Abstract
BACKGROUND:
Supplemental oxygen therapy has been shown to improve exercise performance in patients with chronic obstructive pulmonary disease (COPD). It is unknown whether the magnitude of this benefit would be affected by participation in a pulmonary rehabilitation program.
OBJECTIVE:
To compare the effects of supplemental oxygen on exercise capacity in nonhypoxemic COPD patients before and after participation in a pulmonary rehabilitation program.
METHODS:
Sixteen patients with COPD underwent two pairs of constant-load exercise tests before and after participation in a three-month outpatient pulmonary rehabilitation program. Each pair of exercise tests consisted of a test performed with room air and a second test performed with 50% supplemental oxygen, in random order. The primary outcome was the difference in exercise duration between tests performed with supplemental oxygen and with room air. This difference was compared before and after participation in a pulmonary rehabilitation program.
RESULTS:
Supplemental oxygen therapy improved exercise duration by 75 s before participation in a pulmonary rehabilitation program and by 153 s following pulmonary rehabilitation. Rehabilitation alone improved exercise duration by 28 s, but rehabilitation appeared to augment the exercise benefits of supplemental oxygen therapy by a mean of 78 s (95% CI 11 s to 145 s; P=0.03).
CONCLUSION:
The effects of supplemental oxygen therapy were augmented after pulmonary rehabilitation. The improvement in exercise duration with supplemental oxygen following rehabilitation was greater than either supplemental oxygen or pulmonary rehabilitation alone.
Keywords: Chronic obstructive pulmonary disease, Pulmonary rehabilitation, Supplemental oxygen
Abstract
HISTORIQUE :
Il a été démontré que l’oxygénothérapie améliore le rendement à l’effort chez les patients qui souffrent de maladie pulmonaire obstructive chronique (MPOC). On ignore si la participation à un programme de réadaptation pulmonaire peut modifier l’ampleur de ce bienfait.
OBJECTIF :
Comparer les effets de l’oxygénothérapie sur le rendement à l’effort chez des patients atteints de MPOC non hypoxémiques avant et après leur participation à un programme de réadaptation pulmonaire.
MÉTHODES :
Seize patients atteints de MPOC ont subi deux paires de tests à l’effort sous résistance constante avant et après leur participation à un programme de réadaptation pulmonaire ambulatoire d’une durée de trois mois. Chaque paire de tests à l’effort comportait une épreuve à l’air ambiant et une épreuve sous oxygène à 50 % selon un ordre aléatoire. Le paramètre principal était la différence quant à la durée de l’exercice selon qu’il était fait avec ou sans oxygénothérapie. Cette différence a ensuite été comparée avant et après la participation à un programme de réadaptation pulmonaire.
RÉSULTATS :
L’oxygénothérapie a amélioré de 75 secondes la durée de l’exercice avant la participation à un programme de réadaptation pulmonaire et de 153 secondes après la réadaptation pulmonaire. La réadaptation seule a amélioré de 28 secondes la durée de l’exercice, mais elle a semblé intensifier les bienfaits de l’oxygénothérapie sur le rendement à l’effort, en moyenne de 78 secondes (IC à 95 %, 11 s à 145 s; P = 0,03).
CONCLUSION :
Les effets de l’oxygénothérapie ont été rehaussés par la réadaptation pulmonaire. La durée de l’épreuve d’effort à été plus longue avec l’oxygénothérapie après la réadaptation qu’avec l’oxygénothérapie ou la réadaptation pulmonaire seules
Chronic obstructive pulmonary disease (COPD) is a common and potentially disabling disease. Despite advances in bronchodilator therapy over the past decade, many patients with severe COPD continue to experience significant functional limitation due to dyspnea. For these patients, pulmonary rehabilitation and supplemental oxygen therapy may be considered. Rehabilitation has become clearly established as a proven and recommended therapy for patients with COPD (1). Unfortunately, the availability of pulmonary rehabilitation in Canada is limited (2). Oxygen therapy, although expensive, is widely available and has clearly been shown to improve mortality in COPD patients with resting hypoxia (3,4). However, the role of supplemental oxygen in patients with less severe COPD is less definitive. Although oxygen therapy does not affect mortality in this population (5), it may have a role in alleviating dyspnea and improving exercise performance. Several studies (6–8) have demonstrated that supplemental oxygen therapy can reduce ventilation and hyperinflation in patients with COPD. The reduction in ventilation may be secondary to a combination of diminished hypoxic drive and reduced metabolic acidosis (8,9). Increased oxygen delivery to peripheral muscles may reduce anaerobic metabolism during exercise, thus decreasing the development of metabolic acidosis, which is a strong stimulus for ventilation. It should be noted, however, that the magnitude of the effect of supplemental oxygen therapy on exercise performance is highly variable among individual patients (7,9).
