Results shown represent mean ± SEM of 6 mice per group from 2 independent experiments. *P<0.05 compared with MSA stimulation.
3.2. Evaluation of the effectiveness of the combined CD40/TLR agonists as an adjuvant in generating TFA-specific T cell responses
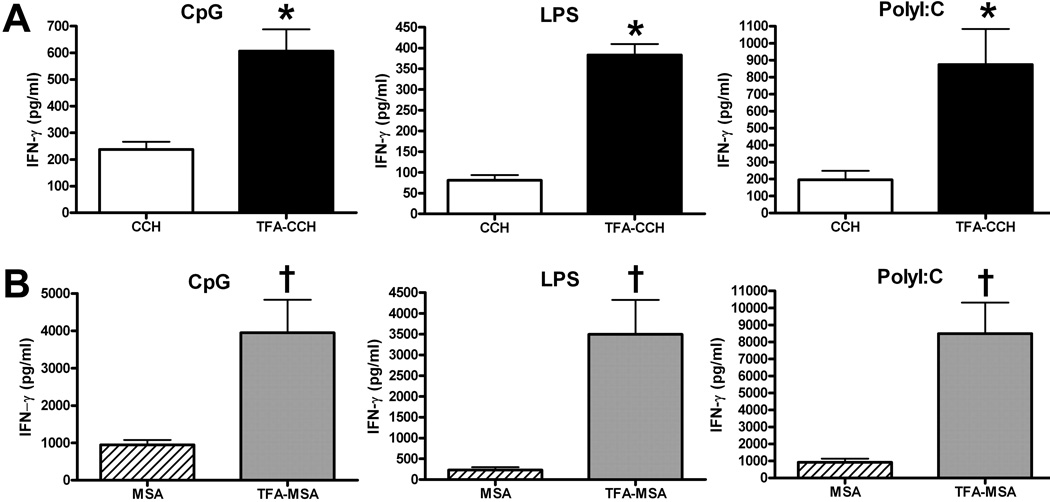
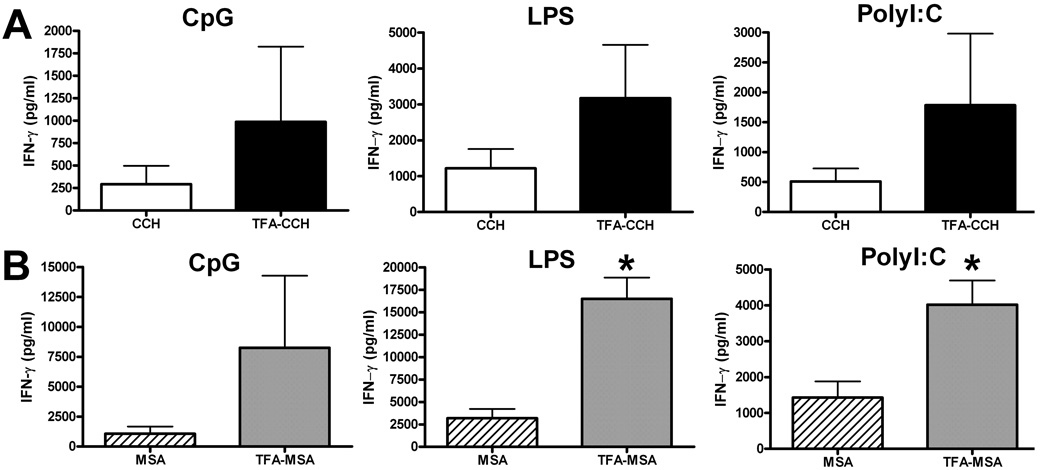
The combination of an anti-CD40 Ab and a TLR agonist has been shown to induce potent CD8+ T cell responses in mice (Ahonen et al., 2004). This approach is adopted in the present study to investigate whether the combination of anti-CD40 Ab with 1 of the 3 TLR agonists: LPS, polyI:C and CpG, represents an effect adjuvant in the generation of TFA-specific immune responses in mice. Female BALB/cByJ mice were i.p. injected with TFA-MSA, anti-CD40 Ab and either LPS, polyI:C or CpG. After one week, splenocytes were isolated and ex vivo re-stimulated with TFA-MSA, MSA, TFA-CCH or CCH. IFN-γ production in the supernatant was measured by ELISA to determine TFA-specific T cell responses. The data revealed that cells re-stimulated with TFA-MSA or TFA-CCH produced significantly higher levels of IFN-γ compared with those stimulated with the corresponding native protein (MSA or CCH) (Fig. 2). All 3 TLR agonists were potent in eliciting TFA-specific T cells, although polyI:C appeared to be slightly more effective. These results indicate that, superior to CFA, the combined CD40/TLR agonists were able to induce TFA-specific, rather than carrier protein-specific immune responses.
