Abstract
Cells exploit autophagy for survival to metabolic stress in vitro as well as in tumors where it localizes to regions of metabolic stress suggesting its role as a survival pathway. Consistent with this survival function, deficiency in autophagy impairs cell survival, but also promotes tumor growth, creating a paradox that the loss of a survival pathway leads to tumorigenesis. There is evidence that autophagy is a homeostatic process functioning to limit the accumulation of poly-ubiquitinated proteins and mutant protein aggregates associated with neuronal degeneration.2,3 Interestingly, we found that deficiency in autophagy caused by monoallelic loss of beclin1 or deletion of atg5 leads to accelerated DNA damage and chromosomal instability demonstrating a mutator phenotype.4 These cells also exhibit enhanced chromosomal gains or losses suggesting that autophagy functions as a tumor suppressor by limiting chromosomal instability. Thus the impairment of survival to metabolic stress due to deficiency in autophagy may be compensated by an enhanced mutation rate thereby promoting tumorigenesis. The protective role of autophagy may be exploited in developing novel autophagy modulators as rational chemotherapeutic as well as chemopreventive agents.
Keywords: autophagy, metabolism, beclin1, atg5, DNA damage, chromosomal instability, cancer
Autophagy is a catabolic process that recycles macromolecules and organelles. As an evolutionarily conserved response to metabolic stress, autophagy facilitates cell survival during starvation by recycling intracellular constituents, but may also provide a means to cell death when it proceeds uninterrupted.5 Mammalian homologues of many of the proteins that orchestrate this process have been identified from yeast genetic screens, of which the essential autophagy proteins Beclin1 and Atg5 are most well studied. The process of autophagy is characterized by the formation of double membrane vesicles known as autophagosomes that encapsulate and facilitate the transport of protein aggregates and other macromolecules and organelles to lysosomes where they are degraded and the resulting components recycled, generating ATP.6 The formation of phagophores, the precursors of autophagosomes, is initiated by the class III phosphatidylinositol (PI)-3 kinase-Beclin1 complex. Phagophores expand and entrap the cargo in subsequent steps mediated by the Atg12-Atg5-Atg16 complex and LC3-II, a lipidated form of LC3. Once the autophagosome structures are completed, these proteins leave the outer membrane and autophagosomes fuse with lysosomes to form autolysosomes where the sequestered cargo is degraded in the lumen.7 Thus, autophagosomes are the best markers of autophagy, visualized in electron microscopy as well as with fluorescently tagged LC3 protein by fluorescence microscopy.
ROLE OF CONSTITUTIVE AUTOPHAGY IN TISSUE MAINTANANCE AND HOMEOSTASIS
Autophagy as a cellular garbage disposal mechanism
Although autophagy is primarily a starvation response, constitutive autophagy is required to perform normal housekeeping functions such as elimination of defective proteins and organelles, and may also aid in the removal of pathogens. Consistent with this, modest constitutive levels of autophagy are observed in transgenic mice expressing the autophagy marker EGFP-LC38 as well as in cells in tissue culture.1
Autophagy and protein turnover
The ubiquitin-proteasome pathway and macro-autophagy (referred to hereafter as autophagy) are the two major pathways of intracellular protein degradation. Whereas proteosomes target ubiquitinated proteins for degradation, autophagy-mediated protein recycling serves to eliminate damaged and aggregation-prone proteins that are not good proteasomal substrates. By doing so, autophagy is also utilized as an alternative means to generate ATP during times of nutrient limitation. The importance of constitutive autophagy in normal cell function is demonstrated elegantly by the targeted deletion of atg5 and atg7 in the central nervous system, which promotes neurodegeneration associated with the accumulation of poly-ubiquitinated proteins.2,3 Autophagy also facilitates the clearance of aggregation-prone proteins such as huntingtin and A53T α-synuclein in cell and animal models, that are implicated in Huntington’s and familial Parkinson’s diseases, respectively.9
Autophagy and organelle quality control
Perhaps the major difference between ubiqutin-proteasome mediated protein turnover and autophagy is that autophagy is able to degrade not only proteins, but also damaged organelles and macromolecular structures. In response to long-term starvation, immortalized baby mouse kidney epithelial (iBMK) cells competent for autophagy exhibit a dramatic decrease in cell size suggesting that functional autophagy leads to effective clearance of cytosol and organelles to aid survival when apoptosis is blocked. If autophagy is impaired, this instead leads to failure of organelle clearance and accumulation of cellular debris in the cytoplasm.1,4,10 Moreover, autophagy plays a critical role in mitochondrial maintenance in yeast model systems11 and the mitochondrial permeability transition induces autophagy in rat hepatocytes.12 This is important because accumulation of defective cellular organelles such as damaged mitochondria and peroxisomes, can lead to degenerative diseases, accelerated aging, and cancer. Therefore, autophagy may exert its protective role by eliminating these dysfunctional organelles.12–14
Autophagy and immune defense
Autophagy plays important roles in innate and adaptive immune responses. Autophagy augments innate immunity by combating bacterial and viral pathogens15 and adaptive immunity by promoting T-cell maturation.16 However, how these immunogens are identified and targeted by autophagy, is not clearly understood.