Pulmonary rehabilitation has also been demonstrated to have a significant impact on exercise physiology in COPD patients. It has been shown to reduce ventilatory demand and resting hyperinflation (10–12). The reduction in ventilatory demand may be due to a combination of increased mechanical efficiency (11) and reduced lactic acid production (10). Mechanisms behind the reduction in resting hyperinflation are not completely understood, although alterations in breathing pattern and improvements in inspiratory muscle strength have been hypothesized (10,12).
Although several studies (13) have explored the use of oxygen during pulmonary rehabilitation, no study has specifically assessed the effect of pulmonary rehabilitation on the response to oxygen in this patient population. It is certainly possible that the effects of pulmonary rehabilitation and supplemental oxygen therapy are additive. However, given that both of these interventions produce improvements in exercise performance via similar effects on respiratory physiology (ie, reduced ventilatory demand, reduced hyperinflation and reduced lactic acidosis), it is also possible that the benefit of supplemental oxygen is minimized following completion of a pulmonary rehabilitation program.
Consequently, we assessed whether the physiological benefits of oxygen therapy were influenced by participation in a pulmonary rehabilitation program. The primary objective of the present study was to measure the effect of oxygen therapy on constant-load exercise duration before and after participation in a pulmonary rehabilitation program. Constant-load exercise duration was chosen as the primary end point because it has been demonstrated to be reproducible and responsive to therapy. Additionally, it has been used as an end point for previous studies evaluating the physiological effects of supplemental oxygen therapy. We chose to focus on COPD patients without resting hypoxia or significant exertional desaturation because this population would not be candidates for supplemental oxygen therapy according to Canadian funding criteria. With regard to the intervention, we chose to administer oxygen at a fraction of inspired oxygen (FiO2) of 0.5. It is acknowledged that administration of an FiO2 of 0.50 may not be practical in an outpatient setting. However, a previous study by Somfay et al (6) demonstrated that the benefits of supplemental oxygen were maximal at an FiO2 of 0.50. Initially, we chose this level for our study to maximize the benefits of supplemental oxygen and increase the likelihood of detecting physiological differences.
It should be emphasized that the present study was not designed to compare pulmonary rehabilitation with supplemental oxygen therapy because the benefits of pulmonary rehabilitation are well established and extend beyond its effects on exercise performance (14).
METHODS
Patients
Patients with clinically stable COPD, who were accepted into the pulmonary rehabilitation program at The Ottawa Hospital Rehabilitation Centre (Ottawa, Ontario), were studied. All patients had a clinical diagnosis of COPD (confirmed by the physician supervising the rehabilitation program as well as the primary investigator) and evidence of chronic airflow obstruction on pulmonary function testing (forced expiratory volume in 1 s [FEV1]/forced vital capacity ratio of less than 0.7, and FEV1 less than 70% predicted). All patients were initially referred for pulmonary rehabilitation for improvement of exertional dyspnea that was not satisfactorily managed by pharmacological therapy alone. To assess for possible relationships between severity of airway obstruction and treatment response, the study inclusion criteria allowed for a relatively wide range of disease severity. However, patients with either resting hypoxia or exertional desaturation during ambulation (oxygen saturation less than 89% on pulse oximetry) were excluded because many of these patients would already be prescribed supplemental oxygen therapy and may have had other indications for oxygen therapy (ie, reduced mortality in patients with resting hypoxia). Patients with nonpulmonary conditions that could affect their exercise performance (eg, angina, congestive heart failure, severe musculoskeletal problems) or lung diseases other than COPD (eg, asthma or bronchiectasis) were excluded. Patients with recent COPD exacerbations or changes in their regular medications within the past two months were also excluded because these factors could influence exercise capacity. All regular medications were continued throughout the study period. The protocol was approved by The Ottawa Hospital Research Ethics Board and all patients provided written informed consent.
