Abstract
It is controversial whether worldwide increases in melanoma incidence represent a true epidemic. Dramatic increases in incidence in the setting of relatively stable mortality trends have also been attributed to expanded skin screening and detection of biologically indolent tumors with low metastatic potential. To better understand how melanoma incidence trends varied by severity at diagnosis and factors relevant to screening access, we assessed recent United States incidence and mortality trends by histologic type, tumor thickness and area-level socioeconomic status (SES). We obtained population-based data regarding diagnoses of invasive melanoma among non-hispanic whites from nearly 291 million person-years of observation by the Surveillance Epidemiology and End Results (SEER) program (1992–2004). Age-adjusted incidence and mortality rates were calculated for SEER and a subset (California) for which small-area SES measure was available. Overall, melanoma incidence increased at 3.1% (p<0.001) per year. Statistically significant rises occurred for tumors of all histologic subtypes and thicknesses, including those >4mm. Melanoma incidence rates doubled in all SES groups over a 10-year period while melanoma mortality rates did not increase significantly. We conclude that screening-associated diagnosis of thinner melanomas cannot explain the increasing rates of thicker melanomas among low SES populations with poorer access to screening.
INTRODUCTION
Malignant melanoma is one of the fastest growing cancers worldwide; studies from Europe (de Vries and Coebergh, 2004; Lasithiotakis et al., 2006; Mansson-Brahme et al., 2002; Stang et al., 2006), Singapore (Koh et al., 2003) Canada (Ulmer et al., 2003) and the United States (Dennis, 1999; Geller et al., 2002; Hall et al., 1999) suggest consistent and dramatic increases in incidence since the 1950s. Yet the underlying causes of these observed trends are widely debated (Florez and Cruces, 2004; Lamberg, 2002; Swerlick and Chen, 1996, 1997), with some authors attributing the rapid rises to environmental risk factors and sun exposure behavior (Diffey, 2004), and others maintaining they result from expanded screening, biopsy (Welch et al., 2005), and reporting of lower-risk melanomas to cancer registries (Hall et al., 2003; Swerlick and Chen, 1996, 1997), notions supported by relatively stable trends in melanoma mortality for most groups. Promotion of skin screening programs (i.e. population-based screening, skin self-examination practices, and routine, opportunistic physician exams) may inflate the numbers of cases, particularly those diagnosed at early stage in the initial phases of program implementation, but may also preferentially detect slow-growing or clinically insignificant disease. Therefore, to quantify the true burden of clinically relevant disease, incidence patterns of thicker melanomas may be more informative than those for all melanomas combined.
There are several reasons that examining melanoma trends according to socioeconomic status (SES) may be informative. Socioeconomic status may be associated with both knowledge about melanoma, as well as access to physician skin screening, and low SES correlates with poorer healthcare access in the United States (Saraiya et al., 2004; Weissman and Schneider, 2005). In the US, patients from higher SES groups are more likely to be diagnosed with melanoma, but patients from lower SES groups, and those with no or substandard health insurance are more likely to have an advanced stage at diagnosis and to die from melanoma (Ortiz et al., 2005; Pollitt et al., 2008; Roetzheim et al., 1999). Therefore, a detailed assessment of time trends in melanoma incidence and mortality among lower SES groups can be informative about the underlying changes in cancer rates among a potentially more poorly screened population with more severe disease. Because information on SES is generally not collected for individual patients by cancer registries, assessments of melanoma trends by SES and tumor thickness have not been published, to our knowledge. Moreover, prior analyses of melanoma incidence trends have assessed trends for all persons of white race, regardless of ethnicity; however this classification includes two distinct groups: non-Hispanic whites who account for over 90% of cutaneous melanoma cases in the US, and Hispanic whites in whom melanoma rates are very low, but increasing (Cockburn et al., 2006). It is unclear to what extent this mixing of two groups with heterogeneous melanoma risks have biased prior estimations of melanoma rate trends.
The primary aim of this analysis was to update our understanding of melanoma trends in recent years, with specific attention to their support of the notion of a possible epidemic. Using data from the national Surveillance Epidemiology and End Results (SEER) program, we analyzed incidence and mortality rates for invasive melanoma among non-Hispanic whites according to tumor thickness and histologic type, as well as by area SES in a subset of SEER data.
RESULTS
In 2004, 7,046 new cases of malignant melanoma were reported in the 13 SEER registry areas of the United States. The vast majority of cases occurred among non-Hispanic whites (N=6,569, 93%), while 3% occurred in Hispanic whites, 1% in Asians and Pacific islanders and less than 1% in blacks and American Indians respectively. Melanoma affected both sexes with a male: female ratio of 3:2. Data on tumor thickness was available for 85% of cancer patients; of these, most were ≤1mm (69%) with other thickness distributions as follows: 17% 1.01–2mm, 9% 2.01–4mm and 5% >4mm.