ROLE OF AUTOPHAGY IN THE CELLULAR RESPONSE TO METABOLIC STRESS
Autophagy as a survival mechanism in normal and cancer cells
The full spectrum of functional implications of autophagy is only beginning to emerge. However, multiple lines of evidence suggest that autophagy is an extremely important cellular process that supports survival in response to metabolic stress. Metabolic stress robustly induces autophagy in iBMK cells, immortalized mouse mammary epithelial cells (iMMEC) and lymphocytes, and cells defective for autophagy are rendered susceptible to metabolic stress, resulting in death by metabolic catastrophe when apoptosis is blocked.1,4,10,17 Growth factor deprivation induces metabolic crisis in hematopoietic cells and stimulates autophagy-mediated ATP production, which is crucial for cell survival.10 Autophagy is important early in mammalian development as monoallelic deletion of beclin1 (ATG6/VPS30) results in embryonic lethality.18 Targeted deletion of the essential autophagy gene atg5 renders mice susceptible to neonatal starvation, resulting in early perinatal death.19 These observations support the general requirement for autophagy in maintenance of cell metabolism and survival, and suggest that the inability to maintain metabolism due to defective autophagy impairs development and leads to cell death.
Solid tumors exploit autophagy for survival under metabolic stress
Metabolic stress is a common feature of solid tumors and exactly how this impacts tumor growth is currently not known. We have recently shown that autophagy acts as a survival mechanism in tumors in vivo where it localizes to regions of metabolic stress.1,4,17 Thus, as a major survival pathway during starvation, autophagy enables cancer cells to tolerate metabolic stress in vivo. Consequently, autophagy may provide a temporary survival advantage to cells in hypoxic regions of tumors until proper vasculature is established. This observation is important, as human tumors often contain hypoxic regions due to aberrant or insufficient vasculature. Moreover, anti-angiogenic therapy aimed at starving cancer cells to death is an important cancer therapeutic tool. Whereas the survival-promoting function of autophagy would be expected to facilitate tumorigenesis, remarkably defects in autophagy are associated with enhanced tumor growth.
THE AUTOPHAGY PARADOX—DEFECTS IN THE AUTOPHAGY SURVIVAL PATHWAY LEAD TO TUMOR PROGRESSION
In stark contrast to the prediction that deficiency in autophagy in tumors would display decreased survival, defective autophagy is often associated with enhanced tumor progression. Monoallelic loss of beclin1 is frequently associated with human breast, ovarian and prostate cancers, and mice with allelic loss of beclin1 are tumor-prone, suggesting that autophagy functions as a tumor suppressor mechanism.18,20 Furthermore, allelic loss of beclin1 in iBMK and iMMEC cells promotes tumorigenesis.1,17 There is further evidence that supports the contention that autophagy defects favor tumor formation. For example, the oncogenic Type I PI-3 kinase pathway and its downstream effectors, such as Akt and mTOR, suppress autophagy, whereas its negative regulator, the tumor suppressor PTEN, activates autophagy.21 The PI-3 kinase pathway regulates cell growth in response to nutrient and growth factor availability, and therefore dissociating the two can lead to cell death by metabolic catastrophe: constitutive signaling of cell growth in the absence of nutrients.22 Interestingly, loss of the PTEN tumor suppressor and constitutive activation of the PI-3 kinase pathway are among the most commonly observed alterations in human cancers.23 These collective observations create a conundrum where the loss of a survival pathway leads to tumorigenesis.