Pulmonary function and exercise testing
Before they commenced exercise training, eligible patients were referred to the study by the rehabilitation physiotherapist after their acceptance into the pulmonary rehabilitation program. At their first visit, patients were provided detailed information by the study personnel regarding the study and were assessed by the study physician to confirm their diagnosis and eligibility. If the patients consented to participation, they underwent spirometry and a symptom-limited incremental-load cardiopulmonary exercise test (SensorMedics Vmax 229 Series with VMax/Spectra Software Version 07-2B), on an electronically braked cycle ergometer (Ergoline – ergometrics 800). Before the test, patients were familiarized with the modified Borg scale for reporting of dyspnea and leg fatigue during exercise. Additionally, practice ‘inspiratory capacity (IC) manoeuvres’ (inhalation to total lung capacity after a normal exhalation) were performed to ensure reproducibility. After 3 min of rest and 1 min of unloaded pedalling, the work rate was increased in a stepwise fashion, at a rate of 20 W every 2 min. The exercise load for all subsequent constant-load exercise tests was set at 75% of the peak work rate achieved by the patient during the incremental test. After completion of the incremental exercise test and a rest period, a ‘practice’ constant-load exercise test was also performed to familiarize the subjects with the protocol and to increase reproducibility (reduce learning effects for future tests). The data from this practice test were not analyzed.
After the initial visit, but before commencement of rehabilitation, the subjects returned for a set of two symptom-limited constant-load exercise tests. These tests were performed on separate days (ie, between 24 h and 48 h apart). Starting at least 10 min before each test and throughout the test, the subjects inhaled a gas mixture from a 20 L Douglas reservoir bag. For one test, the gas mixture consisted of room air; for the other test, the mixture contained an FiO2 of 0.50. The order of testing was randomized. Both the patient and the supervising physician were blinded to the nature of the inhaled gas. The 10 min rest period allowed for equilibration with the inhaled gas. Additionally, three practice IC manoeuvres were performed. After the 10 min rest period, patients performed 1 min of unloaded pedalling. After 1 min, the exercise load for each subject was increased to 75% of the peak work rate achieved during the incremental test performed at the first visit. Patients then exercised at this constant load for as long as tolerated (ie, symptom-limited). During the constant-load exercise, symptoms and IC were measured during every 2 min of loaded pedalling. Symptoms were assessed by asking the patient to point a number betweeen 0 and 10 on the modified Borg scale that best described the severity of their dyspnea and leg fatigue (measured separately) at that moment. The Borg measurements were made at the 60 s to 90 s interval of each 2 min period. IC measurements were made at the 90 s to 120 s interval of each 2 min period.
Another set of two, symptom-limited, constant-load exercises were performed (24 h to 48 h apart) within four weeks after completion of the 12-week pulmonary rehabilitation program. Once again, one test was performed with room air, the other with 50% oxygen, according to a computer-generated randomization schedule. The methodology and workload was identical to the prerehabilitation tests.
Pulmonary rehabilitation
All patients attended a consecutive 12-week outpatient pulmonary rehabilitation program at The Ottawa Hospital Rehabilitation Centre. The personalized endurance exercise program consisted of treadmill and stationary bicycle training, as well as paced walking. Upper and lower extremities strengthening, breathing retraining, postural correction and lung secretion clearance techniques supplemented the program as indicated. Patients underwent two 1 h to 2 h physiotherapist-supervised training sessions per week. Additionally, patients were encouraged to walk or use their own exercise equipment for an additional one to two sessions per week during the rehabilitation period. The patients received individualized education and group education sessions on self-management skills. The multidisciplinary team provided additional counselling and education sessions as indicated.
