Abstract
Methods to prepare pure, bioactive recombinant human vascular endothelial growth inhibitor (rhVEGI), a potent inhibitor of angiogenesis potentially applicable in antiangiogenic cancer therapy, are in urgent demand for preclinical investigation as well as future clinical trials of the protein. Here, we report expression and purification of rhVEGI-192, a recombinant VEGI isoform, comparatively using host strains BL21 (DE3) pLysS and Origami B (DE3) with IPTG-induction and autoinduction techniques. Our study identified that a combined use of Origami B (DE3) strain and autoinduction expression system gave rise to a high yield of purified rhVEGI-192 at 105.38 mg/L culture by immobilized-metal affinity chromatography on Ni-NTA column. The antiangiogenic activity was effectively restored after the insoluble fractions being dissolved in 8M urea and subsequently subjected to a gradient-dialysis refolding process. Functional tests demonstrated that the purified rhVEGI-192 potently inhibited endothelial growth, induced endothelial apoptosis and suppressed neovascularization in chicken chorioallantoic membrane, indicating that the developed method allows preparation of rhVEGI-192 with high yield, solubility, and bioactivity. Most importantly, our study also demonstrates that VEGI-192 is capable of forming polymeric structure, which is possibly required for its antiangiogenic activity.
Keywords: VEGI, autoinduction, oligomerization, protein refolding, angiogenesis
Introduction
Angiogenesis, which involves outgrowth of new blood vessels from existing vasculature, is a normal event in certain biological settings such as embryonic development and wound healing, but a pathological process mechanistically underlying many diseases, including particularly cancer.1 It is recognized that tumor development and progression largely depends on neovascularization, through which nutrients and oxygen are supplied and tumor cells disseminate from their primary sites to distal organs.2 Physiological angiogenesis is controlled by a balance between endogenous pro- and antiangiogenic factors. Pathological angiogenesis, on the other hand, is poorly regulated, often with an increase of proangiogenic factors and decrease of antiangiogenic factors. Use of endogenous antiangiogenic factors has been a promising approach to cancer therapy, including a number of antiangiogenic agents that are under clinical studies.1
Vascular endothelial growth inhibitor (VEGI), a cytokine member of the tumor necrosis factor superfamily-15 (TNFSF-15) expressed largely in endothelial cells, has been identified as a specific endogenous inhibitor of angiogenesis.3 Its three splicing isoforms, namely, VEGI-174, VEGI-251, and VEGI-192, have been found to display various degrees of antiangiogenic activities when tested in their natural or recombinant protein forms.4–8 Among them, the VEGI-192 isoform consists of 192 amino acids and was identified recently.7 In vitro experiments showed that VEGI-192 was 20-fold more potent than endostatin in inhibiting the proliferation of adult bovine aortic endothelial cell and formation of capillary-liked structures.7–9 In vivo study demonstrated a robust inhibition of tumor angiogenesis and growth when systemically administering recombinant human VEGI-192 to tumor-bearing mice, and displayed no detectable toxicity to the liver, kidney and EC in the normal, quiescent vasculature.8 These observations have warranted further investigation of the potential of employing VEGI-192 as a promising antiangiogenic and anticancer agent.
On the other hand, however, the functional and structural biology of VEGI, such as the receptor-binding property and the crystal structure of VEGI-192, has not been clearly defined. Moreover, the toxicological profile and pharmacokinetics of the agent, which are keys to moving VEGI into the core stages of preclinical investigation, are to be determined. All these tasks would depend on the availability of sufficient amount of purified recombinant VEGI, and previous attempts to develop VEGI as an anticancer therapeutic agent have been hindered by the difficulty of producing adequate quantities of VEGI protein with high level of bioactivity despite efforts in developing various expression and purification systems.4,5,8 Such a difficulty largely lies in the fact that the VEGI protein exhibits a strong tendency to aggregate and precipitate, which speculatively results from three possible causes: (1) disulfide bond formation may be strictly required for functional expression; (2) the isoelectric point of VEGI-192 protein (∼ 7.8) is close to the physiological pH value; and (3) VEGI-192 might be a type II transmembrane protein as deduced from its primary structure,3 and overexpression of membrane proteins of eukaryotic origin can be toxic to Escherichia coli. To deal with these difficulties, here we report an efficient autoinduction expression system to express rhVEGI-192 in E. coli, with which production of large quantities of soluble recombinant VEGI-192 protein was achieved. Using the soluble rhVEGI-192 product, we further investigated the relationship between structure and function of VEGI-192.