Fig. 2. Immunization of BALB/cByJ mice with TFA-MSA and the combined CD40/TLR agonist generated strong TFA-specific immune responses.
Female BALB/cByJ mice were injected i.p. with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab, and 1 of the 3 TLR agonists: LPS, polyI:C or CpG, (100 µg). One week later, mice were sacrificed and splenocytes isolated. The cells (10 × 106 cells/well, 24-well plates) were re-stimulated with 50 µg /ml of the various antigens including TFA-CCH or CCH (A), and TFA-MSA or MSA (B) for 48 h. The supernatants were collected to measure IFN-γ production. The measurements were carried out in triplicate and the results shown represent mean ± SEM of at least 6 mice per group. *P<0.05 compared with cells stimulated with CCH. †P<0.05 compared with cells stimulated with MSA.
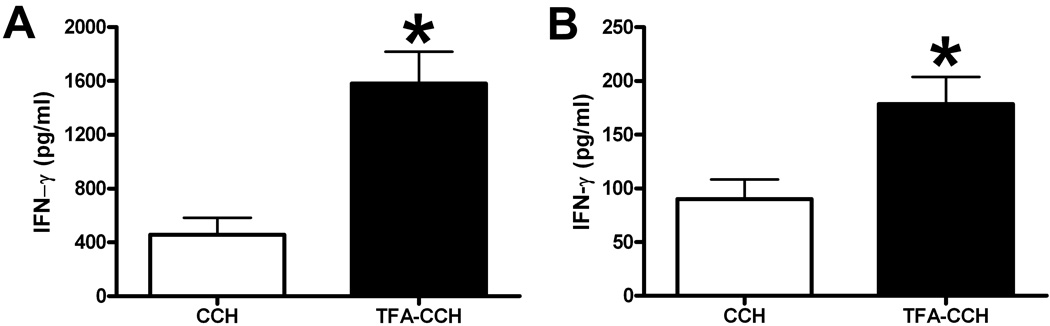
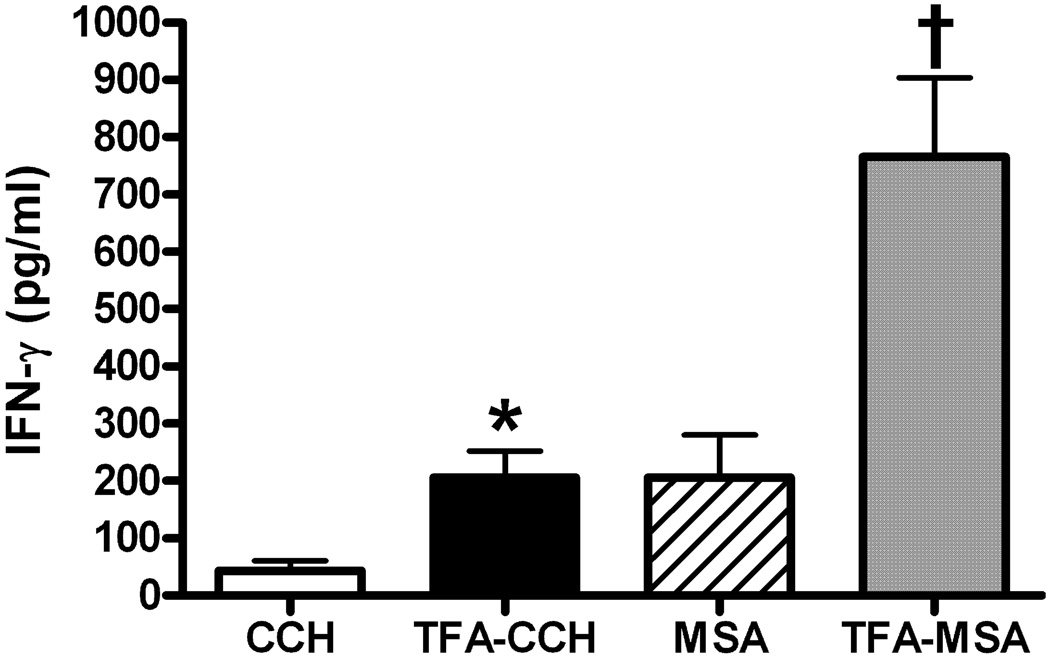
To delineate the subtype of activated TFA-specific T cells, CD4+ and CD8+ T cells were further purified from splenocytes isolated from mice immunized with TFA-MSA in conjunction with CD40/CpG agonist. Ex vivo re-stimulation of the two subsets of T cells by TFA-CCH induced a similar fold-increase in IFN-γ production compared with those stimulated with CCH. Although CD4+ T cells produced higher levels of IFN-γ than CD8+ T cells, this is due to the difference in the number of cells isolated and cultured between the two types (Fig. 3). In addition to splenocytes, LMNC isolated from mice immunized with TFA-MSA and CD40/CpG agonist also exhibited TFA-specific activation when re-stimulated ex vivo with TFA-CCH (Fig. 4).
