Abstract
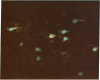
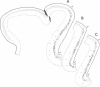
During fetal development, the regional distribution of callosal projection neurons in the rhesus monkey's postcentral gyrus changes from a uniform to a discontinuous pattern. To determine if this developmental change reflects the retraction of transient callosal projections, two different fluorescent tracers were injected into the brain of fetal monkeys of known gestational ages. Fast blue was injected into the entire postcentral gyrus of one hemisphere, whereas a second tracer (rhodamine latex beads or diamidino yellow) was injected into the caudal portion of the postcentral gyrus of the other hemisphere. The rostral portion of the postcentral gyrus (contralateral to the hemisphere injected with fast blue) was subsequently examined for the presence of labeled cells. In animals injected early in fetal development, on embryonic day 110 or younger and sacrificed 4 weeks later, there were numerous cells labeled with both tracers. In contrast, very few double-labeled cells were found in fetuses injected at an older age, embryonic day 135. We interpret these findings as showing that early in fetal development, when callosal projection neurons in the postcentral gyrus show a continuous distribution pattern, single cells in the rostral portion of this gyrus possess at least two collaterals, one projecting to the contralateral hemisphere and the other to the caudal portion of the gyrus. Subsequently, many of these neurons retract callosal collaterals while maintaining ipsilateral projections. Thus, process elimination accounts for the establishment of the discontinuous distribution of callosal neurons found in the postcentral gyrus of the mature primate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. A., Asanuma C., Cowan W. M. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: a study using retrogradely transported fluorescent dyes. J Comp Neurol. 1985 Feb 22;232(4):443–455. doi: 10.1002/cne.902320403. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Baumbach H. D., Lawson R. Callosal projections of the striate cortex in the neonatal rabbit. Exp Brain Res. 1981;42(2):122–126. doi: 10.1007/BF00236899. [DOI] [PubMed] [Google Scholar]
- Dehay C., Kennedy H., Bullier J., Berland M. Absence of interhemispheric connections of area 17 during development in the monkey. Nature. 1988 Jan 28;331(6154):348–350. doi: 10.1038/331348a0. [DOI] [PubMed] [Google Scholar]
- Finlay B. L., Slattery M. Local differences in the amount of early cell death in neocortex predict adult local specializations. Science. 1983 Mar 18;219(4590):1349–1351. doi: 10.1126/science.6828866. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S., Kraftsik R. Interchange of callosal and association projections in the developing visual cortex. J Neurosci. 1986 May;6(5):1384–1409. doi: 10.1523/JNEUROSCI.06-05-01384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy G. O., Akers R. M., Killackey H. P. Differential distribution of callosal projection neurons in the neonatal and adult rat. Brain Res. 1979 Sep 21;173(3):532–537. doi: 10.1016/0006-8993(79)90247-6. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Chalupa L. M. Ontogenetic change in the distribution of callosal projection neurons in the postcentral gyrus of the fetal rhesus monkey. J Comp Neurol. 1986 Feb 15;244(3):331–348. doi: 10.1002/cne.902440306. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Gould H. J., 3rd, Cusick C. G., Pons T. P., Kaas J. H. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol. 1983 Oct 1;219(4):384–419. doi: 10.1002/cne.902190403. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Chang F. L., Land P. W. The development of callosal projections in normal and one-eyed rats. Brain Res. 1984 May;316(1):139–142. doi: 10.1016/0165-3806(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Nelson R. J., Sur M., Felleman D. J., Kaas J. H. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980 Aug 15;192(4):611–643. doi: 10.1002/cne.901920402. [DOI] [PubMed] [Google Scholar]
- Olavarria J., Van Sluyters R. C. Organization and postnatal development of callosal connections in the visual cortex of the rat. J Comp Neurol. 1985 Sep 1;239(1):1–26. doi: 10.1002/cne.902390102. [DOI] [PubMed] [Google Scholar]
- POWELL T. P., MOUNTCASTLE V. B. The cytoarchitecture of the postcentral gyrus of the monkey Macaca mulatta. Bull Johns Hopkins Hosp. 1959 Sep;105:108–131. [PubMed] [Google Scholar]
- Rhoades R. W., Dellacroce D. D. Neonatal enucleation induces an asymmetric pattern of visual callosal connections in hamsters. Brain Res. 1980 Nov 24;202(1):189–195. [PubMed] [Google Scholar]
- Schwartz M. L., Goldman-Rakic P. S. Single cortical neurones have axon collaterals to ipsilateral and contralateral cortex in fetal and adult primates. Nature. 1982 Sep 9;299(5879):154–155. doi: 10.1038/299154a0. [DOI] [PubMed] [Google Scholar]
- Tong L., Spear P. D. Single thalamic neurons project to both lateral suprasylvian visual cortex and area 17: a retrograde fluorescent double-labeling study. J Comp Neurol. 1986 Apr 8;246(2):254–264. doi: 10.1002/cne.902460209. [DOI] [PubMed] [Google Scholar]