Results
Optimization of rhVEGI-192 expression systems
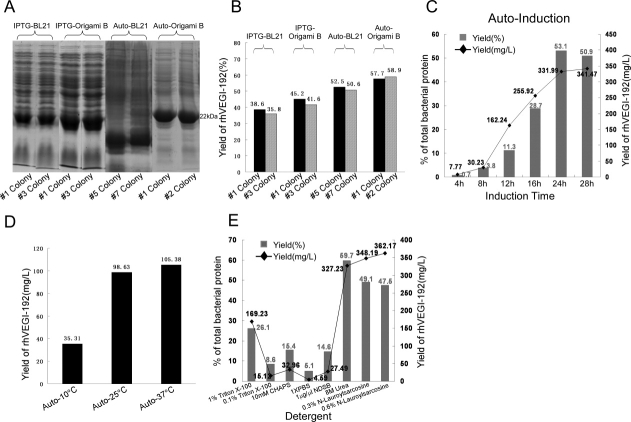
To develop an appropriate system for the expression of rhVEGI-192 protein, we examined IPTG- and autoinduction expression systems, using E. coli strain BL21 (DE3) pLysS and Origami B (DE3) in parallel as the host bacteria. Screening the BL21 (DE3) pLysS and Origami B (DE3) colonies led to two bacterial clones with high-level rhVEGI-192 [Fig. 1(A)]. Abundance of the rhVEGI-192 protein was determined by densitometric scanning of SDS-PAGE gels [Fig. 1(B)], The highest percentage (58.9%) of rhVEGI-192 in total soluble bacterial protein was achieved with the autoinduction system using Origami B (DE3), which also exhibited minimal basal or leaking expression of the recombinant protein.
Figure 1.
Optimization of rhVEGI-192 expression systems. A: Rapid screening of small-scale expression of rhVEGI-192 using BL21 (DE3) pLysS and Origami B (DE3) with IPTG-induction system (IPTG-BL21 and IPTG-Origami B), and autoinduction system (auto-BL21 and auto-Origami B) conducted at 25°C. Two colonies expressing the highest level of rhVEGI-192 were chosen from each induction system-host strain combination and are shown here. B: Quantification of rhVEGI-192 expression level by the chosen colonies was determined by densitometric scanning of SDS-PAGE gels. C: Time course analysis of expression using the autoinduction system and host strain Origami B (DE3). Induction conducted at 25°C for 4, 8, 12, 16, 24, and 28 h. D: Yield of purified rhVEGI-192 protein by auto induction at 10°C (auto-10°C), 25°C (auto-25°C), 37°C (auto-37°C), respectively, using host strain Origami B (DE3). The yield of purified rhVEGI-192 was quantified using BCA assay. E: Effects of detergents on solubilization. For panels (B, C, E), yield of rhVEGI-192 (%) was calculated as the amount of rhVEGI-192 as percentage of the total soluble bacterial protein using band intensity values, and yield of rhVEGI-192 (mg/L) was calculated as milligrams of rhVEGI-192 protein, as quantitated using the intensity of 2 μg BSA band as standard, obtained from 1 L of bacterial culture.
We next studied the conditions for the expression of rhVEGI-192 protein with the IPTG-induction system using host strain Origami B (DE3). It was previously reported that a high yield of rhVEGI-192 could be generated in an IPTG-induction system when induced with 0.5 mM IPTG for 3 h.8 In this study, expression of rhVEGI-192 was experimentally induced with various IPTG concentrations (0, 0.1, 0.4, 0.7, and 1 mM) and induction time lengths (1, 2, 3, and 4 h). We found that when induced with 1 mM IPTG for 4 h, the expression of rhVEGI-192 achieved a level of accounting for 34.1% of the total bacterial protein. Longer induction or higher IPTG concentration did not significantly increase the expression of rhVEGI-192 further.
