Abstract
Angiotensin II (Ang II) produces oxidative stress and endothelial dysfunction in blood vessels. The vasculature from females may be protected against deleterious effects of Ang II. We tested the hypothesis that manganese superoxide dismutase (MnSOD) protects against Ang II-induced endothelial dysfunction. Experiments were performed in C57Bl/6, wild-type (MnSOD+/+) and MnSOD deficient (MnSOD+/−) mice treated systemically with vehicle or Ang II. Basilar arteries were isolated from C57Bl/6 mice treated for one week with a non-pressor dose of Ang II (0.28 mg/kg × day). Ang II treatment produced superoxide-mediated impairment of responses to the endothelium-dependent vasodilator acetylcholine (Ach)(P<0.05). In male, but not female MnSOD+/+ mice, Ang II modestly inhibited responses to ACh (P<0.05). In contrast, Ang II selectively impaired these responses by up to 70% in male MnSOD+/− mice (P<0.05) and this effect was reversed by tempol (P<0.05). Ang II had no effect on ACh responses in MnSOD+/− female mice. Vascular superoxide levels following treatment with an inhibitor of CuZn- and extracellular-SOD, were higher in Ang II-treated vs vehicle-treated MnSOD+/− mice. Thus, a non-pressor dose of Ang II produces endothelial dysfunction in male mice only, suggesting that the female vasculature is protected from Ang II. In male, but not female mice, MnSOD deficiency enhanced endothelial dysfunction, suggesting that MnSOD normally protects the vasculature during disease states in which Ang II contributes to vascular dysfunction.
Keywords: genetically-altered mice, cerebral arteries, mitochondria, oxidative stress
INTRODUCTION
Chronic hypertension has many deleterious effects on blood vessels, particularly in the cerebral circulation where hypertension is a major risk factor for stroke and a leading cause of cognitive decline. The renin-angiotensin system and its main effector angiotensin II (Ang II) underlie many of the changes in vascular structure and function that occur in several forms of hypertension 1–4. Pharmacological inhibitors of the renin-angiotensin system are very useful clinically in the treatment of hypertension 5. In addition to mediating many of the negative effects of hypertension, Ang II also contributes to vascular disease in other states including atherosclerosis and aging 6, 7.
Ang II increases production of reactive oxygen species (ROS) in vascular cells via several mechanisms 4, 8–12 including increased formation in mitochondria 13–15. Mitochondria may be a particularly important source of superoxide in the cerebral vasculature as the mitochondrial content in cerebral endothelium is relatively high 16. Steady-state levels of superoxide in mitochondria are dependent on both its rate of production and activity of manganese superoxide dismutase (MnSOD), which converts superoxide to hydrogen peroxide (H2O2)17. MnSOD is more abundantly expressed in endothelial cells compared to other cell types 17, 18, suggesting it may be particularly important in protecting against endothelial dysfunction. Although expression and activity of MnSOD in blood vessels can change in disease states including hypertension 17, the functional importance of MnSOD in relation to Ang II and hypertension is unknown.
The goal of this study was thus to examine the hypothesis that MnSOD protects against Ang II-induced vascular dysfunction. For our approach, we used mice genetically deficient in MnSOD to examine the role of this form of SOD 18. These studies were performed using a non-pressor dose of Ang II administered systemically. Recent studies suggest that inhibitory effects of Ang II on vascular responses are much less in females 19, 20. A second goal was thus to test the hypothesis that Ang II produces vascular dysfunction in females in the presence of MnSOD deficiency.
METHODS
Experimental animals
Animals for study were derived from breeding MnSOD+/+ and MnSOD+/− mice, thus providing both genotypes and littermate controls as described 21. For some protocols, additional C57BL/6 male mice were studied (see below). Mice had access to regular chow and water ad libitum. All protocols and procedures conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Administration of Ang II and measurement of blood pressure
Following anesthesia with ketamine/xylazine (87.5 mg/kg and 12.5 mg/kg sc, respectively), an osmotic minipump (Alzet, model 1002) was placed subcutaneously in the mid-scapular region to administer vehicle (isotonic saline) or Ang II (0.28 mg/kg × d for 7 days) 22. Systolic blood pressure (BP) was measured using an automated tail-cuff device (BP-2000, Visitech Systems). Prior to surgery, mice were trained for 5 days and baseline BP was recorded, followed by implantation of minipumps and measurements of BP.
