Abstract
A novel defensin-like antimicrobial peptide named longicornsin was isolated from the salivary glands of the hard tick, Haemaphysalis longicornis, using a 10-kDa cut-off Centriprep filter and reversed-phase high-performance liquid chromatography (RP-HPLC). Its amino acid sequence was determined as DFGCGQGMIFMCQRRCMRLYPGSTGFCRGFRCMCDTHIPLRPPFMVG by Edman degradation. The cDNA encoding longicornsin was cloned by cDNA library screening. The predicted protein from the cDNA sequence was composed of 78 amino acids including a mature longicornsin. It showed similarity with defensin-like peptides from other ticks by BLAST search. Different from most other tick defensin-like peptides, longicornsin had a C-terminal extension. Purified longicornsin exerted potent antimicrobial activities against bacteria and fungi. Interestingly, it even showed strong antimicrobial ability against drug-resistant microorganisms and Helicobacter pylori. The results of this study indicated that longicornsin is a potential candidate for novel antimicrobial drug design.
Keywords: tick, antimicrobial peptide, defensin, salivary gland, Haemaphysalis longicornis
Introduction
During the past decade, endogenous antimicrobial peptides have become recognized as important, ubiquitous, and ancient contributors to the innate mechanisms that permit animals and plants to resist infection.1–8 A large number of antimicrobial peptides have been found in insects, amphibians, and mammals, with many still being discovered. Antimicrobial peptides encompass a wide variety of structural motifs. So far known antimicrobial peptides can be divided into several groups: linear peptides forming amphipathic and hydrophobic helices, cyclic peptides and small proteins forming β-sheet structures, peptides with unique amino acid compositions, cyclic peptides with thio-ether groups in the ring, lipopeptides terminating in an amino alcohol and macrocyclic knotted peptides.8
Ticks are second only to mosquitoes as vectors of disease-causing agents to humans, and they are the most important arthropod that transmits pathogens to other animal species.9–11 Despite the importance of ticks as vectors of disease, very little is known of their immune system. Reports indicate that ticks have the ability to control infections when challenged with various bacteria.12–17 They are a reservoir for pharmaceutical molecules with several defensin- and hebraein-like antimicrobial peptides having been found in tick hemolymph and saliva.18–27 Recently, two antimicrobial peptides with unique structural motifs, ixosin and ixosin-B, were identified from salivary glands of Ixodes sinensis.28,29 Antimicrobial peptides may help overcome host defensive responses, or keep the blood sterile in the tick body. Here, we report the characterization of a novel defensin-like antimicrobial peptide, possessing a unique c-terminal extension in its primary structure from the hard tick, Haemaphysalis longicornis.
Results
Purification of antimicrobial peptides
More than 40 peaks were eluted from the supernatant extract of tick salivary glands by C8 RP-HPLC as shown in Figure 1. The peak indicated by an arrow in Figure 1 was found to have significant antimicrobial activity against tested microorganisms. This peak was collected and studied further.
Figure 1.
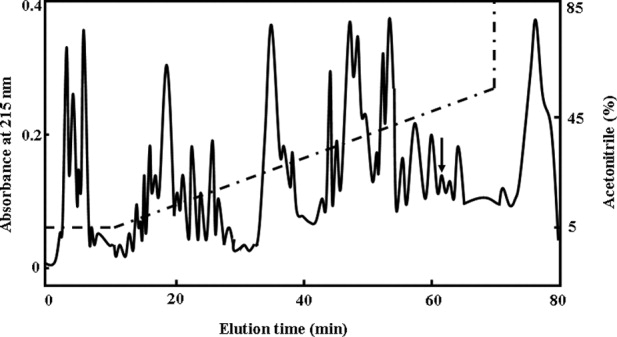
Isolation of longicornsin, from the hard tick, H. longicornis salivary glands. The lyophilized SGE sample (0.2 g) was dissolved in 5 mL 0.1 M phosphate buffer solution, pH 6.0, and filtered through a 10-kDa cut-off Centriprep filter (Millipore, Bedford, CA) and the filtrate was lyophilized. Lyophilized filtrate was applied to a Hypersil BDS C8 RP-HPLC column (4.6 mm x 250 mm) equilibrated with 0.1% (v/v) trifluoroacetic acid/water. The elution was performed with the indicated gradient of acetonitrile in Figure 1 at a flow rate of 0.7 mL/min, and purified antimicrobial peptide was indicated by an arrow.