Fig. 3. Both CD4+ and CD8+ subsets of TFA-specific T cells were generated in BALB/cByJ mice immunized by TFA-MSA and the combined CD40/CpG agonist.
Female BALB/cByJ mice were injected i.p. with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab, and 100 µg of CpG. One week later, mice were sacrificed and splenocytes isolated. CD4+ or CD8+ T cells were further purified by using a T cell enrichment kit. Subsequently, 2.5× 106 of CD4+ T cells (A) and 1.25× 106 of CD8+ T cells(B) per well of 48-well plate were cultured with naïve splenocytes (1 × 106 cells/well), which served as APCs, in the presence of various antigens (50 µg /ml) for 48 h. IFN-γ production was measured in the supernatant by ELISA. The measurements were carried out in triplicate and the results shown represent mean ± SEM of 3 mice per group. *P<0.05 compared with the LMNC stimulated with CCH. The data shown is the representative of two independent experiments.
Fig. 4. Immunization of BALB/cByJ mice with TFA-MSA and the combined CD40/TLR9 agonist generated TFA-specific T cells in the liver.
Female BALB/cByJ mice were injected i.p. with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab, and 100 µg of CpG. One week later, mice were sacrificed and LMNC isolated. The cells (5 × 105 cells/well) were cultured in the presence of various antigens (50 µg /ml) for 48 h. IFN-γ production was measured in the supernatant by ELISA. The measurements were carried out in triplicate and the results shown represent mean ± SEM of 3 mice per group. *P<0.05 compared with LMNC stimulated with CCH. †P<0.05 compared with LMNC stimulated with MSA. The data shown is the representative of two independent experiments.