RESOLVING THE PARADOX—MECHANISMS BY WHICH SUPPRESSION OF AUTOPHAGY FACILITATES TUMOR GROWTH
Autophagy defects reveal a mutator phenotype
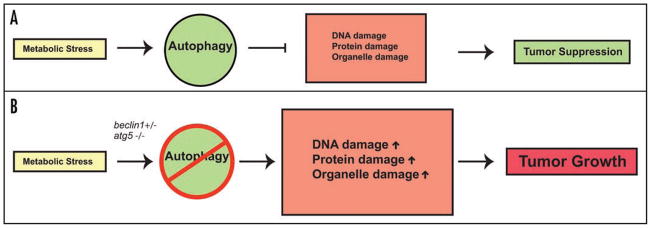
In addition to imbalanced energy homeostasis and accumulation of damaged proteins, defective autophagy is also associated with a mutator phenotype. iBMK and iMMEC cells defective for autophagy either by monoallelic loss of beclin1 or atg5 deletion possess profound chromosomal instability, associated with DNA double strand breaks, gene amplification and chromosomal gains and losses when p53 and pRb checkpoints are inactivated.4,17 These phenotypes are accentuated in an apoptosis-defective background, suggesting that autophagy functions to limit chromosomal instability, and these abnormalities are preferentially manifested in cells with defective apoptosis. How does maintenance of metabolism through autophagy protect the genome? There are several non-mutually exclusive possibilities that include the requirement for autophagy for organelle and protein quality control, maintenance of energy homeostasis and limiting necrotic cell death. Failure of autophagy in any or all of these activities may directly or indirectly lead to impairment of genomic integrity (Fig. 1).
Figure 1.
Role of autophagy in tumorigenesis. (A) Autophagy may function as a homeostatic mechanism preventing damaged protein, organelle and DNA accumulation, thereby limiting tumor progression. (B) Defects in autophagy caused by monoallelic loss of beclin1 or deficiency in atg5 leads to accumulation of DNA, protein and organelle damage, thereby facilitating chromosomal instability and tumorigenesis.
Autophagy-mediated garbage disposal and housekeeping functions facilitate the clearance of damaged mitochondria and other organelles that are potential sources of genotoxic stress.11,13 For example, damaged mitochondria that have lost their membrane integrity are the primary sources of superoxide radicals in cells.24 By degrading damaged mitochondria, autophagy may protect against chronic genotoxic damage and aging. In contrast, cells with a compromised autophagy pathway experience DNA damage and chromosomal instability, which in turn may be the manifestation of the mutator phenotype, leading to tumorigenesis. Inefficient autophagy, chronic oxidative stress, DNA damage and accelerated aging go hand in hand.25 Consistent with this, reduced levels of autophagy correlate with chronic oxidative damage and accelerated aging in mammals26 and inhibiting autophagy limits life-span in Caenorhabditis elegans.27 Peroxisomes are another potential candidate for similar autophagy-mediated garbage disposal, the failure of which can promote oxidative stress.14 These hypotheses are testable, and would predict that cells defective for autophagy accumulate damaged mitochondria and peroxisomes and are also likely to harbor elevated levels of reactive oxygen species (ROS) as a potential source of DNA damage. In fact, recent studies suggest that this is indeed the case for damaged mitochondria in budding yeast.11
Autophagy contributes to the recycling of poly-ubiquitinated or mutant aggregate-prone proteins, and defects in autophagy may result in accumulation of damaged or misfolded proteins that may interfere with essential cellular functions such as mitosis and DNA repair. As these are complex cellular processes that are choreographed by proteins whose functions are precisely regulated, accumulation of aberrant toxic protein aggregates is a potential source of division abnormalities and DNA damage. Indeed, autophagy is protective against neurodegenerative disorders, by enhancing the clearance of mutant aggregate-prone proteins as mentioned above.9 Moreover, small molecule inhibitors that activate autophagy and enhance the clearance of these proteins demonstrate protective effects against progression of these diseases.28 In addition, as many essential cellular processes are ATP-dependent, and autophagy defects often lead to energy depletion and ATP deficiency under metabolic stress, this may also contribute to cellular dysfunction and DNA damage accumulation.