Studies of basilar arteries
Isolation and preparation of basilar arteries are described previously 10. In most experiments we recorded changes in diameter of the basilar artery in response to KCl (50 mmol/L). To examine responses to acetylcholine and nitroprusside, arteries were constricted submaximally (~40% of the response to KCl) using U46619. After development of a stable baseline diameter, dose-response curves were obtained. In some experiments, vessels were treated with tempol (100μmol/L, 30 min) prior to the addition of acetylcholine. Papaverine (100 μmol/L) was added at the end of each experiment to produce maximal vasodilation.
Measurement of ROS
Vascular superoxide levels were measured in aorta using 5μmol/L lucigenin-enhanced chemiluminesence. Following measurements under basal conditions, NADPH (100 μmol/L) was added to stimulate NADPH oxidase-dependent superoxide formation. Tiron (10 mmol/L, a superoxide scavenger) inhibited the lucigenin signal by ~80–85% (data not shown). To minimize potential confounding effects of other SODs, some experiments were performed in the presence of an inhibitor of CuZnSOD and ECSOD [diethyldithiocarbamate (DETC), 10 mmol/L]. 17
Drugs
Acetylcholine, angiotensin II, nitroprusside, papaverine, and tempol, were dissolved in saline. Tiron, lucigenin, NADPH and DETC were dissolved in Dulbecco’s phosphate buffered saline. Most drugs were obtained from Sigma (St. Louis, MO). U46619 was obtained from Cayman Chemical (Ann Arbor, MI) and dissolved in 100% ethanol, with subsequent dilutions being made with Krebs buffer.
Statistical analysis
All data are expressed as mean±SE. Vasodilator responses are expressed as % dilation (% of induced tone), with 100% representing the difference between the resting value under basal conditions and the constricted value with U46619. Vasoconstriction to KCl is expressed as % change in diameter over baseline. For experiments using lucigenin-enhanced chemiluminesence, data were normalized to tissue dry weight, and expressed as RLU (relative light units)/s × mg. Changes in BP were calculated by subtracting the baseline BP from the average BP over days 5–7 of treatment. Comparisons of vasodilation and vasoconstriction, superoxide levels, and BP were made using ANOVA with Student-Newman Keuls post hoc-test, or Students t-test, as appropriate. Statistical significance was accepted at P<0.05.
RESULTS
Effects of Ang II in male C57Bl/6 mice
To establish the protocol in this model, we first studied male C57Bl/6 mice. Resting BP was similar in vehicle (118±7 mmHg, n=10) and Ang II-treated (112±5 mmHg, n=10) mice. Vehicle and Ang II treatment had no significant effect on BP (Δ BP in vehicle-treated mice: 1±4 mmHg, n=10; Δ BP in Ang II-treated mice: 5±6 mmHg, n=10; P>0.05). These results are consistent with previous work 23. Body weight was unaffected by vehicle (weight before treatment: 25±1 g; weight after treatment: 26±1 g, n=10) or Ang II (weight before treatment: 27±1 g; weight after treatment: 28±1 g, n=10) treatment.
Baseline diameter of the basilar artery in C57Bl/6 mice treated with vehicle and Ang II was 137±4 μm (n=8) and 137±5 μm (n=10), respectively (P>0.05). Ang II treatment impaired vasodilator responses to acetylcholine compared to vehicle treatment (Figure 1a). Responses to nitroprusside (Figure 1b), KCl, and papaverine (Supplemental Table 1, http://hyper.ahajournals.org) were not affected by Ang II. Ang II-induced impairment of vasodilation to acetylcholine was reversed by tempol, suggesting the endothelial dysfunction was superoxide-mediated (Supplemental Figure 1, http://hyper.ahajournals.org).
Figure 1.