Incidence and mortality trends among non-Hispanic whites 1992–2004
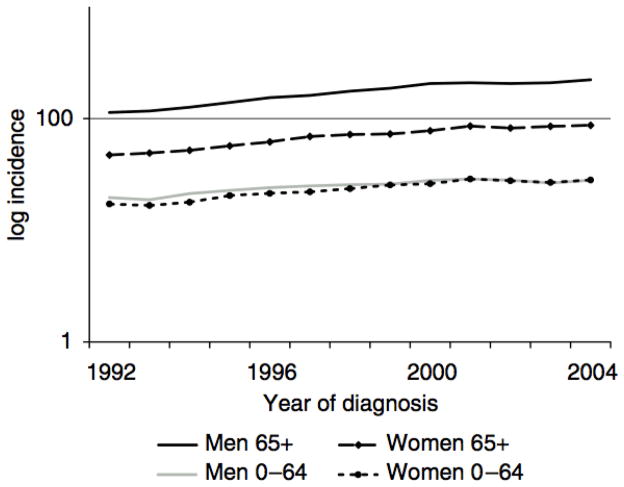
A total of 70,596 new cases of malignant melanoma were diagnosed over the study period, which included 290,913,376 person years of observation. Incidence rates of malignant melanoma increased significantly since 1992 (Figure 1), with an overall 45% increase and estimated 3.1% annual percent change (APC) (p<0.001). In 1992, the incidence rate was 18.2 (95% CI 17.7–18.8) per 100,000 while in 2004 it was 26.3 (95% CI 25.7–27.0) per 100,000. Incidence rates varied more than 10 fold according to age and gender groups. Among those younger than 65 years, incidence rates were 18.8 cases per 100,000 men and 17.9 cases per 100,000 women. Among men 65 years and older, 73.2 (95% CI 68.4–78.4) new cases per 100,000 were diagnosed in 1992, giving this group both the highest absolute rate as well as the fastest growing incidence rate (APC 4.50 % per year), which reached 126.1 (95 % CI 120.2–132.4) per 100,000 in 2004.
Figure 1.
Age adjusted incidence of malignant melanoma per 100,000 according to age and sex 1992–2004
Among non-Hispanic whites, 37.5% had superficial spreading melanoma (SSM, N=26,461), 7.4% had nodular melanoma (NM, N=5,264), 7.3% had lentigo maligna melanoma (LMM, N=5,141), 0.9% had acral lentiginous melanoma (ALM, N=616) and 4.4% had other subtypes (N=3,137). Over 40% of cases (N=29,977) were missing histologic subtype classification (e.g., NOS). Regardless, no significant differences in the proportions of tumors according to histologic subtype were evident over time. The incidence rates per 100,000 of SSM increased from 7.6 in 1992 to 8.5 in 2004; incidence rates of NM increased from 1.5 in 1992 to 1.7 in 2004, and rates of LMM rose from 1.2 to 2.0 over the same period.
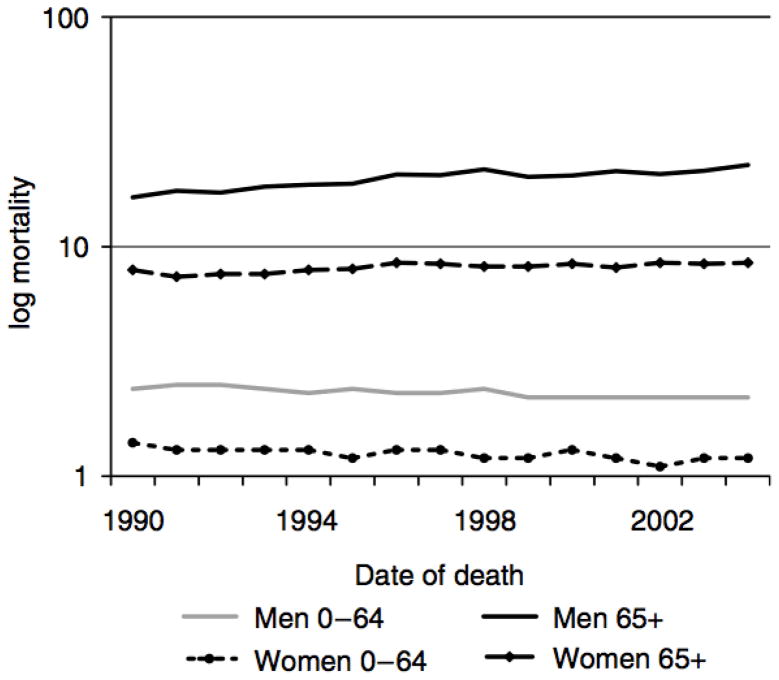
Overall mortality from malignant melanoma increased at an annual rate of 0.4% from 1990 to 2004 (Table 4). For men and women over 65 years of age, mortality increased by 1.7% annually, reaching 14.3 deaths per 100,000 in 2004. Men over 65 years had the fastest increase in mortality over this period (APC 1.9%) reaching 22.7 deaths per 100,000 in 2004. Mortality rates decreased among persons younger than 65 years at diagnosis (APC - 0.9%) remaining low at 1–2 deaths per 100,000. (Figure 2)
Table 4.