3.3. Investigation of strain-dependent variations in the susceptibility to developing TFA-specific immune responses
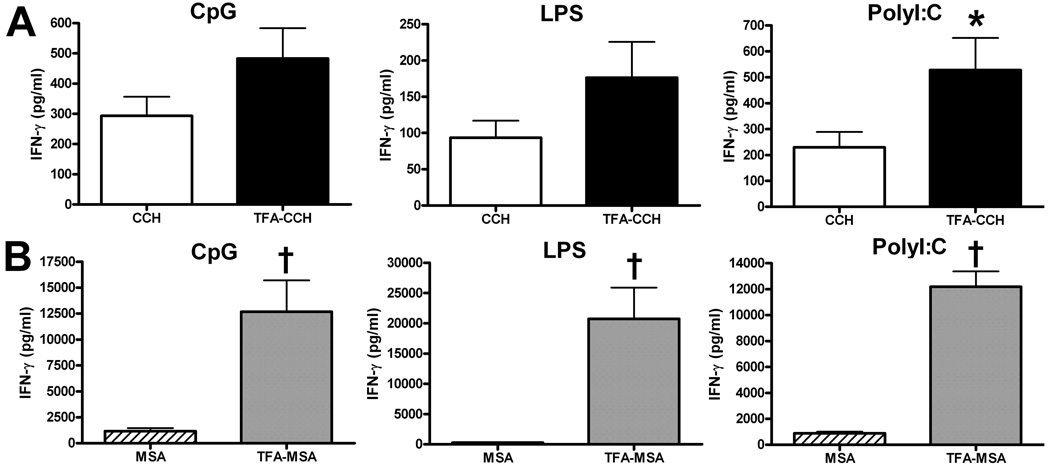
In the previous study, we found that female BALB/cByJ and DBA/1 mice, rather than C57BL/6J mice, can develop halothane induced acute liver injury (You et al., 2006). To examine whether the combined CD40/TLR agonist has varying effects on different strains of mice, female DBA/1 and C57BL/6J mice were immunized with TFA-MSA in combination an anti-CD40 Ab with and a TLR agonist. The data showed that this regimen was not able to induce TFA-specific T cell reactions in C57BL/6J mice (Fig. 6), and that DBA/1 mice only responded to the combined CD40/polyI:C agonist immunization, but not the other two TLR agonists (Fig. 5).
Fig. 6. Immunization of C57BL/6J mice with TFA-MSA and the combined CD40/TLR agonists did not generate TFA-specific immune responses.
Female C57BL/6J mice were injected i.p. with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab, and 1 of the 3 TLR agonists: LPS, polyI:C or CpG, (100 µg). One week later, mice were sacrificed and splenocytes isolated. The cells (10 × 106 cells/well, 24-well plates) were re-stimulated with 50 µg /ml of various antigens, including TFA-CCH or CCH (A), and TFA-MSA or MSA (B) for 48 h. The supernatants were collected to measure IFN-γ production. The measurements were carried out in triplicate and the results shown represent mean ± SEM of at least 6 mice per group. *P<0.05 compared with cells stimulated with MSA.
Fig. 5. Immunization of DBA/1 mice with TFA-MSA in combination with CD40/polyI:C, but not CD40/LPS or CD40/CpG generated TFA-specific immune responses.
Female DBA/1 mice were injected i.p. with 0.5 mg of TFA-MSA, 40 µg of anti-CD40 Ab, and 1 of the 3 TLR agonists: LPS, polyI:C or CpG, (100 µg). One week later, mice were sacrificed and splenocytes isolated. The cells (10 × 106 cells/well, 24-well plates) were re-stimulated with 50 µg /ml of various antigens, including TFA-CCH or CCH (A), and TFA-MSA or MSA (B) for 48 h. The supernatants were collected to measure IFN-γ production. The measurements were carried out in triplicate and the results shown represent mean ± SEM of at least 6 mice per group. *P<0.05 compared with cells stimulated with CCH. †P<0.05 compared with cells stimulated with MSA.
4. Discussion
IADRs represent a significant economic burden and safety concern to the health care community and the pharmaceutical industry. The key to predicting and preventing IADR is a thorough understanding of the underlying mechanisms. Progress at this front has been hampered by the scarcity of good animal models. Several models, including D-penicillamine-induced lupus in Brown Norway rats (Donker et al., 1984;Masson and Uetrecht, 2004), nevirapine-induced skin rash in rats (Shenton et al., 2003), sulfonamide-induced hypersensitivity in dogs (Trepanier, 2004;Lavergne and Trepanier, 2007), and propylthiouracil-induced autoimmunity in cats (Waldhauser and Uetrecht, 1996), resemble the reactions occur in humans; however their utility in studying the underlying pathogenesis is associated with practical limitations. Tools and reagents are not readily available for genetic and immunological studies in animal species except mouse. Therefore, it is our aim to develop a murine model of IADRs and this report describes the successful completion of a crucial step in this endeavor.