We then focused on exploring appropriate induction conditions for rhVEGI-192 expression in the autoinduction system using host strain Origami B (DE3). Through time-course analysis, it was found that when induced at 25°C, the highest level of rhVEGI-192 was produced if the induction time was set to 24 h, as assessed by SDS-PAGE and scanning densitometer, while longer induction did not further increase the percentage of rhVEGI-192 protein in total bacterial protein and offered little improvement in the total yield [Fig. 1(C)]. To identify an appropriate temperature for the autoinduction, the expression of rhVEGI-192 was examined at 10, 25, and 37°C, respectively. While the yield of rhVEGI-192 when autoinduced at 37°C (105.38 mg/L culture) was marginally higher than that at 25°C (98.63 mg/L culture) [Fig. 1(D)], a later activity tests revealed that autoinduction at 25°C led to a significantly higher bioactivity of rhVEGI-192 [Fig. 3(A)]. Thus, autoinduction at 25°C for 24 h appeared to be an optimal condition for the expression of rhVEGI-192.
Figure 3.
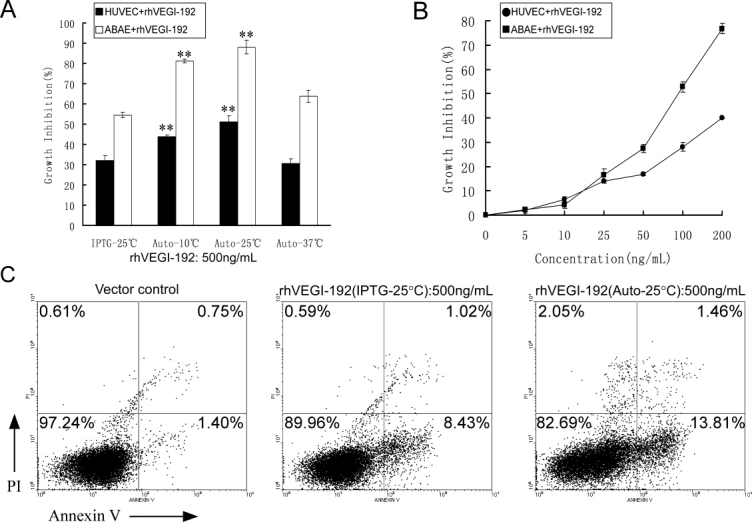
Angiogenesis inhibition by purified rhVEGI-192 protein in vitro. A: Effect of rhVEGI-192 on the growth of HUVEC (transparent columns) and ABAE (black columns). B: Dose-response curve of rhVEGI-192 (auto-25°C) on the growth of HUVEC and ABAE after treatment for 48 h. Student's two-tailed t test was performed, and statistical differences are shown with asterisks (**P < 0.01). C: Annexin V-FITC/PI staining of HUVEC cells treated with rhVEGI-192. Cells were exposed to vector control (left panel), rhVEGI-192 derived from IPTG- (middle panel) or autoinduction (right panel) for 8 h. Cells collected were subjected to Annexin V-FITC/PI double staining and analyzed using flow cytometry.
To identify an appropriate detergent that provides the best solubility for rhVEGI-192, we tested six types of detergents, namely, Triton X-100 (1 or 0.1%), CHAPS (10 mM), 1× PBS, NDSB (1 μg/μL), urea (8M), and N-lauroylsarcosine (0.3 or 0.6%). As shown in Figure 1(E), 8M urea was demonstrated to yield the highest percentage level of soluble rhVEGI-192 in the host bacteria. It is of note that while lauroylsarcosine sodium salt appeared to dissolve as much inclusion bodies containing rhVEGI-192 as 8M urea, it also dissolved a significant amount of heterogeneous proteins, adding unnecessary burden to the downstream purification procedures.
Yield and solubility of purified rhVEGI-192 protein
Purification products of rhVEGI-192 were examined using reducing and nonreducing SDS–PAGE, as well as Western blotting analysis. A predominant protein of 22 kDa, corresponding to the expected molecular weight of rhVEGI-192 was detected as a single band by reducing SDS-PAGE [Fig. 2(A)], suggesting that the purification protocol achieved a high degree of purity for the target protein. Furthermore, Western blotting analysis of nonreducing SDS-PAGE [Fig. 2(B)] revealed protein bands that indicated partial oligomerization of VEGI-192 when autoinduced at 10 and 25°C in strain Origami B (DE3). The potential oligomer band was far weaker in the preparation IPTG-induced in strain Origami B (DE3). Moreover, reduction of purified protein with 30 mM DTT resulted in complete conversion to a monomer band [Fig. 2(C)], suggesting that the expressed rhVEGI-192 was capable of being oligomerized through disulfide bonding.