Deaths from malignant melanoma, age adjusted (2000 US standard) mortality rate among non-Hispanic whites for 2004; Annual percent change in mortality rates 1990–2004
| 0–64 years | 65+ years | 0–85+ years | |
|---|---|---|---|
| Men | |||
| No. of deaths from melanoma in 2004 | 2168 | 2799 | 4967 |
| Mortality rate per 100,000 in 2004 | 2.2 (2.1–2.3) | 22.7 (21.9–23.6) | 4.8 (4.6–4.9) |
| Trend APC (%), p value | −1.0* | 1.9* | 0.6* |
| Women | |||
| No. of deaths from melanoma in 2004 | 1156 | 1531 | 2687 |
| Mortality rate per 100,000 in 2004 | 1.2 (1.1–1.2) | 8.5 (8.0–8.9) | 2.1 (2.1–2.2) |
| Trend APC (%), p value | −0.9* | 0.8* | −0.1 |
| All | |||
| No. of deaths from melanoma in 2004 | 3324 | 4330 | 7654 |
| Mortality rate per 100,000 in 2004 | 1.7 (1.6–1.7) | 14.3 (13.8–14.7) | 3.3 (3.2–3.3) |
| Trend APC (%), p value | −0.9* | 1.7* | 0.4* |
Statistically significant difference p <0.05, APC=Annual Percent Change in mortality rate
Figure 2.
Age adjusted mortality rates from melanoma per 100,000 according to age and sex 1990–2004
Incidence trends by tumor thickness
Melanoma incidence rates increased across all groups of tumor thickness. The overall annual increase in incidence ranged from 0.43% to 6.88% per year across the four groups of tumor thickness (Table 1). The overall incidence of tumors >4mm thick increased by 3.86% each year (4.10% men, 3.30% women). The annual percent increase in incidence was highest for men over 65 years old, 4.50% overall and per thickness categories: 6.88% for melanomas ≤1mm, 4.76% for 1.01–2mm, 3.86% for 2.01–4mm, and 5.67% for >4mm tumors.
Table 1.
Age adjusted (2000 US standard) incidence of malignant melanoma among non-Hispanic whites and 95% confidence intervals (CI) for 2004 according to age and tumor thickness, SEER 1992–2004
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Tumor thickness | No. of cases (%) | Age-adjusted incidence rate per 100,000 in 2004 | Trend APC (%)1992–2004 | No. of cases (%) | Age-adjusted incidence rate per 100,000 in 2004 | Trend APC (%)1992–2004 |
| Age | ||||||
| 0–85+ years | ||||||
| ≤1mm | 2306 (61%) | 19.7 (18.9–20.6) | 4.84* | 1869 (66%) | 14.9 (14.3–15.6) | 4.68* |
| 1.01–2mm | 499 (13%) | 4.3 (3.9–4.7) | 2.39* | 389 (14%) | 3.0 (2.7–3.3) | 3.16* |
| 2.01–4mm | 318 (8%) | 2.8 (2.5–3.1) | 2.54* | 153 (5%) | 1.1 (0.9–1.3) | 1.80 |
| ≥4.01mm | 200 (5%) | 1.7 (1.5–2.0) | 4.10* | 103 (4%) | 0.7 (0.6–0.9) | 3.30* |
| Unknown | 435 (12%) | 3.8 (3.4–4.1) | −2.88* | 297 (11%) | 2.3 (2.0–2.6) | −2.48* |
| All 1 | 3758 | 32.3 (31.3–33.4) | 2.96* | 2811 | 22.0 (21.2–22.9) | 3.21* |
| 0–64 years | ||||||
| ≤1mm | 1346 (64%) | 12.1 (11.4–12.8) | 3.42* | 1344 (72%) | 12.8 (12.2–13.6) | 4.33* |
| 1.01–2mm | 273 (13%) | 2.5 (2.2–2.8) | 0.44 | 243 (13%) | 2.3 (2.0–2.6) | 2.91* |
| 2.01–4mm | 145 (7%) | 1.3 (1.1–1.5) | 0.84 | 75 (4%) | 0.7 (0.5–0.9) | 0.42 |
| ≥4.01mm | 100 (5%) | 0.9 (0.7–1.1) | 1.96 | 39 (2%) | 0.4 (0.3–0.5) | 1.40 |
| Unknown | 229 (11%) | 2.1 (1.8–2.3) | −3.66* | 177 (9%) | 1.7 (1.4–2.0) | −2.65* |
| All 1 | 2093 | 18.8 (18.0–19.6) | 1.68* | 1878 | 17.9 (17.1–18.7) | 2.97* |
| 65+ years | ||||||
| ≤1mm | 960 (58%) | 72.6 (68.1–77.4) | 6.88* | 525 (56%) | 29.4 (26.9–32.1) | 5.88* |
| 1.01–2mm | 226 (14%) | 17.1 (15.0–19.5) | 4.76* | 146 (16%) | 7.7 (6.4–9.0) | 3.71* |
| 2.01–4mm | 173 (6%) | 13.2 (11.3–15.3) | 3.86* | 78 (8%) | 4.1 (3.2–5.1) | 3.73* |
| ≥4.01mm | 100 (10%) | 7.6 (6.2–9.2) | 5.67* | 64 (7%) | 3.2 (2.5–4.1) | 5.10* |
| Unknown | 206 (12%) | 15.6 (13.6–17.9) | −2.14* | 120 (13%) | 6.4 (5.3–7.7) | −2.17* |
| All 1 | 1665 | 126.1 (120.2–132.4) | 4.50* | 933 | 50.8 (47.5–54.2) | 3.84* |
Includes all malignant melanoma, including those for which thickness was not recorded
Statistically significant difference p <0.05
APC=Annual Percent Change in incidence rate
Because complete reporting of tumor thickness improved over the study period, we quantified the potential impact of this change on observed trends. The proportion of melanoma cases missing thickness information dropped from 20% in 1992–1996 to 12% in 2000–2004, a reduction of approximately 40%. To understand the impact of this change, we reallocated 40% of the missing tumors in 1992–1996 to each of the groups with known thickness (Table 2) in several iterations: 1) proportionally to all thickness categories, which assumes that reporting improved uniformly across all thickness levels; 2) disproportionately (twice as many cases to the 2.01–4mm and 4mm+ groups), which assumes that reporting improved selectively among thicker tumors; and 3) disproportionately to the thinnest tumor category (≤1 mm), which assumes that reporting improved selectively among thin tumors. We considered the latter iteration to be the most realistic, because most of the cases with missing thickness information were high SES groups, which include a higher proportion of thinner tumors. In all iterations, incidence trends remained statistically significant across all thickness levels (Table 2). Therefore, we concluded that trends in missing thickness level did not change the interpretation of our findings.