We chose halothane because the clinical characteristics of halothane hepatitis, including the presence of auto-antibodies and TFA-specific antibodies, eosinophilia, and a greater prevalence after multiple exposures, are consistent with an IADR (Walton et al., 1976;Benjamin et al., 1985;Neuberger and Kenna, 1987). Several attempts have been made previously to generate TFA-specific T cell responses by immunizing various species of animals with TFA modified serum albumin or hepatocytes from halothane-treated animals (Mathieu et al., 1975;Reves and McCracken, Jr., 1976;Neuberger et al., 1987;Chen and Gandolfi, 1997); however, potent immune reactions were not observed. In the hepatocyte preparation, the target antigens may be diluted resulting in reduced immune response. In the case of TFA-serum albumin, the response was stronger against the carrier proteins rather than to TFA (Reves and McCracken, Jr., 1976). Several strategies were employed in the current study to optimize the generation of TFA-specific T cell reactions. First, the use of MSA as the carrier protein can minimize background reactions because MSA is a self-antigen and is not expected to elicit an immune response in mice. Our previous study demonstrated that treatment of mice with the nitroso reactive metabolite of sulfamethoxazole (SMX-NO) induced SMX-NO-specific T cell reactions in the spleen and lymph nodes (Cheng et al., 2008). MSA adducts of SMX-NO were also detected in these lymphoid tissues, suggesting a strong propensity of drug-MSA conjugates to elicit drug-specific immune responses. Second, the TFA-MSA adducts synthesized and used in this study had high hapten/carrier protein ratios, as greater than 50% of the lysine residues of MSA were adducted by TFA. We found that if TFA modification accounted for less than 30% of total lysine residues, the adducts would not induce significant T cell responses (data not shown). This finding is consistent with the hypothesis that the hapten/carrier protein ratio is a key determinant of the strength of the immune response that can be induced against the hapten.
Third, we used the combination of an anti-CD40 Ab and a TLR agonist as an adjuvant to significantly enhance the immunogenicity of TFA-MSA. The data demonstrated that the combined CD40/TLR agonist was superior to a common adjuvant, CFA, which has been widely used in immunological research and the development of several mouse models of autoimmune diseases, such as RA (Courtenay et al., 1980) and EAE (Fritz et al., 1983). We found that lymphocytes, isolated from the spleen and liver of TFA-MSA/CFA-immunized mice, produced larger amounts of IFN-γ and proliferated to a greater extent when the cells were re-stimulated ex vivo with TFA-MSA than with MSA (Fig. 1). However, no response was observed when the cells were stimulated by TFA-CCH. These data suggest that the use of CFA as adjuvant generates a population of T cells that recognize MSA-derived specific peptides and that these cells do not respond to TFA-modified peptides derived from a different protein. This finding is consistent with a previous report that immunization of guinea pigs induced more potent immune response against the carrier protein rather than to TFA (Reves and McCracken, Jr., 1976). In contrast, TFA-specific and carrier protein independent T cell reactions were elicited when mice were immunized with TFA-MSA in conjunction with the combined CD40/TLR agonist. Ample evidence support that TLR agonists can trigger dendritic cell maturation and cytokine production, thereby bridging the innate and adaptive immunities (Pasare and Medzhitov, 2004). As such, the potential of TLR agonists as vaccine adjuvants has been intensely investigated. However, many studies have shown that the use of a single TLR agonist as adjuvant could not generate T cell responses to a similar degree as those observed during an actual infection (Butz and Bevan, 1998;Busch and Pamer, 1999;Tscharke et al., 2005). A recent study examined the outcome of combining various TLR agonists and demonstrated that TLR3 and TLR4 agonists synergized with TLR7, TLR8 and TLR9 agonists, thereby stimulating enhanced and sustained T helper