Figure 2.
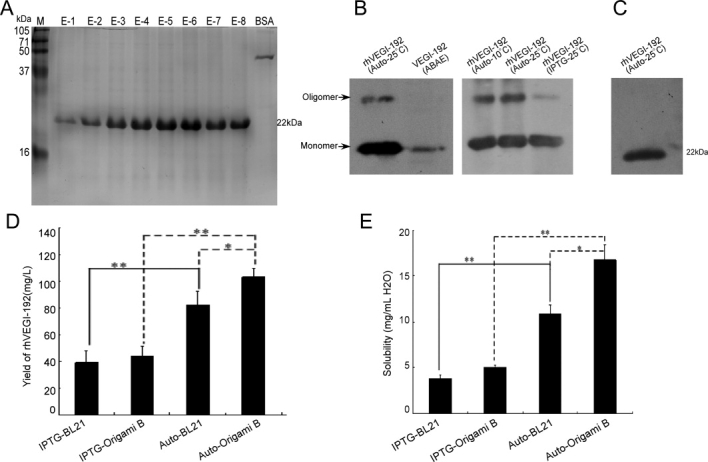
SDS-PAGE and Western blotting analysis of the purified rhVEGI-192 protein. (A) Reducing SDS-PAGE analysis of purified fractions. Lanes E1–8, elution fractions from Ni-NTA column; Lane M, protein molecular weight marker; Lane BSA: 2 μg BSA loaded. (B, C) Western blotting analysis of the purified rhVEGI-192 by nonreducing (B) and reducing (C) SDS-PAGE (2 μg rhVEGI-192 protein was loaded). Endogenous VEGI of ABAE cell was included in panel B for comparison. (D, E) Yield (D) and solubility (E) of purified rhVEGI-192 protein. Results in (D) and (E) are presented as means ± SD. Statistical analysis (Student's two-tailed t test) revealed the differences in yields (D) and solubilities (E) among IPTG-induction and autoinduction at 25°C using BL21 (DE3) pLysS or Origami B (DE3) as host bacteria (*P < 0.05 and **P < 0.01).
The yield of purified rhVEGI-192 was quantified using BCA assay [Fig. 2(D)]. The highest yield was observed with the Origami B (DE3) strain by autoinduction (103.2 mg/L) at 25°C, and a relatively high level of expression was also shown in BL21 (DE) pLysS with autoinduction (82.4 mg/L). Expression in Origami B (DE3) and BL21 (DE3) pLysS induced with IPTG seemed to result in suboptimal outcome as the two host strains yielded 43.8 mg/L and 39.1 mg/L, respectively. It is noteworthy that the endotoxin concentration in each rhVEGI-192 preparation was less than 5 EU/mg (data not shown), and that the control sample prepared from E. coli cells that contained only the vector plasmid without the VEGI-192 expression cassette did not exhibit any antiangiogenic activity, suggesting that the observed antiangiogenic activities in following experiments were not relevant to bacterial LPS.
Our results also demonstrated that the rhVEGI-192 product expressed and purified from autoinduced Origami B (DE3) presented the highest level of solubility (16.7 mg/mL in deionized H2O), while those from autoinduced BL21 (DE3) pLysS, IPTG-induced Origami B (DE3), and IPTG-induced BL21 (DE3) pLysS combinations gave rise to solubilities of 10.9 mg/mL, 5.1 mg/mL, and 3.8 mg/mL, respectively [Fig. 2(E)]. These data suggest that the autoinduction system using Origami B (DE3) as host strain not only generated high yields of crude as well as purified rhVEGI-192 but also provided a high degree of solubility of the recombinant protein. It may thus represent an appropriate and practically applicable expression system for a large scale preparation of rhVEGI-192 protein.