Table 2.
Sensitivity analysis assessing the impact of improved reporting of tumor thickness on melanoma trends according to tumor thickness
| Total | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Missing before correction | 15 | 23 | 21 | 20 | 17 | 16 | 15 | 15 | 14 | 13 | 13 | 12 | 12 | 11 |
| % Missing after correction | 12 | 13 | 11 | 11 | 9 | 8 | 15 | 15 | 14 | 13 | 13 | 12 | 12 | 11 |
| Annual percent Change | 1. Underreporting in all thicknesses | 2. Selective underreporting of thick tumors | 3. Selective underreporting of thin tumors | |||||||||||
| 1mm | 3.85* | 4.14* | 3.40* | |||||||||||
| 1–2mm | 1.41* | 1.76* | 2.68* | |||||||||||
| 2–4mm | 1.54* | 0.25 | 2.22* | |||||||||||
| 4mm+ | 3.37 | 1.85* | 3.86* | |||||||||||
| Missing thickness | 3.14 | 3.44 | 3.08* | |||||||||||
| Overall | 2.97* | 3.08* | 2.16 | |||||||||||
Trends according to socioeconomic status in California
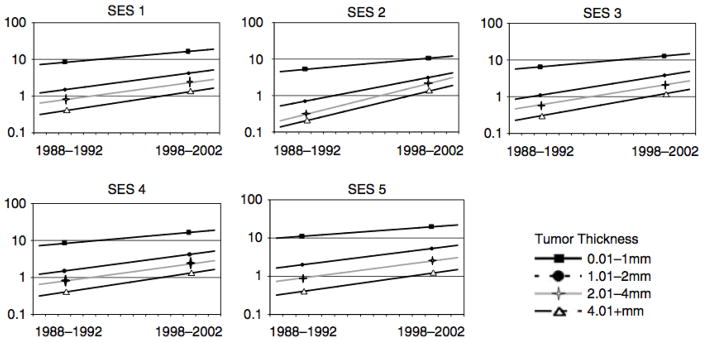
A total of 29,792 cases of cutaneous melanoma in the state of California were included in the SES analyses. Persons living in the highest quintile of SES were at higher absolute risk of all melanomas. As shown in Figure 3, increasing trends in incidence rates over time were observed across all SES groups and tumor thickness levels. For each of the SES groups and tumor thickness classifications, there was at least a 2-fold increase in rates comparing the period of 1988–1992 to 1998–2002. Persons living in low SES areas experienced the highest increases in melanoma incidence, and among the lowest SES group, the steepest rises in melanoma incidence were observed for thicker tumors (2.01–4mm and ≥4.01 mm) (Table 3). Mortality rates among different socioeconomic groups did not change significantly between the two time periods (data not shown).
Figure 3.
Age adjusted incidence of melanoma according to levels of SES (1–5) and by tumor thickness.
Table 3.