Structural characterization
Purified antimicrobial peptide indicated by an arrow in Figure 1 was named longicornsin. It was subjected to complete amino acid sequence analysis by automated Edman degradation. Its amino acid sequence was DFGCGQGMIFMCQRRCMRLYPGSTGFCRGFRCMCDTHIPLRPPFMVG, composed of 47 amino acid residues, and its molecular weight was 5370.1 analyzed by fast atom bombardment FAB mass spectrometry. There were six half-cystines in its sequence as in other defensin-like peptides. Its molecular weight matched well with the theoretical molecular weight (5370.4). There were multiple basic amino acids in the sequence of longicornsin as those found in other antimicrobial peptides. Analysis using the ExPASy MW/pI tool (http://www.expasy.ch/tools/pi_tool.html) showed that it had predicted pI of 8.98.
cDNA cloning
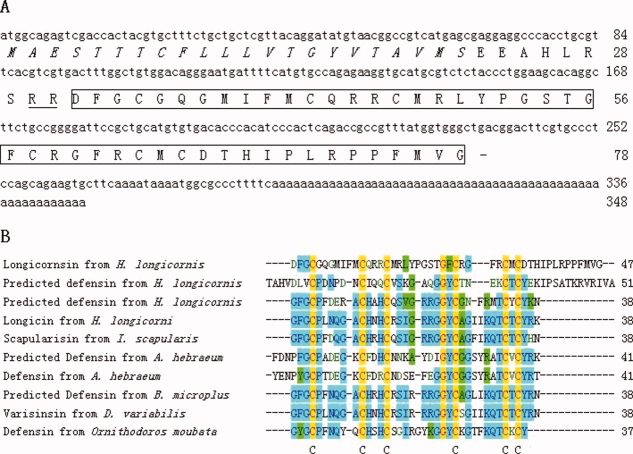
A clone, which contained an insert around 348 base pairs, was identified and isolated. Both strands of the clone were sequenced (Fig. 2, GenBank accession EU627689). It was found to have an open reading frame that encodes a polypeptide composed of 78 amino acids including the mature longicornsin sequence. The amino acid sequence deduced from the cDNA sequence matched well with the amino acid sequence determined by Edman degradation. By BLAST search, it shared similarity with other defensin-like antimicrobial peptides found in ticks [Fig. 2(B)].
Figure 2.
(A) The nucleotide sequence encoding longicornsin and the deduced amino acid sequence of the precursor polypeptide. The sequence of mature longicornsin is boxed. The predicted signal peptide is italic. The predicted processing site (—RR—) for protease is double underlined. The stop codon is indicated by a bar. B: Sequence alignment of longicornsin with other tick defensin-like peptides. The sources of sequences are: two predicted defensins from H. longicornis,[21] longicin,[27] scapularisin,[23] defensin from Amblyommahebraeum,[18] predicted defensin from Boophilus microplus (gi|28864187|gb|AAO48943.1|[28864187]), varisinsin from Dermacentor variabilis,[12] defensin from Ornithodoros moubata.[26] Gaps (−) have been introduced to optimize the sequence homology. The conserved half-cystines are indicated by stars (*). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Antimicrobial activity
Purified longicornsin exhibited potent antimicrobial activity against the tested strains as shown in Table I. Beside its antimicrobial activities against standard strains, longicornsin also had strong antimicrobial ability against drug-resistant strains. Among the tested drug-resistant strains, Pseudemonas aeruginosa and Staphylococcus aureus were the most sensitive to longicornsin. Longicornsin could exert anti-Helicobacter pylori activity with a minimal inhibitory concentration (MIC) of 6.4 μg/mL. The sensitive strains were not capable of resuming growth on agar plates after a 6 h treatment with concentrations above the corresponding MICs. MIC was defined as the lowest concentration of peptide that completely inhibits growth of the microbe determined by visual inspection or spectrophotometrically growth percentage was less than 5% compared to that of negative control.
Table I.
