Abstract
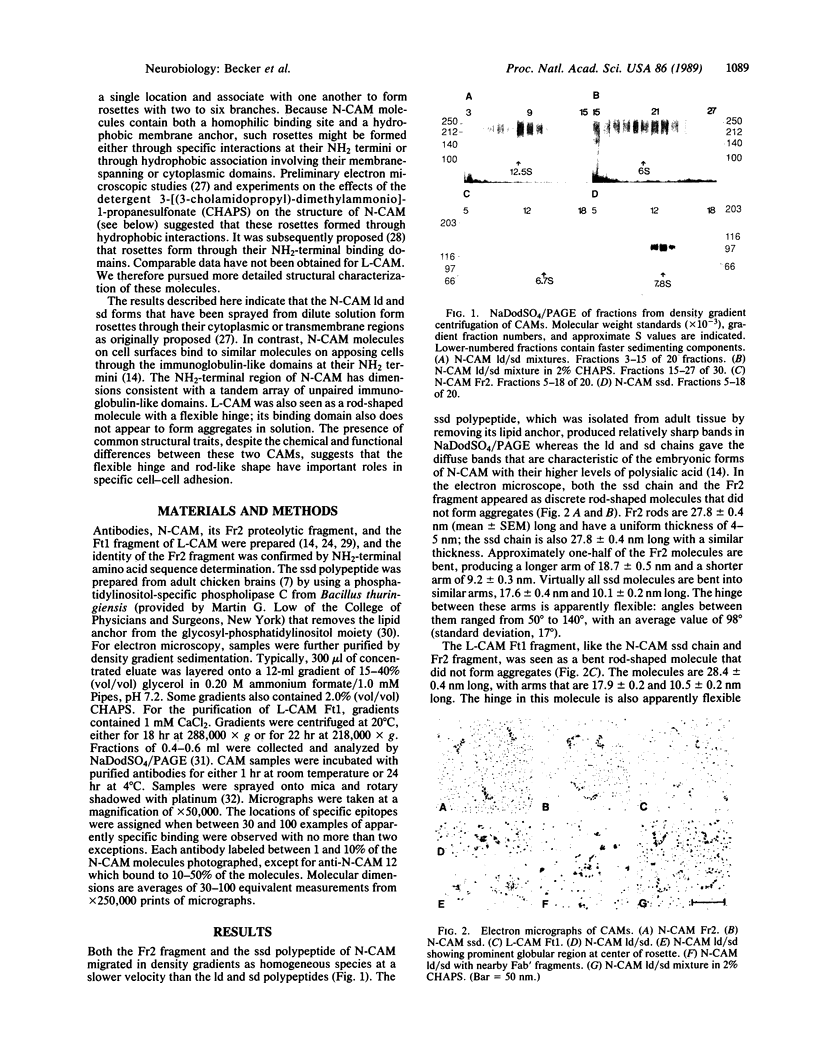
The neural cell adhesion molecule (N-CAM) exists in two major forms [ld (large cytoplasmic domain) peptide and sd (small cytoplasmic domain) peptide] that contain transmembrane segments and different cytoplasmic domains and in a third form [ssd (small surface domain) peptide] that lacks transmembrane and cytoplasmic regions. All forms have the same extracellular region of more than 600 amino acid residues, a region also found in a fragment (Fr2) that can be released from cells by proteolysis. The liver cell adhesion molecule (L-CAM) is expressed as a single species that is distinct from N-CAM, but its extracellular region can also be obtained as a proteolytic fragment (Ft1). Examination of the various forms of N-CAM and the Ft1 fragment of L-CAM by electron microscopy of rotary shadowed molecules indicated that they all have rod-shaped structures that contain a hinge region which is apparently flexible. Both the ssd chain and the Fr2 fragment of N-CAM are single rods bent into arms approximately 18 and 10 nm long. The ld and sd chains are longer bent rods that form rosettes comprising two to six branches; detergent treatment disrupts these rosettes into single rods. Specific antibodies that block homophilic N-CAM binding labeled the distal ends of the branches of the ld/sd rosettes and the ends of the longer arm of both the ssd chain and the Fr2 fragment. Antibodies that bind to the sialic acid-rich region of N-CAM bound near the hinge. These data indicate that the N-CAM rosettes are formed by interaction between their transmembrane or cytoplasmic domains and not by interactions involving their homophilic binding sites. The L-CAM Ft1 fragment is also a bent rod with an apparently flexible hinge; like the ssd chain and the Fr2 fragment of N-CAM, it does not form aggregates. The similarities between L-CAM and N-CAM, despite their differences in amino acid sequence, suggest that their general configuration and the presence of a flexible hinge are important elements in assuring effective and specific cell-cell adhesion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Arquint M., Roder J., Chia L. S., Down J., Wilkinson D., Bayley H., Braun P., Dunn R. Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987 Jan;84(2):600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4225–4229. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Carrell N. A., Fitzgerald L. A., Steiner B., Erickson H. P., Phillips D. R. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985 Feb 10;260(3):1743–1749. [PubMed] [Google Scholar]
- Chuong C. M., Edelman G. M. Alterations in neural cell adhesion molecules during development of different regions of the nervous system. J Neurosci. 1984 Sep;4(9):2354–2368. doi: 10.1523/JNEUROSCI.04-09-02354.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Edelman G. M., Cunningham B. A. Mapping of three carbohydrate attachment sites in embryonic and adult forms of the neural cell adhesion molecule. J Cell Biol. 1984 Nov;99(5):1848–1855. doi: 10.1083/jcb.99.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hoffman S., Rutishauser U., Hemperly J. J., Edelman G. M. Molecular topography of the neural cell adhesion molecule N-CAM: surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci U S A. 1983 May;80(10):3116–3120. doi: 10.1073/pnas.80.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Leutzinger Y., Gallin W. J., Sorkin B. C., Edelman G. M. Linear organization of the liver cell adhesion molecule L-CAM. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5787–5791. doi: 10.1073/pnas.81.18.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]