Trends over time according to SES and tumor thickness: Incidence rate ratio1 comparing period 2 (1998–2002) to period 1 (1988–1992)
| Thickness | ||||||
|---|---|---|---|---|---|---|
| SES 2 | Women | Men | All | |||
| 0–64 yrs | 65+ yrs | 0–64 yrs | 65+ yrs | 0–64 yrs | 65+ yrs | |
| ≤1mm | ||||||
| 1 | 2.24 | 2.01 | 2.22 | 2.70 | 2.26 | 2.42 |
| 2 | 1.88 | 1.97 | 1.98 | 2.38 | 1.93 | 2.19 |
| 3 | 1.96 | 1.96 | 1.89 | 2.18 | 1.94 | 2.13 |
| 4 | 2.08 | 2.13 | 1.76 | 2.13 | 1.93 | 2.17 |
| 5 | 1.67 | 1.84 | 1.73 | 1.97 | 1.69 | 1.95 |
| 1.01–2mm | ||||||
| 1 | 5.25 | 7.00 | 4.00 | 3.97 | 4.4 | 4.89 |
| 2 | 3.8 | 3.89 | 3.83 | 8.11 | 3.5 | 5.56 |
| 3 | 3.14 | 3.58 | 3.09 | 4.68 | 3.11 | 4.24 |
| 4 | 2.36 | 4.14 | 2.54 | 4.06 | 2.42 | 4.16 |
| 5 | 2.55 | 2.44 | 2.47 | 3.28 | 2.5 | 2.98 |
| 2.01–4mm | ||||||
| 1 | - | 12.25 | 3.33 | 6.52 | 5.00 | 8.3 |
| 2 | 4.00 | 8.83 | 5.67 | 9.75 | 4.33 | 9.88 |
| 3 | 2.67 | 4.45 | 2.83 | 4.88 | 3.00 | 5.27 |
| 4 | 2.50 | 2.57 | 2.25 | 4.39 | 2.33 | 3.48 |
| 5 | 2.25 | 3.33 | 2.29 | 3.05 | 2.00 | 3.23 |
| ≥4.01mm | ||||||
| 1 | 4.0 | 4.0 | 13.0 | 5.48 | 8.0 | 5.38 |
| 2 | - | 4.5 | 3.33 | 11.29 | 4.0 | 6.71 |
| 3 | 4.0 | 4.0 | 3.33 | 6.69 | 3.5 | 5.44 |
| 4 | 2.0 | 4.13 | 2.67 | 5.4 | 3.0 | 5.17 |
| 5 | 2.0 | 4.33 | 2.67 | 4.36 | 2.0 | 4.31 |
| Unknown3 | ||||||
| 1 | 0.45 | 0.52 | 0.69 | 0.85 | 0.57 | 0.68 |
| 2 | 0.45 | 0.58 | 0.46 | 0.67 | 0.45 | 0.61 |
| 3 | 0.45 | 0.66 | 0.42 | 0.68 | 0.43 | 0.64 |
| 4 | 0.40 | 0.53 | 0.45 | 0.68 | 0.43 | 0.61 |
| 5 | 0.40 | 0.53 | 0.39 | 0.57 | 0.39 | 0.54 |
| All | ||||||
| 1 | 1.68 | 1.68 | 1.74 | 2.07 | 1.70 | 1.93 |
| 2 | 1.49 | 1.71 | 1.51 | 1.98 | 1.50 | 1.87 |
| 3 | 1.54 | 1.67 | 1.43 | 1.81 | 1.49 | 1.81 |
| 4 | 1.50 | 1.67 | 1.37 | 1.88 | 1.42 | 1.83 |
| 5 | 1.30 | 1.55 | 1.32 | 1.63 | 1.31 | 1.63 |
- used when unable to calculate ratio since denominator equal to zero
Incidence rate ratio was calculated as the number of new cases of melanoma per 100,000 which occurred during period 1 (1998–2002) divided by the number of new cases of melanoma per 100,000 in period 2 (1988–1992)
Socioeconomic status was divided into 5 quintiles based on an index of neighborhood SES based on characteristics of the census tract of patient residence at the time of diagnosis. SES1 (lowest), SES 5 (highest) (Yost et al., 2001)
The percentage of cases with missing thickness across SES groups 1 through 5 is: 32%, 28%, 25%, 22% and 21% respectively
DISCUSSION
Our analysis of almost 300 million person-years and over 70,000 new cases of malignant melanoma, the largest such assessment to date, suggests a continued rise in new cases of malignant melanoma in the US. These findings update through 2004 prior reports indicating persistent rises in incidence from the 1960s in the US (Geller et al., 2002; Ries et al., 2002) and are consistent with mounting evidence of similar trends worldwide (de Vries et al., 2003a; de Vries and Coebergh, 2004; de Vries et al., 2003b; Koh et al., 2003; Lasithiotakis et al., 2006; Mansson-Brahme et al., 2002; Stang et al., 2006; Ulmer et al., 2003). Unlike prior large SEER-based analyses, we were able to assess trends jointly by tumor thickness and SES, which allowed for more precise characterization of thick melanoma trends in populations likely to be unscreened or with limited access to physician screening.
We observed that melanoma incidence increased for both men and women across all categories of tumor thickness, including a significant 3.86% annual increase among the thickest tumors (>4 mm). Interestingly, this increase did not correlate with a disproportionate increase in nodular melanomas, which are characterized by rapid growth and may elude early detection. As with prior reports (Geller et al., 2002), incidence increases were most dramatic for men aged 65 and older. These observations persisted in analyses accounting for improvements in reporting of tumor thickness. When patients were divided according to neighborhood-level SES, increases were noted in all groups, especially those in the lowest two quintiles. Importantly, the lowest SES group demonstrated the steepest rise in the incidence of thick tumors >4mm.
Mortality from melanoma continued to increase, especially among men aged 65 and older (approximately 2% increase annually), consistent with previous reports (Geller et al., 2002; Ries et al., 2002) although mortality rates decreased for men and women younger than 65. For all cancers, age is one of the strongest risk factors, presumably due to accumulating DNA damage over time. Melanoma in the elderly may have a different biology and/or altered host immune response, both of which could contribute to increased incidence and mortality (Balch et al., 2001b). Regardless, the pattern of dramatic increases in melanoma among persons over 65 in the face of decreasing mortality among younger men and women, is notable.
Some have argued that the rapid rises in melanoma incidence are indicative of a true epidemic on the basis of greater ultraviolet radiation-induced carcinogenesis, while others insist that the apparent trends are an artifact of improved surveillance, diagnostic scrutiny (Welch et al., 2005), and regular screening (Swerlick and Chen, 1996) leading to increased diagnosis of thinner tumors with lower or no metastatic potential. Our findings inform this debate by showing persistent increases among more fatal, thick (>4mm) tumors and contest the argument that rising incidence rates are solely attributable to increased diagnosis of thinner tumors.