type 1 immune reactions in vitro (Napolitani et al., 2005). However, the advantage of such combination of TLR agonists in vivo has not been evaluated. Interestingly, it has been shown that immunization of mice with ovalbumin (OVA) in combination with both an anti-CD40 Ab and a TLR agonist induced 10- to 20-fold greater CD8+ T cell responses than immunization with either agonist alone (Ahonen et al., 2004). It appeared that the combination, rather than CD40 Ab alone or TLR agonist alone, could stimulate dendritic cell expression of CD70, which engages with CD27 (expressed on T cells) and plays a critical role in the development of potent cell-mediated immunity. (Hendriks et al., 2000;Taraban et al., 2004;Bullock and Yagita, 2005). The above-described study further showed that all TLR agonists were potent in generating OVA-specific T cell responses when combined with anti-CD40 Ab; nonetheless, each TLR agonists synergized to varying degrees with CD40 (Ahonen et al., 2004). In the present study, we compared 3 agonists: polyI:C (TLR3), LPS (TLR4), and CpG (TLR9), and the data revealed that each TLR agonist, in conjunction with the anti-CD40 Ab, could effectively induce TFA-specific T cell responses in BALB/cByJ mice (Fig. 2). It appeared that polyI:C is slightly more potent than CpG and LPS. Although the previous study focused on CD8+ T cell expansion caused by the combined CD40/TLR agonist immunization (Ahonen et al., 2004), we found that both CD4+ and CD8+ T cells were activated, as both subsets of T cells isolated from the spleen of mice immunized with TFA-MSA and CD40/CpG agonist produced IFN-γ upon ex vivo re-stimulation by TFA-CCH (Fig. 3). This finding is consistent with the report that CpG can stimulate IFN-γ production by antigen-specific CD4+ T cells and increase the number of IFN-γ producing CD8+ effector T cells (Welters et al., 2007).
We found that, aside from splenic T cells, those isolated from the liver of immunized mice were also activated in a TFA-specific manner (Fig. 4). Although our data did not delineate whether the activated T cells within the liver were hepatic resident cells or cells recruited from the system circulation, it is intriguing to detect TFA-specific T cells in the liver because these cells presumably play a crucial role in recognizing and killing TFA-antigen-bearing hepatocytes, thereby resulting in halothane hepatitis. We previously developed a mouse model of halothane-induced acute liver injury and reported a strain-dependent susceptibility to such hepatotoxicity (You et al., 2006). The current study demonstrated that varying immune responses also occur in different strains of mice immunized with TFA-MSA and the combined CD40/TLR agonists. This immunization regiment was not able to induce TFA-specific T cell reactions in C57BL/6J mice or in DBA/1 mice, with the exception of polyI:C (Fig. 5 and Fig. 6). These data indicate that the BALB/cByJ mouse is the strain of choice in developing a model of halothane hepatitis because these mice are the most susceptible to developing TFA-specific immune responses as well as halothane-induced acute liver injury, which is a prerequisite for the subsequent immune-mediated tissue damage.
In summary, we showed that, using the combined CD40/TLR agonists as adjuvant, TFA-specific T cell responses could be generated in mice. The observation that these responses not only occur in the spleen but also in the liver indicates this immunization procedure as a promising approach in further developing a model of halothane hepatitis. Future studies will investigate whether administration of immunized mice with halothane could result in overt tissue injury. The combined findings that BALB/cByJ mice generated the greatest TFA-specific immune responses and that they were the most susceptible strain to halothane-induced acute liver injury provide the basis for employing this strain for the model development. Collectively, the data presented mark the successful completion of a critical step in the effort of developing a mouse model of immune-mediated halothane-induced hepatitis.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant RO1 ES012914 (to C.J.).