Vasodilation to acetylcholine (A: vehicle, n=8; Ang II, n=10), and nitroprusside (B: vehicle, n=9; Ang II, n=10) in C57Bl/6 mice. All data are mean±SE.
Ang II had no significant effect on arterial pressure in male MnSOD+/+ or MnSOD+/− mice
Resting BP was similar in male MnSOD+/+ (117±5 mmHg, n=14) and MnSOD+/− (114±2 mmHg, n=16) mice. Vehicle and Ang II treatment had no significant effect on BP in MnSOD+/+ mice (Δ BP in vehicle-treated mice: 3±6 mmHg, n=7; Δ BP in Ang II-treated mice: 14±7 mmHg, n=7; P>0.05) consistent with results in C57Bl/6 mice. Similarly, treatment with vehicle and Ang II had no significant effect on BP in MnSOD+/− mice (Δ BP in vehicle-treated mice: 9±3 mmHg, n=6; Δ BP in Ang II-treated mice: 7±6 mmHg, n=10; P>0.05), suggesting that MnSOD deficiency does not augment changes in blood pressure in response to a low dose of Ang II. Thus, differences in vascular function observed in MnSOD+/− mice are related to MnSOD deficiency and not due to differences in blood pressure. Body weight in MnSOD+/+ and MnSOD+/− mice was unaffected by vehicle or Ang II treatment (data not shown).
Endothelial dysfunction in response to Ang II was enhanced in male MnSOD+/− mice
Baseline diameter of the basilar artery in MnSOD+/+ mice treated with vehicle and Ang II was 138±4 μm (n=9) and 140±7μm (n=10), respectively (P>0.05). Baseline vessel diameter in MnSOD+/− mice was 132±6μm (n=11; P>0.05 vs vehicle treated MnSOD+/+) in animals treated with vehicle and 149±5 μm (n=14) in mice treated with Ang II (P<0.05 vs vehicle treated MnSOD+/−). Acetylcholine caused similar dilation in arteries from vehicle-treated male MnSOD+/+ and MnSOD+/− mice (Figure 2).
Figure 2.
Vasodilation to acetylcholine in (A) MnSOD+/+ mice treated with vehicle (n=9) and Ang II (n=10); and (B) MnSOD+/− mice treated with vehicle (n=11) and Ang II (n=14). Vasodilation to nitroprusside in (C) MnSOD+/+ mice treated with vehicle (n=6) and Ang II (n=6); and (D) MnSOD+/− mice treated with vehicle (n=8) and Ang II (n=8). All data are from male mice. All data are mean±SE.
Vasodilation to acetylcholine was modestly impaired in male MnSOD+/+ mice treated with Ang II compared to vehicle (Figure 2a). In contrast, Ang II treatment impaired vasodilator responses to acetylcholine in MnSOD+/− mice by as much as 70% (P<0.05, Figure 2b). These findings suggest that partial MnSOD deficiency markedly enhances Ang II-induced endothelial dysfunction.
Vasodilation to nitroprusside (Figures 2c & d) and papaverine (Supplemental Table 2, http://hyper.ahajournals.org) were similar following vehicle and Ang II treatment in MnSOD+/+ and MnSOD+/− mice. Vasoconstriction to KCl was also unaffected by genotype and Ang II treatment (Supplemental Table 2, http://hyper.ahajournals.org). These data suggest the inhibitory effects of Ang II were selective for endothelium-dependent responses.