Increasing incidence of thicker melanomas has been reported in earlier population-based studies (Dennis, 1999; Geller et al., 2002) but was not noted in recent regional studies from France (Lipsker et al., 2007) and Germany (Lasithiotakis et al., 2006). This discrepancy may reflect variations in patterns of disease presentation or study methodology differences including smaller sample sizes in the European studies. Above and beyond diagnostic surveillance, it is possible that reported trends in melanoma have been influenced by patterns of melanoma reporting to cancer registries. Although it is likely that reporting of thin tumors did improve over time as physicians realized their reporting responsibilities, we did not find evidence of selective over-reporting of thin tumors among persons of higher SES groups. Thus, it is unlikely that reporting patterns explain the SES-specific incidence patterns observed here.
Routine screening for skin cancer is currently recommended by the American Cancer Society (Smith et al., 2007). However, the proportion of non-Hispanic white adults who reported ever having a physician skin examination in National Health Interview Surveys conducted in 1992, 1998, and 2000 ranges from only 14–21%, and physician screening is rare among those without health insurance (Saraiya et al., 2004). Our data showing increases in melanoma incidence across all SES groups are consistent with previous reports of melanoma increases by census tract poverty level from 1975 to 1999 (Singh et al., 2003). However, our findings of increasing incidence in low SES groups, who may have reduced access to health prevention education and practices, suggest a true increase in melanoma burden independent of screening access.
Our observations of modest increases in mortality rates in the presence of dramatic increases in incidence are curious, and are probably not attributable to improvements in survival, treatment or early detection alone. Median survival for late-stage melanoma in the US has not changed appreciably over the past 30 years (Barth et al., 1995), nor have there been any major innovations in melanoma treatment (Korn et al., 2008). Although the influence of a stage distribution shift towards thinner, more curable tumors has occurred in recent decades (Geller et al., 2007), the incidence of thicker melanoma has not declined (Jemal et al., 2001) as supported by our findings of persistent increases in melanomas of all thicknesses over the study period.
This study provides a comprehensive analysis of the most recent SEER data covering a large segment of the US population. Additional strengths include a more specific classification of ethnicity of non-Hispanic whites, providing precise estimates of risk of melanoma among those at highest risk. Moreover, calculation of SES-specific incidence trends allowed us to examine trends of thicker tumors in a population with poorer access to screening. Complete SES data were available for the state of California; however, this reflected relative SES of the patient’s neighborhood at the time of diagnosis, and therefore may be misclassified with respect to socioeconomic characteristics measured at the individual-level or for time periods prior to diagnosis. SES-specific data was not available outside the state of California.
Incomplete reporting of certain cancer registry data items, including histologic subtype and thickness, may have biased the ultimate representativeness of our final study cohort. This issue underscores the dependence of accurate melanoma surveillance on both the quality and completeness of melanoma reporting to cancer registries by diagnosing hospitals and physicians (Hall et al., 2003). Under-reporting (Koh et al., 1992; Zippin et al., 1995; Cockburn et al. 2008, in press) and delayed reporting (Clegg et al., 2002) of melanoma to cancer registries have been documented and indicate that even the best assessments of melanoma incidence patterns likely represent underestimates.
We do not believe that improvements in the reporting of thickness information over time could fully explain our observations of increasing incidence trends across all thickness categories, as demonstrated in our sensitivity analyses described above. Furthermore, missing thickness was not associated with patient age, sex, or suggesting that thickness data is largely missing at random.
The absolute magnitude of melanoma incidence in older white men warrants greater public health attention, as these data suggest a contemporary incidence rate exceeding 125 cases per 100,000 men aged 65 and older. This is proportional to the incidence of non-Hodgkin’s lymphoma in the same population, making malignant melanoma the 5th most common cancer in older white men following prostate, lung, colorectal and bladder cancer. If melanoma is truly increasing in all thickness categories and across socioeconomic levels, it will soon become a major concern for an increasingly aging population and their health care providers. Secondary prevention through early detection of melanoma is especially important in reducing mortality in high-risk groups (Geller et al., 2006), including those of lowest SES who demonstrated the sharpest rises in thick melanoma incidence in the California data. Our analysis also highlights the need for continued, detailed surveillance of melanoma occurrence, which in turn, underscores the importance of complete and accurate reporting of all melanoma cases by hospitals and private physicians.
MATERIALS & METHODS
Population
Data on newly diagnosed cases of malignant melanoma were obtained from the National Cancer Institute’s SEER program. Information on mortality from melanoma was obtained from the National Center for Health Statistics (NCHS). SEER data are collected by 13 population based cancer registries including Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles and San Jose-Monterey, rural Georgia and the Alaska Native Tumor Registry. The non-Hispanic white population covered by SEER is somewhat more urban but otherwise generally representative of the overall US population.