Data analysis
The primary outcome was the difference in exercise duration during constant-load exercise tests performed with and without supplemental oxygen. A paired t test was used to compare the benefit of supplemental oxygen therapy before and after participation in the pulmonary rehabilitation program. Secondary outcomes included ventilation, tidal volume, IC, heart rate and symptoms during exercise compared at isotime. For each subject, isotime was the exercise time of the constant-load exercise test with the shortest duration among the four constant-load tests completed by that subject. Data from the same isotime interval were compared across all four constant-load exercise tests. For IC and symptoms (measured at 2 min intervals), the closest available measurements before isotime were used. Paired t tests were used to assess the effects of oxygen and rehabilitation on the secondary outcomes.
Pearson correlation analyses were used to assess for possible relationships between severity of lung function (% predicted FEV1) or exercise capacity (% predicted peak workload) on the primary end point (4). A two-factor ANOVA analysis with repeated measures was performed to study the interaction effect of oxygen and rehabilitation on exercise duration.
RESULTS
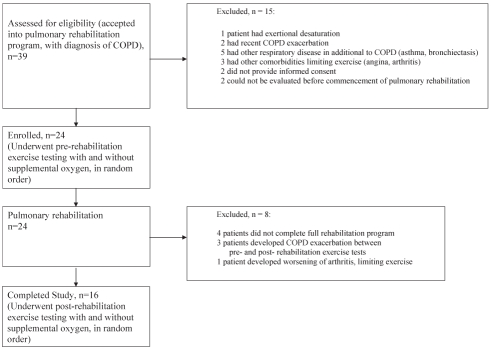
A total of 39 potential candidates were referred to the study by the rehabilitation physiotherapist, of which 15 patients were excluded after assessment by the study team (Figure 1). Twenty-four patients with moderate or severe COPD (FEV1 range 18% to 68%, mean 42% of predicted) met all eligibility criteria and initially enrolled in the study. Of these 24, eight patients were later excluded – four patients did not complete the full rehabilitation program, three patients developed an acute COPD exacerbation during the study period and one patient could not complete the postrehabilitation testing due to worsening osteoarthritis of the knee. Exercise duration data were obtained for the 16 patients who completed the study protocol, the characteristics of whom are presented in Table 1.
Figure 1).
Patient flow diagram. COPD Chronic obstructive pulmonary disease
TABLE 1.
Baseline characteristics of the study population (n=16)
| Characteristic | Mean ± SD |
|---|---|
| Age, years | 65.9±6.6 |
| FEV1, % predicted | 41.5±13.8 |
| VO2/kg, % predicted | 48.8±16.7 |
| Oxygen saturation on resting oximetry, % | 97.1±1.9 |
| Lowest oxygen saturation on ambulation, % | 92.9±2.7 |
FEV1 Forced expiratory volume in 1 s; VO2 Maximal oxygen uptake
Primary end points
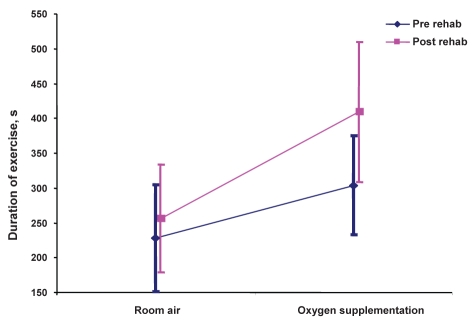
For paired exercise tests performed before participation in pulmonary rehabilitation, supplemental oxygen therapy was associated with a mean 75 s (95% CI 20 s to 131 s; P=0.01) improvement in constant-load exercise duration, compared with the constant-load exercise test performed on room air (Table 2). For paired exercise tests performed after completion of pulmonary rehabilitation, a mean 153 s (95% CI 75 s to 231 s; P=0.0008) improvement was observed between tests conducted with supplemental oxygen and room air. The benefits of supplemental oxygen therapy were significantly greater after rehabilitation than before rehabilitation. Patients exercised for an additional 78 s (95% CI 11 s to 145 s; P=0.03) following participation in pulmonary rehabilitation.
TABLE 2.