Ang II-induced endothelial dysfunction in MnSOD+/− mice was reversed by tempol
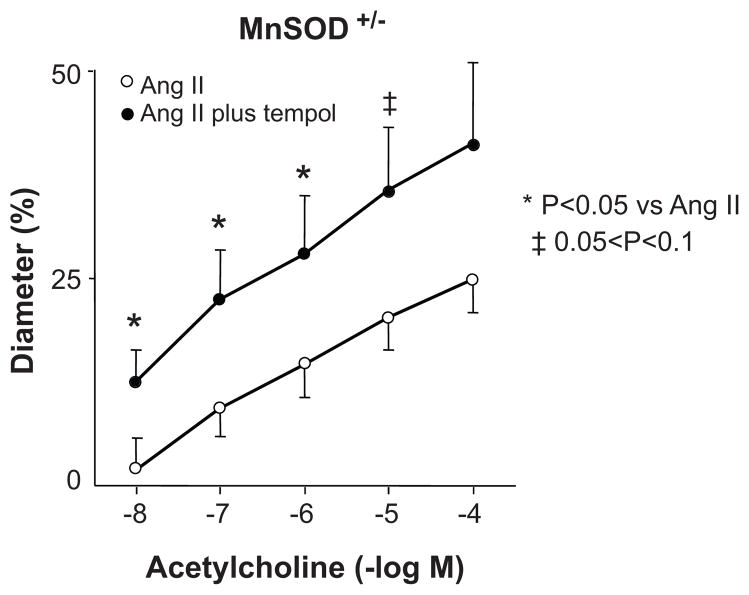
In MnSOD+/− mice, tempol restored acetylcholine-induced vasodilator responses in Ang II-treated mice to normal (Figure 3), whereas tempol had no significant effect on responses to acetylcholine in vehicle-treated mice (data not shown). These data suggest that increases in superoxide mediate endothelial dysfunction in response to Ang II in MnSOD+/− mice.
Figure 3.
Effect of tempol (100 μmol/L) on vasodilation to acetylcholine in Ang II- treated male MnSOD+/− mice (n=8). All data are mean±SE.
Ang II did not cause endothelial dysfunction in female mice
Baseline diameter of the basilar artery in female MnSOD+/+ mice treated with vehicle and Ang II was 143±12 μm (n=7) and 157±5 μm (n=6), respectively (P>0.05). Baseline vessel diameter in female MnSOD+/− mice was 149±4 μm (n=6; P>0.05 vs vehicle treated MnSOD+/+) in animals treated with vehicle and 157±11 μm (n=6) in mice treated with Ang II (P>0.05 vs vehicle treated MnSOD+/−). Body weight in MnSOD+/+ and MnSOD+/− was unaffected by vehicle or Ang II treatment (data not shown).
In contrast to males, Ang II had no significant effect on responses to acetylcholine in female mice of either genotype (Figures 4a & b). Responses to nitroprusside (Figure 4c) were increased in female MnSOD+/+ mice treated with Ang II compared to vehicle, suggesting responses to nitric oxide (NO) were increased. There was no effect of Ang II treatment on nitroprusside responses in MnSOD+/− mice (Figure 4d). In MnSOD+/+ and MnSOD+/− mice, vasoconstriction to KCl (Supplemental Table 2, http://hyper.ahajournals.org) was similar following vehicle and Ang II treatment.
Figure 4.
Vasodilation to acetylcholine in (A) MnSOD+/+ mice treated with vehicle (n=7) and Ang II (n=6); and (B) MnSOD+/− mice treated with vehicle (n=6) and Ang II (n=6). Vasodilation to nitroprusside in (C) MnSOD+/+ mice treated with vehicle (n=6) and Ang II (n=5); and (D) MnSOD+/− mice treated with vehicle (n=6) and Ang II (n=6). All data are from female mice. All data are mean±SE.
Superoxide levels
In both strains of male mice, there were no significant differences in vascular superoxide levels under basal conditions, in response to this low dose of Ang II (MnSOD+/+: vehicle-treated=16±4 RLU/s/mg, n=11; Ang II-treated=21±7 RLU/s/mg, n=11; MnSOD+/−: vehicle-treated=17±8 RLU/s/mg, n=10; Ang II-treated=27±8 RLU/s/mg, n=10), or in the presence of NADPH (data not shown). Superoxide levels were higher in the presence of DETC and NADPH compared to DETC alone (Figure 5). In the presence of DETC, Ang II tended to increase superoxide levels in MnSOD+/− (0.05<P<0.1), but not MnSOD+/+ mice (P=0.31, power=18%) (Figure 5). Superoxide levels in the presence of NADPH (and DETC) were greater in MnSOD+/− than in MnSOD+/+ mice treated with Ang II (Figure 5b), suggesting that MnSOD deficiency increases vascular superoxide production following Ang II treatment.