Because of the disparate incidence of melanoma in fair-skinned populations, previous studies on melanoma trends in the US have restricted analyses to individuals of white ethnicity (Geller et al., 2002; Hall et al., 1999). For more accurate assessment, we limited our analyses to non-Hispanic whites who account for over 90% of melanoma cases in the US. Because of our particular interest in melanoma trends according to tumor thickness, we further restricted our analysis to the time period for which data on these variables were available in the SEER database; from 1992 to 2004 (the most recent year available for which Veterans Administration reporting to SEER was complete). We obtained data regarding all incident cases of invasive cutaneous melanoma (International Classification of Diseases for Oncology (ICDO) topography codes C44.0 through C44.9) including year at diagnosis, tumor thickness, histology, patient age, race and sex. All patient and tumor information was abstracted and coded directly from the medical record or death certificate by trained cancer registry personnel. Assignment of patient Hispanic race/ethnicity was augmented by Hispanic surname lists. ICD-O-3 classification of histology was used to examine trends in superficial spreading melanoma (SSM; 8743), nodular melanoma (NM; 8721), lentigo maligna melanoma (LMM; 8742), acral lentiginous melanoma (ALM; 8744), melanoma not otherwise specified (NOS; 8720) and other histologic types (other; 8723, 8730, 8740, 8745, 8761, 8770–8773 and 8780). Tumor thickness was classified according to 2002 American Joint Committee on Cancer (AJCC) tumor (T) categories as <1mm, 1.01–2mm, 2.01–4mm or > 4mm (Balch et al., 2001a). On average, tumor thickness was not recorded in 15.1% of the SEER melanoma patients. Patients with missing thickness information did not differ significantly from patients with known melanoma thickness by age at diagnosis or gender, although missing thickness information was more common among higher SES groups. The proportion of melanomas with missing thickness information declined over time. We therefore performed sensitivity analyses to assess the impact on our results of improved reporting of thickness over time.
Socioeconomic status index
Three of the SEER member registries, together comprising the statewide California Cancer Registry, developed a measure of neighborhood SES based on characteristics of the census tract of patient residence at the time of diagnosis. This small-area measure has been used previously to predict cancer and other health outcomes (Clarke et al., 2005; Yost et al., 2001). This index incorporates information regarding education, median household income, proportion living 200% below poverty level, proportion of blue-collar workers, proportion older than 16 years and unemployed, median rent and median house value using principal components analysis as described by Yost et al (Yost et al., 2001). The index was divided into quintiles (1=lowest, 5=highest). Complete information on census tract SES index was available for all individuals in this analysis. This was assigned randomly within county of residence at diagnosis for patients with unknown census tract of residence. Because census tract-level population denominators are available from the US Census Bureau for decennial census years only, we examined SES-specific trends for the five-year periods surrounding decennial census years, (e.g., 1988–1992 and 1998–2002).
Statistical Analysis
SEER*Stat software was used to calculate annual melanoma incidence and mortality rates for each year between 1992 and 2004, reported as cases or deaths per 100,000 people, and age-adjusted to the 2000 US population standard. Standard errors, 95% confidence intervals and all tests of statistical significance are 2-sided, p value=0.05. Incidence trends for each classification of tumor thickness were examined using estimated annual percentage changes (APC) from 1992 to 2004, calculated by fitting a least squares regression line to the natural logarithm of the rates, using calendar year as the dependent variable. Because only two time periods were available for SES-specific trends, we calculated SES-specific incidence rate ratios to comparing period 2 (1998–2002) to period 1 (1988–1992).
Acknowledgments
The authors acknowledge the helpful statistical input of Sarah J Shema, MS, Northern California Cancer Center in this analysis.
Footnotes
None of the authors of this manuscript have any conflict of interest to disclose.
CONFLICT OF INTEREST
The authors state no conflict of interest.
Financial Disclosure Information: This study was supported by a developmental cancer research award from the Stanford Comprehensive Cancer Center. The National Institutes of Health grant R25 CA098566 provided salary support for EL. MC was supported in part by NIEHS grant 5P30 ES07048, and National Institutes of Health grant R01 CA121052. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
References
http://seer.cancer.gov/registries/characteristics.html.
- Kleinbaum DG. Applied regression analysis and other multivariable methods. 2. Boston: PSW Kent; 1988. [Google Scholar]
- SEER*Stat software, version 6.1.4. Bethesda MD: Surveillance Research Program, National Cancer institute; 2005. [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Mortality - All COD, Public-Use With State, Total U.S. (1990–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007. Underlying mortality data provided by NCHS. ( www.seer.cancer.gov) ( www.cdc.gov/nchs)
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001a;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001b;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193–201. [PubMed] [Google Scholar]