Exercise duration pre- and postrehabilitation, with and without oxygen (n=16)
| Exercise duration, s (mean ± SD) | |
|---|---|
| Postrehabilitation (with oxygen) | 409.4±205.3 |
| Postrehabilitation (without oxygen) | 256.3±158.4 |
| Prerehabilitation (with oxygen) | 303.5±145.0 |
| Prerehabilitation (without oxygen) | 228.1±156.8 |
For individual patients, however, the improvements associated with supplemental oxygen were highly variable. Consequently, Pearson correlation analyses were performed to determine whether the response to supplemental oxygen therapy could be predicted from baseline patient factors (FEV1 and exercise capacity). An inverse correlation between the severity of lung disease (% predicted FEV1) and response to supplemental oxygen before rehabilitation (Pearson coefficient = −0.66; P=0.005) was identified, indicating that patients with more severe lung impairment had a greater relative improvement in exercise duration with supplemental oxygen. However, this relationship between severity of lung disease and response to supplemental oxygen was not observed in exercise tests performed after rehabilitation (Pearson coefficient = −0.21; P=0.42). No significant relationship was observed between baseline exercise capacity (% predicted VO2) and the response to supplemental oxygen, either before or after rehabilitation.
Secondary end points
For exercise tests performed before rehabilitation, supplemental oxygen therapy was associated with an increase in IC of 0.2 L (95% CI 0.1 L to 0.4 L; P=0.002) as well as a reduction in isotime ventilation of −3.0 L/min (95% CI −5.8 L/min to −0.2 L/min; P=0.04). Again, postrehabilitation exercise testing demonstrated a significant reduction in ventilation with use of supplemental oxygen, although changes in IC were not statistically significant (Table 3).
TABLE 3.
Response to oxygen (pre- and postrehabilitation, and difference)
|
Oxygen – room air |
Difference | P* | ||||
|---|---|---|---|---|---|---|
| Prerehabilitation | P* | Postrehabilitation | P* | |||
| Time, s (n=16) | 75.4 (19.8–131.1) | 0.01 | 153.2 (75.4–230.9) | 0.0008 | 77.8 (10.7–144.8) | 0.03 |
| Pulmonary ventilation, L (n=16)† | −3.01 (−5.8– −0.2) | 0.04 | −4.6 (−6.9– −2.3) | 0.0007 | −1.6 (−4.8–1.5) | 0.28 |
| Tidal volume, L (n=15)† | 0.03 (−0.09–0.2) | 0.58 | −0.04 (−0.1–0.07) | 0.46 | −0.1 (−0.2–0.07) | 0.31 |
| Inspiratory capacity, L (n=14)† | 0.2 (0.1–0.4) | 0.002 | 0.1 (−0.1–0.3) | 0.32 | −0.1 (−0.3–0.02) | 0.07 |
| Heart rate, beats/min (n=16)† | −1.7 (−6.3–2.8) | 0.43 | −3.9 (−8.7–0.9) | 0.11 | −2.1 (−6.7–2.4) | 0.33 |
| BORG scale (breathlessness, n=15)† | −0.4 (−1.2–0.4) | 0.28 | −0.8 (−1.4–0.2) | 0.009 | −0.4 (−1.4–0.6) | 0.37 |
| BORG scale (leg fatigue, n=15)† | −0.3 (−1.3–0.6) | 0.47 | −0.8 (−1.6–0.04) | 0.06 | −0.5 (−1.9–0.9) | 0.49 |
Data are presented as mean (95% CI) unless indicated otherwise.
Paired t test;
Closest complete 30 s isotime
Effects of pulmonary rehabilitation
Because all patients underwent pulmonary rehabilitation, the present study was not designed to specifically assess its benefits. However, the results of the constant-load exercise tests performed on room air were compared pre- and postrehabilitation to provide insight into the differences in results seen with supplemental oxygen pre- and postrehabilitation (Table 4). Pulmonary rehabilitation was associated with a statistically significant reduction in dyspnea and leg fatigue at isotime. Dyspnea decreased by a mean of 1.1 points on the modified Borg scale (95% CI −2.1 to −0.1; P=0.03) and leg fatigue decreased by a mean of 1.8 points (95% CI −2.7 to −0.9; P=0.001). A mean improvement in exercise duration of 28 s (12%) was observed; however, this was not statistically significant (95% CI −84 to 140; P=0.6).
TABLE 4.