Abbreviations
- IADRs
immune-mediated adverse drug reactions
- MSA
mouse serum albumin
- CCH
Concholepas concholepas hemocyanin
- s.c.
subcutaneously
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- PolyI:C
polyinosinic-polycytidylic acid
- TNFR
tumor necrosis factor receptor
- APC
antigen presenting cell
- i.p.
intraperitoneally
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Reference List
- Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J.Exp.Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Mammalian Toll-like receptors. Curr.Opin.Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Benjamin SB, Goodman ZD, Ishak KG, Zimmerman HJ, Irey NS. The morphologic spectrum of halothane-induced hepatic injury: analysis of 77 cases. Hepatology. 1985;5:1163–1171. doi: 10.1002/hep.1840050617. [DOI] [PubMed] [Google Scholar]
- Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J.Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J.Exp.Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz E, Bevan MJ. Dynamics of the CD8+ T cell response during acute LCMV infection. Adv.Exp.Med.Biol. 1998;452:111–122. doi: 10.1007/978-1-4615-5355-7_13. [DOI] [PubMed] [Google Scholar]
- Chen M, Gandolfi J. Characterization of the humoral immune response and hepatotoxicity after multiple halothane exposures in guinea pigs. Drug Metab Rev. 1997;29:103–122. doi: 10.3109/03602539709037575. [DOI] [PubMed] [Google Scholar]
- Cheng L, Stewart BJ, You Q, Petersen DR, Ware JA, Piccotti JR, Kawabata TT, Ju C. Covalent binding of the nitroso metabolite of sulfamethoxazole is important in induction of drug-specific T-cell responses in vivo. Mol.Pharmacol. 2008;73:1769–1775. doi: 10.1124/mol.107.043273. [DOI] [PubMed] [Google Scholar]
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Donker AJ, Venuto RC, Vladutiu AO, Brentjens JR, Andres GA. Effects of prolonged administration of D-penicillamine or captopril in various strains of rats. Brown Norway rats treated with D-penicillamine develop autoantibodies, circulating immune complexes, and disseminated intravascular coagulation. Clin.Immunol.Immunopathol. 1984;30:142–155. doi: 10.1016/0090-1229(84)90015-1. [DOI] [PubMed] [Google Scholar]
- Fritz RB, Chou CH, McFarlin DE. Relapsing murine experimental allergic encephalomyelitis induced by myelin basic protein. J.Immunol. 1983;130:1024–1026. [PubMed] [Google Scholar]
- Goldberger RF. [34] Trifluoroacetylation of [epsilon]-amino groups. In: Hirs HW, editor. Methods in Enzymology Enzyme Structure. Academic Press; 1967. pp. 317–322. [Google Scholar]
- Goldberger RF, Anfinsen CB. The Reversible Masking of Amino Groups in Ribonuclease and Its Possible Usefulness in the Synthesis of the Protein. Biochemistry. 1962;1:401–405. [Google Scholar]
- Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J.Immunol. 2004;172:1501–1507. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat.Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- Kenna JG, Satoh H, Christ DD, Pohl LR. Metabolic basis for a drug hypersensitivity: antibodies in sera from patients with halothane hepatitis recognize liver neoantigens that contain the trifluoroacetyl group derived from halothane. J.Pharmacol.Exp.Ther. 1988;245:1103–1109. [PubMed] [Google Scholar]
- Lavergne SN, Trepanier LA. Anti-platelet antibodies in a natural animal model of sulphonamide-associated thrombocytopaenia. Platelets. 2007;18:595–604. doi: 10.1080/09537100701392913. [DOI] [PubMed] [Google Scholar]
- Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J.Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- Masson MJ, Uetrecht JP. Tolerance induced by low dose D-penicillamine in the brown Norway rat model of drug-induced autoimmunity is immune-mediated. Chem.Res.Toxicol. 2004;17:82–94. doi: 10.1021/tx034195a. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Dipadua D, Kahan BD, Galdabini JJ, Mills J. Correlation between specific immunity to a metabolite of halothane and hepatic lesions after multiple exposures. Anesth.Analg. 1975;54:332–339. doi: 10.1213/00000539-197505000-00014. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat.Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger J, Kenna JG. Halothane hepatitis: a model of immune mediated drug hepatotoxicity. Clin.Sci.(Lond) 1987;72:263–270. doi: 10.1042/cs0720263. [DOI] [PubMed] [Google Scholar]