- Dickson G., Gower H. J., Barton C. H., Prentice H. M., Elsom V. L., Moore S. E., Cox R. D., Quinn C., Putt W., Walsh F. S. Human muscle neural cell adhesion molecule (N-CAM): identification of a muscle-specific sequence in the extracellular domain. Cell. 1987 Sep 25;50(7):1119–1130. doi: 10.1016/0092-8674(87)90178-4. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gallin W. J., Delouvée A., Cunningham B. A., Thiery J. P. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Hoffman S., Chuong C. M., Thiery J. P., Brackenbury R., Gallin W. J., Grumet M., Greenberg M. E., Hemperly J. J., Cohen C. Structure and modulation of neural cell adhesion molecules in early and late embryogenesis. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):515–526. doi: 10.1101/sqb.1983.048.01.056. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Finne J., Finne U., Deagostini-Bazin H., Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983 Apr 29;112(2):482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. K., Rutishauser U. Visualization of neural cell adhesion molecule by electron microscopy. J Cell Biol. 1987 Jun;104(6):1579–1586. doi: 10.1083/jcb.104.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. T., Finne J., Goridis C. Biosynthesis, membrane association, and release of N-CAM-120, a phosphatidylinositol-linked form of the neural cell adhesion molecule. J Cell Biol. 1987 Dec;105(6 Pt 1):2489–2500. doi: 10.1083/jcb.105.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Edelman G. M., Cunningham B. A. cDNA clones of the neural cell adhesion molecule (N-CAM) lacking a membrane-spanning region consistent with evidence for membrane attachment via a phosphatidylinositol intermediate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9822–9826. doi: 10.1073/pnas.83.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Murray B. A., Edelman G. M., Cunningham B. A. Sequence of a cDNA clone encoding the polysialic acid-rich and cytoplasmic domains of the neural cell adhesion molecule N-CAM. Proc Natl Acad Sci U S A. 1986 May;83(9):3037–3041. doi: 10.1073/pnas.83.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Nybroe O., Albrechtsen M., Dahlin J., Linnemann D., Lyles J. M., Møller C. J., Bock E. Biosynthesis of the neural cell adhesion molecule: characterization of polypeptide C. J Cell Biol. 1985 Dec;101(6):2310–2315. doi: 10.1083/jcb.101.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Edelman G. M., Cunningham B. A. Organization of the neural cell adhesion molecule (N-CAM) gene: alternative exon usage as the basis for different membrane-associated domains. Proc Natl Acad Sci U S A. 1987 Jan;84(1):294–298. doi: 10.1073/pnas.84.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger E. A., Hoffman S., Edelman G. M., Cunningham B. A. Four exons encode a 93-base-pair insert in three neural cell adhesion molecule mRNAs specific for chicken heart and skeletal muscle. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9616–9620. doi: 10.1073/pnas.85.24.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Brackenbury R., Cunningham B. A., Edelman G. M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982 Sep 25;257(18):11064–11069. [PubMed] [Google Scholar]
- Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Tissue- and developmental stage-specific forms of a neural cell surface antigen linked to differences in glycosylation of a common polypeptide. EMBO J. 1982;1(10):1239–1244. doi: 10.1002/j.1460-2075.1982.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul R., Hirn M., Deagostini-Bazin H., Rougon G., Goridis C. Adult and embryonic mouse neural cell adhesion molecules have different binding properties. 1983 Jul 28-Aug 3Nature. 304(5924):347–349. doi: 10.1038/304347a0. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Small S. J., Shull G. E., Santoni M. J., Akeson R. Identification of a cDNA clone that contains the complete coding sequence for a 140-kD rat NCAM polypeptide. J Cell Biol. 1987 Nov;105(5):2335–2345. doi: 10.1083/jcb.105.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin B. C., Hemperly J. J., Edelman G. M., Cunningham B. A. Structure of the gene for the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7617–7621. doi: 10.1073/pnas.85.20.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Wagner S. L., Dean W. L., Gray R. D. Effect of a zwitterionic detergent on the state of aggregation and catalytic activity of cytochrome P-450LM2 and NADPH-cytochrome P-450 reductase. J Biol Chem. 1984 Feb 25;259(4):2390–2395. [PubMed] [Google Scholar]
- Wheelock M. J., Buck C. A., Bechtol K. B., Damsky C. H. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987 Jul;34(3):187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]