Effect of pulmonary rehabilitation
|
Rehabilitation |
Difference, mean (95% CI) | P | ||
|---|---|---|---|---|
| Before | After | |||
| Forced expiratory volume in 1 s, L (n=16) | 1.08±0.3 | 1.04±0.3 | −0.04 (−0.1–0.01) | 0.14 |
| Time, s (n=16) | 228.06±156.8 | 256.2±158.4 | 28.2 (−84.1–140.4) | 0.6 |
| Pulmonary ventilation, L (n=16)† | 28.2±8.3 | 28.3±7.4 | 0.14 (−2.7–2.9) | 0.91 |
| Tidal volume, L (n=15)† | 0.98±0.28 | 1.02±0.28 | 0.04 (−0.1–0.2) | 0.59 |
| Inspiratory capacity, L (n=15)† | 1.18±0.31 | 1.3±0.4 | 0.08 (−0.1–0.3) | 0.36 |
| Heart rate, beats/min (n=16)† | 117±15.7 | 116.6±19.3 | −0.37 (−5.6–4.8) | 0.88 |
| BORG scale (breathlessness, n=15)† | 5.3±2.5 | 4.2±1.6 | −1.1 (−2.1–0.1) | 0.03 |
| BORG scale (leg fatigue, n=15)† | 5.9±2.6 | 4.1±1.99 | −1.8 (−2.7–0.9) | 0.001 |
Data presented as mean ± SD unless indicated otherwise.
Paired t test;
Closest complete 30 s to isotime
Interaction between pulmonary rehabilitation and supplemental oxygen
The effects of combination supplemental oxygen and rehabilitation on exercise duration were greater than the individual effects of each therapy. Consequently, the interaction effect of oxygen and rehabilitation on exercise duration was assessed. A significant interaction was found: the benefits of supplemental oxygen therapy were significantly greater after rehabilitation than before rehabilitation (P=0.026 [F test for interaction]) (Figure 2).
Figure 2).
Interaction plot. Effects of supplemental oxygen therapy on exercise before and after pulmonary rehabilitation (rehab), P=0.026 (F test for interaction)
DISCUSSION
Several studies have demonstrated that supplemental oxygen therapy can improve exercise duration in patients with COPD, even in patients with no evidence of resting hypoxia or exertional desaturation. Our data suggest that the beneficial effects of supplemental oxygen therapy may be further augmented by participation in a pulmonary rehabilitation program. Indeed, the magnitude of improvement in exercise duration with combination supplemental oxygen therapy and pulmonary rehabilitation was observed to be considerably greater than the sum of the individual responses to oxygen and rehabilitation alone.
As demonstrated in previous studies, the effect of supplemental oxygen therapy varies considerably among individual patients. It did not appear from the present study that the magnitude of the benefits from supplemental oxygen therapy were predictable from baseline data. Although we found that baseline FEV1 was inversely correlated with response to supplemental oxygen before rehabilitation, neither FEV1 nor baseline exercise capacity could predict postrehabilitation oxygen responses.
It was not possible to provide a definitive physiological explanation for the increased oxygen benefit following rehabilitation from our study data. Oxygen therapy appeared to increase IC and reduce isotime ventilation. This can potentially improve exercise capacity by delaying the onset of ventilatory limitation. These results are consistent with observations from previously published studies (7,8). In our study, pulmonary rehabilitation was associated with a reduction in symptoms (both dyspnea and leg fatigue), without improvements in exercise capacity or detectable changes in ventilatory responses. Although several studies attribute the reduction in dyspnea following pulmonary rehabilitation to changes in ventilation and hyperinflation, some studies (12,15) suggest that desensitization to dyspnea may play a role. It is possible that the combination of supplemental oxygen therapy and rehabilitation increases exercise duration by a combination of increased IC, reduced ventilatory demand and increased tolerance (desensitization) to dyspnea. However, it is more likely that our study was underpowered for the detection of physiological changes with pulmonary rehabilitation.