- Neuberger JM, Kenna JG, Williams R. Halothane hepatitis: attempt to develop an animal model. Int.J.Immunopharmacol. 1987;9:123–131. doi: 10.1016/0192-0561(87)90086-5. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin.Immunol. 2004;16:23–26. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Pohl LR. Drug-induced allergic hepatitis. Semin.Liver Dis. 1990;10:305–315. doi: 10.1055/s-2008-1040486. [DOI] [PubMed] [Google Scholar]
- Pohl LR, Kenna JG, Satoh H, Christ D, Martin JL. Neoantigens associated with halothane hepatitis. Drug Metab Rev. 1989;20:203–217. doi: 10.3109/03602538909103537. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol.Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Ray DC, Drummond GB. Halothane hepatitis. Br.J.Anaesth. 1991;67:84–99. doi: 10.1093/bja/67.1.84. [DOI] [PubMed] [Google Scholar]
- Reves JG, McCracken LE., Jr. Failure to induce hepatic pathology in animals sensitized to a halothane metabolite and subsequently challenged with halothane. Anesth.Analg. 1976;55:235–242. doi: 10.1213/00000539-197603000-00024. [DOI] [PubMed] [Google Scholar]
- Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, Seder RA. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J.Exp.Med. 2002;195:1565–1573. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Martin BM, Schulick AH, Christ DD, Kenna JG, Pohl LR. Human anti-endoplasmic reticulum antibodies in sera of patients with halothane-induced hepatitis are directed against a trifluoroacetylated carboxylesterase. Proc.Natl.Acad.Sci.U.S.A. 1989;86:322–326. doi: 10.1073/pnas.86.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton JM, Teranishi M, Abu-Asab MS, Yager JA, Uetrecht JP. Characterization of a potential animal model of an idiosyncratic drug reaction: nevirapine-induced skin rash in the rat. Chem.Res.Toxicol. 2003;16:1078–1089. doi: 10.1021/tx034064+. [DOI] [PubMed] [Google Scholar]
- Taraban VY, Rowley TF, Al Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J.Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- Trepanier LA. Idiosyncratic toxicity associated with potentiated sulfonamides in the dog. J.Vet.Pharmacol.Ther. 2004;27:129–138. doi: 10.1111/j.1365-2885.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J.Exp.Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani D, Mieli-Vergani G, Alberti A, Neuberger J, Eddleston AL, Davis M, Williams R. Antibodies to the surface of halothane-altered rabbit hepatocytes in patients with severe halothane-associated hepatitis. N.Engl.J.Med. 1980;303:66–71. doi: 10.1056/NEJM198007103030202. [DOI] [PubMed] [Google Scholar]
- Waldhauser L, Uetrecht J. Antibodies to myeloperoxidase in propylthiouracil-induced autoimmune disease in the cat. Toxicology. 1996;114:155–162. doi: 10.1016/s0300-483x(96)03476-2. [DOI] [PubMed] [Google Scholar]
- Walton B, Simpson BR, Strunin L, Doniach D, Perrin J, Appleyard AJ. Unexplained hepatitis following halothane. Br.Med.J. 1976;1:1171–1176. doi: 10.1136/bmj.1.6019.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters MJ, Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. Multiple CD4 and CD8 T-cell activation parameters predict vaccine efficacy in vivo mediated by individual DC-activating agonists. Vaccine. 2007;25:1379–1389. doi: 10.1016/j.vaccine.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J.Exp.Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R, Eade OE, Chisholm M, Hawksley M, Lloyd B, Moles TM, Edwards JC, GArdner MJ. Controlled prospective study of the effect on liver function of multiple exposures to halothane. Lancet. 1975;1:817–820. doi: 10.1016/s0140-6736(75)93000-7. [DOI] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]