Growth inhibition of endothelial cells by rhVEGI-192 protein
The effects of rhVEGI-192 protein on the growth of HUVEC and ABAE were investigated using the MTT assay [Fig. 3(A)]. Purified rhVEGI-192 from both IPTG-induction and autoinduction (induced at 10, 25, or 37°C) expression systems demonstrated various degrees of inhibitory effects, among which the rhVEGI-192 protein derived from the 25°C autoinduced expression system exhibited the highest inhibitory activity on HUVEC and ABAE growth. This preparation of rhVEGI-192, as shown in Figure 3(B), inhibited the growth of cultured HUVEC and ABAE in a dose-dependent manner, with an IC50 of 0.6 μg/mL and 0.1 μg/mL, respectively, for HUVEC and ABAE.
rhVEGI-192 induced apoptosis in HUVEC
To further characterize the inhibitory effect of rhVEGI-192 on the growth of endothelial cells, annexin-V-FITC staining was performed to examine whether rhVEGI-192 triggered endothelial apoptosis. Following treatment with 500 ng/mL of rhVEGI-192 prepared from either the IPTG-induction or the autoinduction expression system on HUVEC cells for 8 h, 8.43 and 13.81% of annexin V+/PI- HUVEC, respectively, were identified, suggesting that the rhVEGI-192 preparation was able to induce endothelial apoptosis, and that rhVEGI-192 with a higher degree of oligomer conformation, which was derived from the autoinduction system, triggered a stronger apoptosis [Fig. 3(C)].
Inhibitory effect of rhVEGI-192 on new blood vessel formation
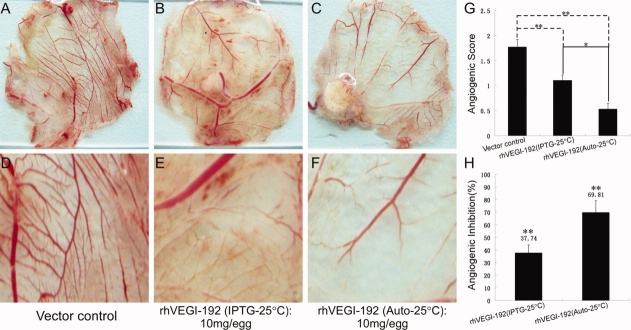
We further investigated whether rhVEGI-192 could effectively suppress formation of neovasculature by using the CAM model. Preparations of rhVEGI-192 protein from IPTG-induction or autoinduction were applied in restricted areas of the chorioallantoic membranes (CAMs) of fertilized eggs, and the angiogenic scores were determined. Treatment with autoinduced and IPTG-induced rhVEGI-192 led to a marked reduction of vessel density [Fig. 4(A–F)]. Quantitative analysis of the vessel density indicated that treatment with the two different preparations at a dosage of 10 mg protein per egg led to 69.81% and 37.74% reduction of vessel density, respectively [Fig. 4(G,H)]. These data demonstrate that the rhVEGI-192 preparations were able to effectively suppress neovascularization in the CAM model, and that rhVEGI-192 with a higher degree of oligomer conformation derived from the autoinduction system, induced a stronger brush-like capillary inhibition.
Figure 4.
Antiangiogenic activity of rhVEGI-192 in chick CAM assay. Direct camera images (A–C) and microscopic images (5×) (D–F) of representative CAMs treated with indicated conditions. (A, D) Vehicle-controlled, n = 6; (B, E) rhVEGI-192 derived from the IPTG induction system, n = 6; (C, F) rhVEGI-192 derived from the autoinduction system, n = 6. (G) Angiogenic score was determined by scoring from 0 to 3 the appearance of the brush-like capillary formation by two independent observers. (H) Inhibition of blood vessel formation within chorioallantoic membrane (CAM) by rhVEGI-192. Data for the quantitative assessment of angiogenesis inhibition were expressed as means ± SD. Statistical analysis (student's two-tailed t test) was performed to show significant differences (*P < 0.05; **P < 0.01). [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
Discussion
As the use of angiogenesis inhibitors is becoming an appealing approach to interrupting new vessel formation, and ultimately to arresting tumor growth, identification of VEGI, a novel endogenous cytokine that inhibits tumor angiogenesis and growth and yet remains relatively nontoxic to the host, has provided a basis for developing new and promising antiangiogenic strategies for the treatment of cancer and other diseases mechanistically attributable to pathological angiogenesis. Preclinical investigations and mechanistic studies on VEGI, which are required for a successful translation of this antiangiogenic drug candidate into clinical application, however, has been impeded by difficulties in obtaining biologically active VEGI protein of high purity, large quantity, and low cost. Aiming at removing this impediment, we carried out this study to explore appropriate conditions to express and purify recombinant VEGI with high-antiangiogenic activity. In this work, we developed a system for efficient expression and purification of intact and biologically active human VEGI-192 protein. Our results indicated that a decent level of production could be achieved by using the autoinduction pET system, giving rise to as high as 105.38 mg/1 L culture pure rhVEGI-192 protein. Interestingly, our data suggest that when used to express rhVEGI-192, the autoinduction system appears to be advantageous over the IPTG-induction system, with the former generating nearly threefold more soluble rhVEGI-192 than the latter system. Furthermore, an efficient and effective purification was achieved by lysing the host bacterial cells and their rhVEGI-192 containing inclusion bodies using high concentration of urea, followed by Ni-NTA affinity chromatography. Our results also demonstrated that a well-designed refolding protocol composed of serial gradient dialysis cascades could efficiently restore the solubility and bioactivity of the rhVEGI-192 protein. To our knowledge, this is the first report on scaled expression and purification of intact and bioactive rhVEGI-192.