- Clarke CA, Glaser SL, Keegan TH, Stroup A. Neighborhood socioeconomic status and Hodgkin’s lymphoma incidence in California. Cancer Epidemiol Biomarkers Prev. 2005;14:1441–1447. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- Cockburn MG, Zadnick J, Deapen D. Developing epidemic of melanoma in the Hispanic population of California. Cancer. 2006;106:1162–1168. doi: 10.1002/cncr.21654. [DOI] [PubMed] [Google Scholar]
- de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003a;107:119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- de Vries E, Coebergh JW. Cutaneous malignant melanoma in Europe. Eur J Cancer. 2004;40:2355–2366. doi: 10.1016/j.ejca.2004.06.003. [DOI] [PubMed] [Google Scholar]
- de Vries E, Schouten LJ, Visser O, Eggermont AM, Coebergh JW. Rising trends in the incidence of and mortality from cutaneous melanoma in the Netherlands: a Northwest to Southeast gradient? Eur J Cancer. 2003b;39:1439–1446. doi: 10.1016/s0959-8049(03)00320-4. [DOI] [PubMed] [Google Scholar]
- Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275–280. doi: 10.1001/archderm.135.3.275. [DOI] [PubMed] [Google Scholar]
- Diffey B. Climate change, ozone depletion and the impact on ultraviolet exposure of human skin. Phys Med Biol. 2004;49:R1–11. doi: 10.1088/0031-9155/49/1/r01. [DOI] [PubMed] [Google Scholar]
- Florez A, Cruces M. Melanoma epidemic: true or false? Int J Dermatol. 2004;43:405–407. doi: 10.1111/j.1365-4632.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969–1999. Jama. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- Geller AC, Miller DR, Swetter SM, Demierre MF, Gilchrest BA. A call for the development and implementation of a targeted national melanoma screening program. Arch Dermatol. 2006;142:504–507. doi: 10.1001/archderm.142.4.504. [DOI] [PubMed] [Google Scholar]
- Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000–2006) and future directions. J Am Acad Dermatol. 2007;57:555–572. doi: 10.1016/j.jaad.2007.06.032. quiz 573–556. [DOI] [PubMed] [Google Scholar]
- Hall HI, Jamison P, Fulton JP, Clutter G, Roffers S, Parrish P. Reporting cutaneous melanoma to cancer registries in the United States. J Am Acad Dermatol. 2003;49:624–630. doi: 10.1067/s0190-9622(03)00885-5. [DOI] [PubMed] [Google Scholar]
- Hall HI, Miller DR, Rogers JD, Bewerse B. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol. 1999;40:35–42. doi: 10.1016/s0190-9622(99)70562-1. [DOI] [PubMed] [Google Scholar]
- Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore Cancer Registry data 1968–97. Br J Dermatol. 2003;148:1161–1166. doi: 10.1046/j.1365-2133.2003.05223.x. [DOI] [PubMed] [Google Scholar]
- Koh HK, Geller A, Miller DR, Clapp RW, Lew RA. Underreporting of cutaneous melanoma in cancer registries nationwide. J Am Acad Dermatol. 1992;27:1035–1036. doi: 10.1016/s0190-9622(08)80285-x. [DOI] [PubMed] [Google Scholar]
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- Lamberg L. “Epidemic” of malignant melanoma: true increase or better detection? Jama. 2002;287:2201. [PubMed] [Google Scholar]
- Lasithiotakis KG, Leiter U, Gorkievicz R, Eigentler T, Breuninger H, Metzler G, et al. The incidence and mortality of cutaneous melanoma in Southern Germany: trends by anatomic site and pathologic characteristics, 1976 to 2003. Cancer. 2006;107:1331–1339. doi: 10.1002/cncr.22126. [DOI] [PubMed] [Google Scholar]
- Lipsker D, Engel F, Cribier B, Velten M, Hedelin G. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007;157:338–343. doi: 10.1111/j.1365-2133.2007.08029.x. [DOI] [PubMed] [Google Scholar]
- Mansson-Brahme E, Johansson H, Larsson O, Rutqvist LE, Ringborg U. Trends in incidence of cutaneous malignant melanoma in a Swedish population 1976–1994. Acta Oncol. 2002;41:138–146. doi: 10.1080/028418602753669508. [DOI] [PubMed] [Google Scholar]
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163–172. [PubMed] [Google Scholar]
- Pollitt RA, Clarke CA, Shema SJ, Swetter SM. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7–13. doi: 10.1016/j.amepre.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries L, Eisner M, Kosary C. SEER Cancer Statistics Review, 1975–2002. Bethesda MD: National Cancer Institute; 2002. [Google Scholar]
- Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- Saraiya M, Hall HI, Thompson T, Hartman A, Glanz K, Rimer B, et al. Skin cancer screening among U.S. adults from 1992, 1998, and 2000 National Health Interview Surveys. Prev Med. 2004;39:308–314. doi: 10.1016/j.ypmed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Singh G, Miller B, Hankey B, Edwards B. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment and Survival, 1975–1999. NCI Cancer Surveillance Monograph Series. 2003:4. [Google Scholar]
- Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- Stang A, Pukkala E, Sankila R, Soderman B, Hakulinen T. Time trend analysis of the skin melanoma incidence of Finland from 1953 through 2003 including 16,414 cases. Int J Cancer. 2006;119:380–384. doi: 10.1002/ijc.21836. [DOI] [PubMed] [Google Scholar]
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881–884. doi: 10.1001/archderm.132.8.881. [DOI] [PubMed] [Google Scholar]
- Swerlick RA, Chen S. The melanoma epidemic: more apparent than real? Mayo Clin Proc. 1997;72:559–564. doi: 10.4065/72.6.559. [DOI] [PubMed] [Google Scholar]
- Ulmer MJ, Tonita JM, Hull PR. Trends in invasive cutaneous melanoma in Saskatchewan 1970–1999. J Cutan Med Surg. 2003;7:433–442. doi: 10.1007/s10227-003-0159-0. [DOI] [PubMed] [Google Scholar]
- Weissman JS, Schneider EC. Social disparities in cancer: lessons from a multidisciplinary workshop. Cancer Causes and Control. 2005;16:71–74. doi: 10.1007/s10552-004-1255-1. [DOI] [PubMed] [Google Scholar]
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. Bmj. 2005;331:481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- Zippin C, Lum D, Hankey B. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2345. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]