Autophagy defects promote necrosis and inflammation when apoptosis is blocked
Under starvation conditions, impairment of autophagy, either by monoallelic deletion of beclin1, RNAi mediated knock-down of atg5 or beclin1, or by constitutive activation of the downstream effecter of the class I PI-3 kinase pathway, Akt, diverts cells to necrotic cell death when apoptosis is blocked.1,10 With defects in apoptosis being common in tumors, compound defects in autophagy prevent survival to metabolic stress, forcing cells into metabolic catastrophe and the default cell death mechanism by necrosis. Necrosis, in turn, is a potent stimulator of the inflammatory response which can influence tumor growth.1 Necrosis is common in advanced treatment-refractory solid tumors in humans, and is often associated with inflammation, macrophage infiltration, NFκB activation and poor prognosis.29 How does chronic necrosis promote tumor growth and worsen prognosis? One possibility is that chronic inflammation and macrophage infiltration may create a genotoxic microenvironment that facilitates DNA damage and a wound healing response resulting in angiogenesis, providing a cell non-autonomous mechanism for tumor progression.30
IT IS NOT THE NUMBER, BUT THE QUALITY OF SURVIVING CELLS THAT MATTERS
The two apparently contradictory functions of autophagy, namely the survival and tumor suppressing functions, can be reconciled by the observation that autophagy functions to limit genomic instability. On one hand, defects in autophagy may limit the ability of tumor cells to survive metabolic stress in the tumor microenvironment, but on the other hand, autophagy enhances the mutation rate that manifests as chromosomal instability, the infamous hallmark of the neoplastic process (Fig. 1). Thus, impairment in autophagy compromises tumor cell survival in starvation, although the DNA damage sustained in the remaining surviving cells may ultimately drive tumor progression.4,5,17
AUTOPHAGY AS A POTENTIAL CANCER THERAPEUTIC TARGET
How can autophagy be harnessed for therapeutic benefit? Since autophagy is primarily a survival pathway in tumors in response to nutrient limitation, there is enormous potential for the development of autophagy inhibitors to acutely sensitize cancer cells to metabolic stress.22 In apoptotic-competent cells, this may be an effective strategy to induce apoptosis, where combination therapies with angiogenesis inhibitors and promoters of metabolic stress may further augment the efficacy. Chloroquine, a widely used antimalarial drug that impairs autophagy by raising intralysosomal pH, is a promising autophagy inhibitor as a chemotherapeutic adjuvant. In a myc-driven lymphoma model, inhibition of autophagy by chloroquine or Atg5 short hairpin RNA, results in activation of apoptosis and tumor cell death, augmenting chemotherapeutic efficacy.31 More importantly, in apoptosis-defective cells, inhibition of autophagy may acutely sensitize cancer cells to metabolic catastrophe, inducing acute necrotic cell death.1,22 However, inhibition of autophagy in normal cells may have deleterious effects that need to be investigated further. Moreover, the nature of inflammatory, and possibly tumor-promoting effects of, necrosis warrants further investigation. Whereas chronic necrotic cell death in tumors may stimulate tumor growth, induction of acute necrotic cell death by the inhibition of autophagy under metabolic stress conditions in apoptosis-defective tumor cells may be therapeutically beneficial.
Additionally, autophagy’s function to maintain metabolism and limit the accumulation of DNA damage has important implications in chemoprevention of cancer. In breast, ovarian and prostate cancers, which are prone to defects in autophagy, therapeutic promotion of autophagy may restrict progression by minimizing chronic oxidative stress. Similar strategies to activate autophagy using small-molecule enhancers facilitate the clearance of mutant proteins in mammalian cell models of Huntington’s disease.28 The mTOR inhibitor rapamycin is an example of a widely used drug that is also an activator of autophagy, currently under phase III clinical trials for gliomas and advanced renal carcinomas.32,33 It will be of interest to directly test if part of the anti-tumor activity of mTOR inhibitors derives from the ability to stimulate autophagy.
References
- 1.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 4.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin S, White E. Role of autophagy in cancer: Management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Xiao G. Autophagy and NF-κB: Fight for fate. Cytokine Growth Factor Rev. 2007;18:233–43. doi: 10.1016/j.cytogfr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravikumar B, Rubinsztein DC. Role of autophagy in the clearance of mutant huntingtin: A step towards therapy? Mol Aspects Med. 2006;27:520–7. doi: 10.1016/j.mam.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: Characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–46. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- 12.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–7. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 13.Jin S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy. 2006;2:80–4. doi: 10.4161/auto.2.2.2460. [DOI] [PubMed] [Google Scholar]
- 14.Monastyrska I, Klionsky DJ. Autophagy in organelle homeostasis: Peroxisome turnover. Mol Aspects Med. 2006;27:483–94. doi: 10.1016/j.mam.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo MI. Autophagy: A pathogen driven process. IUBMB Life. 2007;59:238–42. doi: 10.1080/15216540701230503. [DOI] [PubMed] [Google Scholar]
- 16.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007:21. doi: 10.1101/gad.1565707. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–82. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 20.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 21.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–6. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 22.Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–83. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Lengauer C, Velculescu VE. Colorectal cancer: Mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 24.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 25.Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med. 2006;27:411–25. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy. 2005;1:131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 27.Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, Liu LF. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–5. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL, Rubinsztein DC. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol. 2007;3:331–8. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeh HJ, IIIrd, Lotze MT. Addicted to death: Invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A north central cancer treatment group study. J Clin Oncol. 2005;23:5294–304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 33.Lee VW, Chapman JR. Sirolimus: Its role in nephrology. Nephrology (Carlton) 2005;10:606–14. doi: 10.1111/j.1440-1797.2005.00493.x. [DOI] [PubMed] [Google Scholar]