Figure 5.
Superoxide levels in the presence of (A) DETC (10 mmol/L, n=5–8); and (B) DETC and NADPH (100 μmol/L, n=5–9) in aorta from vehicle and Ang II-treated MnSOD+/+ and MnSOD+/− male mice. Note the change in scale between A and B. All data are mean±SE.
DISCUSSION
The major new finding of the present study was that Ang II-induced endothelial dysfunction in a cerebral artery is markedly enhanced in male heterozygous MnSOD deficient mice, suggesting that MnSOD is a critical component of mechanisms that protect the vasculature against Ang II. Cerebral arteries like the basilar artery are important resistance vessels in brain 24. To our knowledge, this is the first data of its kind for any blood vessel. The inhibitory effect of Ang II was reversed by a superoxide scavenger, and vascular superoxide levels following Ang II treatment were increased in MnSOD+/− mice. The effects on vascular function were seen with a low dose of Ang II that had little effect on arterial pressure. Interestingly, Ang II had no effect on vascular function in MnSOD+/+ or MnSOD+/− female mice, supporting the concept that the female cerebral vasculature is protected from deleterious effects of Ang II 19, 20 even in the presence of MnSOD deficiency. Protection against the deleterious effects of Ang II in the cerebral vasculature in females may occur at the level of sources of superoxide (NADPH oxidase) 25. Suppression of ROS formation in the cerebral vascular wall may be estrogen-dependent 19.
Ang II-induced oxidative stress and endothelial dysfunction
Scavengers of superoxide and other reactive oxygen species protect against endothelial dysfunction in carotid arteries and cerebral blood vessels and impairment of functional hyperemia in brain in response to acute and chronic treatment with Ang II, and in a genetic model of Ang II-dependent hypertension 4, 9, 10, 26, 27. One mechanism by which superoxide impairs endothelium-dependent relaxation is by decreasing bioavailability of NO 2, 17. Since Ang II is a major stimulus for production of superoxide in the vasculature 11, 12, 28, impaired endothelium-dependent vasodilation in response to Ang II may be due in large part to increased superoxide levels.
In the present study, we found that Ang II produced endothelial dysfunction that was reversed by a superoxide scavenger, suggesting superoxide was the mediator of the dysfunction. We observed no significant changes in arterial pressure with this low dose of Ang II, consistent with previous work 23. These observations suggest that Ang II-induced endothelial dysfunction may be largely independent of increased blood pressure. Direct effects of Ang II to impair responses of carotid artery and cerebral blood vessels to endothelium-dependent stimuli and functional hyperemia have been seen in several models 4, 9, 11, 26, 27. The current findings, that Ang II impairs endothelium-dependent responses, support these findings and the concept that cerebral blood vessels are particularly sensitive to Ang II.
MnSOD haploinsufficiency enhances Ang II-induced oxidative stress and endothelial dysfunction
Consistent with previous findings 18, 21, 29, deletion of a single copy of the gene for MnSOD did not alter responses in male or female cerebral arteries under baseline conditions. Although Ang II is known to increase ROS in mitochondria in endothelial cells 14, the functional importance of this effect in relation to regulation of vascular tone has been unknown. MnSOD is more abundantly expressed in endothelial cells relative to other cell types 17,18 and the mitochondrial content in cerebral endothelium is greater than that in other cells 16. Mitochondria may be a particularly important source of superoxide and thus MnSOD may play an important role in the cerebral circulation during oxidative stress. Perhaps the most important finding of this study is the observation that a dose of Ang II which produced only modest impairment of endothelial function in male wild-type mice, produced marked impairment of endothelial function in male MnSOD-deficient mice. Thus, MnSOD is an important mediator of vascular protection in response to Ang II.