Antimicrobial Activity of Longicornsin
| Microorganisms | MICs (μg/mL) |
|---|---|
| E. coli ATCC25922 | 3.2 |
| E. coli 23Aa | 6.4 |
| E. coli 27Aa | 6.4 |
| S. aureus ATCC2592 | 0.8 |
| S. aureus 6Ab | 0.8 |
| S. aureus 15Ab | 1.6 |
| Pseudemonas aeruginosa 3Ac | 3.2 |
| P. aeruginosa 7Ac | 0.8 |
| H. pylori NCTC11637 | 6.4 |
| C. albicans ATCC2002 | 25.6 |
MIC, minimal peptide concentration required for total inhibition of cell growth in liquid medium. These concentrations represent mean values (±25%) of three independent experiments performed in duplicates.
Clinical-isolated strain resisitant to ampicillin, cephalothin I, II, III, and IV
Clinical-isolated strain resisitant to methicillin, amoxicillin, ampicillin
Clinical-isolated strain resisitant to methicillin, amoxicillin, ampicillin, cephalothin I, II, III, and IV.
Hemolytic activity
Some antimicrobial peptides exhibit hemolytic activities.7,8 Rabbit red blood cells were used to examine hemolytic capability in our experiments. As a result, longicornsin had little hemolytic activity (1.2%) on red blood cells even with peptide concentrations up to 200 μg/mL.
Discussion
Ticks and other arthropods do not contain lymphocytes, thymus, or antibodies. They can live harmoniously with microbes, relying heavily on antimicrobial peptides for defense against microbes without the help of adaptive immunity.30 Ticks have many opportunities to encounter microbes because of their special feeding behavior. Antimicrobial peptides/proteins are an innate immune system to kill pathogenic microbes. In terms of coevolution, ticks might have developed multiple antimicrobial factors because they have to encounter a large diversity of pathogenic microbes including bacteria, fungi, and viruses from their hosts in their different life stages. With the growing problem of conventional antibiotic resistance of human pathogenic organisms, ticks are becoming promising sources of pharmacological substances to treat infections. Four types of antimicrobial peptide/protein including defensin-, ixosin-, ixosin-B-, and hebraein-like peptides were reported from ticks. Defensins form a unique family of cysteine-rich cationic and structured polypeptides with three or four disulfide bridges; hebraein is composed of about one hundred amino acids and contains six half-cysteines and multiple histidines. Ixosin and Ixosin-B are linear peptides containing no cysteines.18,19,28,29
In this article, a defensin-like peptide named longicornsin was identified from the hard tick, H. longicornis. As far as we know, four defensin-like peptides have been identified from H. longicornis. Two of them were deduced from the cDNA sequences,26 one of which, longicin, was found to exert a remarkable ability to inhibit the proliferation of merozoites, an erythrocyte blood stage of equine Babesia equi, by killing the parasites.27 Different from most tick defensin-like peptides, longicornsin was found to compose of 47 amino acid residues with a C-terminal extension, which was similar to one of the predicted defensins from H. longicornis.26 Two interesting findings about longicornsin in this study were: (1) longicornsin had potential antimicrobial activities against drug-resistant microorganisms; (2) longicornsin also showed strong anti-H. pylori activity.
Although the actual antimicrobial mechanism remains still unclear, as a novel class of antibiotics, antimicrobial peptides have attracted considerable attention, especially for the treatment of antibiotic-resistant pathogens. More diversity of antimicrobial peptides provides more opportunities for designing novel and effective antimicrobial agents. The discovery of tick longicornsin with its interesting activities not only adds a new member to the family of antimicrobial proteins/peptides but also provides pharmacologists a novel candidate for anti-infection drug design.
Materials and Methods
Animals
Unfed and fed adult hard ticks of both sexes (H. longicornis) were kept in the laboratory according to the method of Kaufman and Phillips,31,32 and were maintained at 26°C and >90% humidity.
Salivary gland dissection
Ticks were glued to the bottom of a Petri dish and placed on ice for 20 min. They were then incised along the dorsal-lateral margin, and the dorsal integument was removed. The salivary gland was excised and transferred into 0.1 M phosphate buffer solution, pH 6.0, and kept in the same solution at −20°C.