Limitations of the present study include the small sample size, lack of a definitive conclusion regarding physiological mechanisms and the relatively small magnitude of physiological improvement following pulmonary rehabilitation. Recruitment was difficult because patients were rigorously screened for comorbidities that could affect exercise performance. Additionally, many patients were excluded due to either ‘unstable’ lung disease (COPD exacerbation or medication change within two months of starting pulmonary rehabilitation) or hypoxia (a significant proportion of patients enrolled in our rehabilitation program had either hypoxia at rest or during ambulation). We could not provide a definitive physiological explanation for the improvements in oxygen response following rehabilitation. This is due to the relatively small sample size, as well as the heterogeneity of results. The latter is likely related to our decision to include a wide range of disease severities. This enabled us to determine whether disease severity was associated with the response to supplemental oxygen therapy (there was no relationship observed in postrehabilitation studies).
Finally, we acknowledge that the effects of pulmonary rehabilitation on exercise duration observed in our study were smaller than that of other published studies (12% improvement in exercise duration, P=0.6). This may be because our rehabilitation program was less intense and shorter in duration than that used in other published studies (our rehabilitation program involved two supervised exercise sessions per week for a three-month period, while other studies have used exercise training three times per week for up to six months). The present study was also not powered to detect a significant effect on exercise duration following pulmonary rehabilitation. Furthermore, our chosen primary end point (exercise duration on constant-load exercise) may be less sensitive to the effects of pulmonary rehabilitation than other exercise measurements (eg, 6 min walk test distance). However, it should be noted that even if larger effects on exercise duration were observed following pulmonary rehabilitation, our primary conclusion would likely not be changed. Indeed, it could be argued that the combined effects of pulmonary rehabilitation and supplemental oxygen could have been even larger had we been able to observe better rehabilitation results.
It should be emphasized again that the present study was not designed to be a comparison between pulmonary rehabilitation and supplemental oxygen therapy. We believe that this was unnecessary because pulmonary rehabilitation has been clearly established as a useful treatment modality for patients with COPD. If anything, the present study underscores the importance of pulmonary rehabilitation in maximizing the benefits of supplemental oxygen in COPD patients without resting hypoxia.
Footnotes
FUNDING: The present study was funded by a block term grant from the Ontario Thoracic Society.
CONFLICTS OF INTEREST: All authors have no conflicts of interest to declare.
REFERENCES
- 1.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007;14:5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks D, Sottana R, Bell B, et al. Characterization of pulmonary rehabilitation programs in Canada in 2005. Can Respir J. 2007;14:87–92. doi: 10.1155/2007/951498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 4.Nocturnal Oxygen Therapy Trial Group Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease: A clinical trial. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 5.Gorecka D, Gorzelak K, Sliwinski P, et al. Effects of long-term oxygen therapy on survival in patients with chronic obstructive lung disease with moderate hypoxaemia. Thorax. 1997;52:674–9. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somfay A, Porszasz J, Lee SM, et al. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18:77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Care Crit Med. 2001;163:892–8. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 8.Somfay A, Porszasz J, Lee SM, et al. Effect of hyperoxia on gas exchange and lactate kinetics following exercise onset in nonhypoxemic COPD patients. Chest. 2002;121:393–400. doi: 10.1378/chest.121.2.393. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Resp Crit Care Med. 1997;155:530–5. doi: 10.1164/ajrccm.155.2.9032190. [DOI] [PubMed] [Google Scholar]
- 10.Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell DE, McGuire M, Samis L, et al. The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Resp Crit Care Med. 1995;152:2005–13. doi: 10.1164/ajrccm.152.6.8520769. [DOI] [PubMed] [Google Scholar]
- 12.Gigliotti F, Coli C, Bianchi R, et al. Exercise training improves exertional dyspnea in patients with COPD. Chest. 2003;123:1794–802. doi: 10.1378/chest.123.6.1794. [DOI] [PubMed] [Google Scholar]
- 13.Nonoyama ML, Brooks D, Lacasse Y, et al. Oxygen therapy during exercis training in chronic obstructive pulmonary disease (Review) Cochrane Database Syst Rev. 2007:1–11. doi: 10.1002/14651858.CD005372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Resp Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 15.Carrieri-Kohlman V, Gormley JM, Douglas MK, et al. Exercise training decreases dyspnea and the distress and anxiety associated with it. Chest. 1996;110:6–35. doi: 10.1378/chest.110.6.1526. [DOI] [PubMed] [Google Scholar]