Our data with tempol suggest that increased superoxide mediates the deleterious effects of Ang II under control conditions and in the presence of MnSOD deficiency. Ang II increased vascular superoxide levels in MnSOD-deficient mice under conditions where CuZnSOD and ECSOD were inhibited, suggesting that Ang II increased superoxide production. Furthermore, superoxide levels in the presence of NADPH (and DETC) were greater in MnSOD-deficient vs MnSOD+/+ mice treated with Ang II. This finding is consistent with the concept that Ang II-induced activation of NADPH oxidase leads to mitochondrial ROS production 14, 30. There are several isoforms of the catalytic subunit of NADPH oxidase (Nox1, Nox2, Nox4, Nox5) and the subcellular distribution of these proteins varies with cell type and tissue 2, 28. There has not been much evidence for NADPH oxidase localization in mitochrondria to our knowledge. However, a recent study has suggested that Nox4 may be present in mitochondria 31. Mitochondrial-derived ROS may activate NADPH oxidase 14, resulting in increased superoxide production and reduced NO bioavailability. Such a sequence would be consistent with our result showing enhanced NADPH-induced superoxide production in the presence of Ang II under conditions of MnSOD deficiency.
MnSOD deficiency does not enhance vascular dysfunction following treatment with Ang II in females
It is well established that premenopausal women have a lower incidence of cardiovascular disease, compared to age-matched men. Female gender is associated with vascular protection 19, 20, 32. Pressor doses of Ang II impair cerebral vascular responses to endothelium-dependent agonists in male, but not in female mice 19, 20. Our finding that Ang II had little effect on endothelial function in female mice is consistent with these previous reports. Our finding that even in the presence of MnSOD deficiency, endothelial function is not impaired following Ang II treatment in females is novel and further emphasizes the marked gender-dependent effects of Ang II on blood vessels.
Perspectives
ROS are thought to play a major role in vascular disease. The present study supports that concept and provides the first evidence that MnSOD deficiency promotes endothelial dysfunction in response to Ang II in males. Thus, MnSOD may be an important mediator of vascular protection during hypertension, but may also play a role in other states where Ang II is thought to contribute to vascular disease such as atherosclerosis and aging 6, 7. Because we observed significant vascular effects following deletion of only one copy of the MnSOD gene, our findings have implications for disease states or genetic polymorphisms that cause decreased expression or activity of MnSOD. We also demonstrated for the first time that even in the face of MnSOD deficiency, female mice are protected against vascular effects of Ang II.
Supplementary Material
Acknowledgments
We are grateful to the University of Iowa transgenic core for genotyping mice and to Dr. Sean Didion for help with maintenance of the MnSOD mouse colony.
SOURCES OF FUNDING
This work was supported by National Institutes of Health grants HL-62984, HL-38901, and NS-24621, American Heart Association grants 0575092N and 0725643Z, and by a CJ Martin Fellowship from the National Health and Medical Research Council of Australia (359282).
Footnotes
DISCLOSURES
Sophocles Chrissobolis NONE, Frank M. Faraci NIH grant HL-62984
References
- 1.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 5.Touyz RM. Reactive oxygen species as mediators of calcium signaling by Angiotensin II: implications in vascular physiology and pathophysiology. Antiox Redox Signalling. 2005;7:1302–1314. doi: 10.1089/ars.2005.7.1302. [DOI] [PubMed] [Google Scholar]
- 6.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 8.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: New insights from genetic models. J Cereb Blood Flow Metab. 2006;26:449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 11.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 12.Miller AA, Drummond GR, Schmidt HHW, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: Comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 14.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 15.Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, Ide T, Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens. 2008;26:2176–2184. doi: 10.1097/HJH.0b013e32830dd5d3. [DOI] [PubMed] [Google Scholar]
- 16.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM, Didion SP. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 18.Andresen JJ, Faraci FM, Heistad DD. Vascular responses in Mn-SOD deficient mice. Am J Physiol Heart Circ Physiol. 2004;287:H1141–H1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Anrather J, Milner TA, Iadecola C. Estrous cycle dependent neurovascular dysfunction induced by angiotensin II in the mouse neocortex. Hypertension. 2009;54:302–307. doi: 10.1161/HYPERTENSIONAHA.109.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol. 2007;294:H156–H163. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- 21.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- 22.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: Role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 24.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 26.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 27.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 28.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 29.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: Evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 30.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 31.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.