Peptide purification
The salivary glands from 2000 ticks were homogenized using a glass homogenizer in 0.1 M buffer solution, pH 6.0, containing protease inhibitor cocktail (Sigma, P2714). The salivary glands were homogenized in 0.1 M phosphate buffer solution, pH 6.0, and centrifuged at 5000 g for 10 min. The supernatant termed as salivary gland extract (SGE) was lyophilized. The lyophilized SGE sample (0.2 g) was dissolved in 5 mL 0.1 M phosphate buffer solution, pH 6.0, and filtered through a 10 kDa cut-off Centriprep filter (Millipore, Bedford, CA) and the filtrate was lyophilized. Lyophilized filtrate was applied to a 4.6 mm x 250 mm Hypersil BDS C8 RP-HPLC column equilibrated with 0.1% (v/v) trifluoroacetic acid/water. Elution (0.7 mL/min) was performed using 0.1% (v/v) TFA/water over 10 min, followed by a linear gradient of 0–60% acetonitrile containing 0.1% (v/v) TFA in 0.1% (v/v) TFA/water over 70 min, and final elution with 85% acetonitrile containing 0.1% (v/v) TFA. UV absorbing peaks were collected and lyophilized, and their effects on microorganisms were detected.
Structural analysis
Complete peptide sequencing was undertaken by Edman degradation on an Applied Biosystems pulsed liquid-phase sequencer, model 491. Fast atom bombardment (FAB) mass spectrometry was carried out on an Autospec-3000 spectrometer (VG, Manchester, England), equipped with a high field magnet, using glycerol:3-nitrobenzyl alcohol:dimethyl sulphoxide (1:1:1, v:v:v) as mixed matrix. The ion gun was operated at 25 kV with a current of 1 micro A, using Cs+ as the bombarding ion.
Construction and screening of a cDNA library
Standard recombinant DNA techniques were used as described.33 mRNAs were prepared from the total RNA of H. longicornis salivary glands by oligo(dT) cellulose chromatography. A directional cDNA library was constructed with a plasmid cloning kit (SuperScript™ Plasmid System, GIBCO/BRL) following the instructions of the manufacturer, producing a library of about 2.3 × 105 independent colonies.
A PCR-based method for high stringency screening of DNA libraries was used for screening and isolating the clones with some modifications. Two oligonucleotide primers, S1, 5′- GA(T/C)TT(T/C)GG(A/T/C/G)TGTGG(A/T/C/G)CA(A/G)GG(A/T/C/G)ATG-3′, in the sense direction), a specific primer designed according to the amino acid sequence determined by Edman degradation and a vector SP6 promoter primer (5′-CATACGATTTAGGTGACACTATAG-3′, in the antisense direction) located in the 3′ part of the cloned insert, were used in PCR reactions. All the oligonucleotide primers for PCR were prepared with a DNA synthesizer (Model 381A, Applied Biosystems). The PCR conditions were: 2 min at 94°C, followed by 30 cycles of 10 sec at 92°C, 30 sec at 52°C, 60 sec at 72°C. DNA sequencing was performed on an Applied Biosystems DNA sequencer, model ABI PRISM 377.
Antimicrobial assays
Standard bacterial and fungal strains used in antimicrobial assays, Gram-positive bacterium Staphylococcus aureus (ATCC2592), Gram-negative bacterium Escherichia coli (ATCC25922), fungus Candida albicans (ATCC2002), and other clinical-isolated drug-resistant microorganisms were obtained from Kunming Medical College. Bacteria were first grown in LB (Luria-Bertani) broth to an OD600 nm of 0.8. A 10 μL aliquot of the bacteria was then taken and added to 8 mL of fresh LB broth with 0.7% agar and poured over a 90 mm Petri dish containing 25 mL of 1.5% agar in LB broth. After the top agar hardened, a 20 μL aliquot of the test sample filtered on a 0.22 μm Millipore filter was dropped onto the surface of the top agar and completely dried before being incubated overnight at 37°C. If an examined sample contained antimicrobial activity, a clear zone formed on the surface of the top agar representing inhibition of bacterial growth. The MIC was determined in liquid LB medium as in a previous report.7 The MIC at which no visible growth occurred was recorded. The peptides were quantified by UV absorbance at 215 and 225 nm using the formula: concentration (mg/mL) = (A215 nm − A225 nm) × 0.144.
Anti-H. pylori testing
Anti-H. pylori testing was performed using the methods described by Chen et al.34 H. pylori NCTC11637 was obtained from Third Military Medical University and used for in vitro experiments. It was cultured at 37°C in an incubator in a microaerobic atmosphere consisting of 5% O2, 10% CO2, and 85% N2, either in special plates for 48 h or special broth shaken for 24 h. Briefly, special broth was prepared containing 85% volume Brucella broth,10% volume newborn calf serum, 5% volume 100 g/L glucose. Special plates were prepared with 1.5% weight agar powder in Brucella broth, and 10% volume newborn calf serum, 10% volume 100 g/L glucose, 1% volume antibiotics mixture were added before pouring into the plates.35,36
Hemolysis assays
Hemolysis assays were undertaken using rabbit red blood cells in liquid medium as reported.37 Serial dilutions of the peptide were used, and after incubation at 37°C for 30 min, the cells were centrifuged and the absorbance in the supernatant was measured at 595 nm. Maximum hemolysis was determined by adding 1% Triton X-100 to a sample of cells.
References
- 1.Barra D, Simmaco M. Amphibian ska promising resource for antimicrobial peptides. Trends Biotechnol. 1995;13:205–209. doi: 10.1016/S0167-7799(00)88947-7. [DOI] [PubMed] [Google Scholar]
- 2.Simmaco M, Mignogna G, Barra D. Antimicrobial peptides from amphibian skwhat do they tell us? Biopolymers. 1999;47:435–450. doi: 10.1002/(SICI)1097-0282(1998)47:6<435::AID-BIP3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Zasloff M. A class of antimicrobial peptides from Xenopus skisolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992;4:3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Li J, Han Y, Yang H, Liang J, Lu Q, Lai R. Two antimicrobial peptides from skin secretions of Rana grahami. Toxicon. 2006;47:459–464. doi: 10.1016/j.toxicon.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Li J, Lu Q, Yang H, Zhang Y, Lai R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon. 2006;47:249–253. doi: 10.1016/j.toxicon.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Xu X, Xu C, Zhou W, Zhang K, Yu H, Zhang Y, Zheng Y, Rees HH, Lai R, Yang D, Wu J. Anti-infection peptidomics of amphibian skin. Mol Cell Proteomics. 2007;6:882–894. doi: 10.1074/mcp.M600334-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action, Biochimica. Biophys Acta. 1999;462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 9.Bior AD, Essenberg RC, Sauer JR. Comparison of differentially expressed genes in the salivary glands of male ticks, Amblyomma americanum and Dermacentor andersoni. Insect Biochem Mol Biol. 2002;32:645–655. doi: 10.1016/s0965-1748(01)00143-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu ZG, Ye BH, Zhu QX. Histological changes in the midgut epithelium of Ixodes sinensis after biting naive and immunized New Zealand white rabbits. Acta Entomologica Sinica. 2004;47:534–537. [Google Scholar]
- 11.Liu J, Liu Z, Zhang Y, Yang X, Gao Z. Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol. 2005;36:131–138. doi: 10.1007/s10493-005-1271-1. [DOI] [PubMed] [Google Scholar]
- 12.Johns R, Ohnishi J, Broadwater A, Sonenshine DE, De Silva AM, Hynes WL. Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae) J Med Entomol. 2001;38:99–107. doi: 10.1603/0022-2585-38.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Johns R, Sonenshine DE, Hynes WL. Control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae): evidence for the existence of antimicrobial proteins in tick haemolymph. J Med Entomol. 1998;35:458–464. doi: 10.1093/jmedent/35.4.458. [DOI] [PubMed] [Google Scholar]
- 14.Johns R, Sonenshine DE, Hynes WL. Response of the tick Dermacentor variabilis (Acari: Ixodidae) to hemocoelic inoculation of Borrelia burgdorferi (Spirochetales) J Med Entomol. 2000;37:265–270. doi: 10.1603/0022-2585-37.2.265. [DOI] [PubMed] [Google Scholar]
- 15.Motoyashiki T, Tu AT, Azimov DA, Ibragim K. Isolation of anticoagulant from the venom of tick, Boophilus calcaratus, from Uzbekistan. Thromb Res. 2003;110:235–241. doi: 10.1016/s0049-3848(03)00409-2. [DOI] [PubMed] [Google Scholar]
- 16.Podboronov VM. Antibacterial protective mechanisms of ixodid ticks. In: Dusbabek F, Bukva V, editors. Modern acarology. Hague: SPB Academic Pub; 1991. pp. 375–380. [Google Scholar]
- 17.Rego RO, Kovar V, Kopacek P, Weise C, Man P, Sauman I, Grubhoffer L. The tick plasma lectin, Dorin M, is a fibrinogen-related molecule. Insect Biochem Mol Biol. 2006;36:291–299. doi: 10.1016/j.ibmb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Lai R, Lomas LO, Jonczy J, Turner PC, Rees HH. Two novel non-cationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. Biochem J. 2004;379:681–685. doi: 10.1042/BJ20031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai R, Takeuchi H, Lomas LO, Turner PC, Jonczy J, Rees HH. A new type of antimicrobial protein with multiple histidines from the hard tick, Amblyomma hebraeum. FASEB J. 2004;18:1447–1449. doi: 10.1096/fj.03-1154fje. [DOI] [PubMed] [Google Scholar]
- 20.Rudenko N, Golovchenko M, Grubhoffer L. Gene organization of a novel defensin of Ixodes ricinus: first annotation of an intron/exon structure in a hard tick defensin gene and first evidence of the occurrence of two isoforms of one member of the arthropod defensin family. Insect Mol Biol. 2007;16:501–507. doi: 10.1111/j.1365-2583.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Liao M, Ueda M, Gong H, Xuan X, Fujisaki K. Sequence characterization and expression patterns of two defensin-like antimicrobial peptides from the tick Haemaphysalis longicornis. Peptides. 2007;28:1304–1310. doi: 10.1016/j.peptides.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Ceraul SM, Dreher-Lesnick SM, Gillespie JJ, Rahman MS, Azad AF. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect Immun. 2007;75:1973–1983. doi: 10.1128/IAI.01815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes WL, Ceraul SM, Todd SM, Seguin KC, Sonenshine DE. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med Vet Entomol. 2005;19:339–344. doi: 10.1111/j.1365-2915.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 24.Fogaça AC, Lorenzini DM, Kaku LM, Esteves E, Bulet P, Daffre S. Cysteine-rich antimicrobial peptides of the cattle tick Boophilus microplus: isolation, structural characterization and tissue expression profile. Dev Comp Immunol. 2004;28:191–200. doi: 10.1016/j.dci.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Ceraul SM, Sonenshine DE, Ratzlaff RE, Hynes WL. An arthropod defensin expressed by the hemocytes of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Insect Biochem Mol Biol. 2003;33:1099–1103. doi: 10.1016/s0965-1748(03)00122-x. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima Y, Van Der Goes Van Naters-Yasui A, Taylor D, Yamakawa M. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol Biol. 2002;11:611–618. doi: 10.1046/j.1365-2583.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji N, Battsetseg B, Boldbaatar D, Miyoshi T, Xuan X, Oliver JH, Fujisaki K. Babesial Vector Tick Defensin against Babesia sp. Parasites Infect Immun. 2007;75:3633–3640. doi: 10.1128/IAI.00256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu D, Sheng Z, Xu X, Li J, Yang H, Liu Z, Rees HH, Lai R. A novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Peptides. 2006;27:31–35. doi: 10.1016/j.peptides.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Liu H, Liu X, Wu X. Purification and cloning of a novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:557–561. doi: 10.1016/j.cbpb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman WR, Phillips JE. Ion and water balance in the ixodid tick Dermacentor andersoni: I. Routes of ion and water excretion. J Exp Biol. 1973;58:523–536. [Google Scholar]
- 32.Lomas LO, Turner PC, Rees HH. A novel neuropeptide-endocrine interaction controlling ecdysteroid production in ixodid ticks. Proc R Soc Lond B Biol Sci. 1997;1264:589–596. doi: 10.1098/rspb.1997.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Chen L, Li Y, Li J, Xu X, Lai R, Zou Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides. 2007;28:1527–1531. doi: 10.1016/j.peptides.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Dai GF, Cheng N, Dong L, Muramatsu M, Xiao SD, Wang MW, Zhu DX. Bactericidal and morphological effects of NE-2001, a novel synthetic agent directed against Helicobacter pylori. Antimicrob Agents Chemother. 2005;49:3468–3473. doi: 10.1128/AAC.49.8.3468-3473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar MR, Pike J. Bactericidal activity of antimicrobial agents against slowly growing Helicobacter pylori. Antimicrob Agents Chemother. 2005;32:185–187. doi: 10.1128/aac.36.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bignami GS. A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon. 1993;31:817–820. doi: 10.1016/0041-0101(93)90389-z. [DOI] [PubMed] [Google Scholar]