In this study, the autoinduction system provides an expression platform that facilitates the improvement of both yield and solubility of the end product protein. In our optimized protocol, the autoinduction media contains three carbon sources, namely, glucose, glycerol, and lactose. Host bacteria Origami B (DE3) possess active lactose permease (lac Y) to import lactose, and β-galactosidase (lac Z) to convert lactose to allolactose, the inducer of expression. In the initial phase, glucose in the medium prevents uptake of lactose, and thereby the expression of target protein is suppressed during the growth and multiplication of the host bacterial cells.10 On exhaustion of glucose, lactose-dependent induction begins and persists till high-culture densities (OD600 ≥ 20) are reached, producing more target protein per volume of culture than the IPTG induction (usually OD600 ≈ 6 at induction). Such a course might provide sufficient time for the target protein to express and to refold correctly, therefore be of great utility for membrane protein production. Moreover, the autoinduction system offers a tight expression control, which prevents leaking basal expression of the target protein, and saves the needs to monitor cell density and to add exogenous inducers.
To choose an appropriate host E. coli strain that could efficiently express rhVEGI-192, we have comparatively studied the expression efficiencies derived from the BL21 (DE3) pLysS strain and the Origami B (DE3) strain. These strains, being deficient in both ATP-dependent proteinase Lon and outer membrane proteinase OmpT, were expected to prevent degradation of heterologous proteins expressed in these strains and thus increase protein stability.11 BL21 (DE3) pLysS is a high-stringency expression host, and DE3 lysogens contain a derivative of phage lambda that supplies T7 RNA polymerase by transcription from the lacUV5 promoter that provides a control to basal expression. On the other hand, Origami B (DE3) is a general expression host derived from a lacZY mutant of BL21, containing tuner lac permease mutation that enables more precise control of the induction step and eliminates contamination of β-galactosidase. It also carries mutations in both the thioredoxin reductase (trxB) and glutathione reductase (gor) genes, thus greatly enhances the formation of disulfide bonds in proteins within the E. coli cytoplasm. Using a combination of the Origami B (DE3) host strain and the autoinduction expression strategy, we are able to obtain highly bioactive rhVEGI-192 protein at a robust expression level. Furthermore, such an expression strategy also has resulted in a high degree of solubility (16.7 mg/mL deionized H2O), as compared with those purified from the autoinduced BL21 (DE3) pLysS system (10.9 mg/mL), from the IPTG-induced Origami B (DE3) system (5.1 mg/mL) and from the IPTG-induced BL21 (DE3) pLysS system (3.8 mg/mL).
Partitioning of a given target protein into the soluble versus insoluble fractions largely depends on the nature of the buffer used to lyse the host bacteria. It is also noteworthy that proteins containing hydrophobic or membrane associated domains tend to be partitioned into the insoluble fraction, but not necessarily in the inclusion bodies. Proteins in the insoluble fraction due to association with bacterial lipids or membranes may often be converted to the soluble fraction by adding millimolar amounts of nonionic or zwitterionic detergents to the lysis buffer.12 Thus, selection of a detergent(s) for the solubilization of a target protein remains an empirical work, and our trials with various detergents have led to the identification of urea (8M) as an effective agent that efficiently solubilizes the aggregated rhVEGI-192, which also has led to effective restoration of protein bioactivity during the subsequent refolding procedures.
It is of note that VEGI-192 contains two cysteine residues essential for the formation of disulfide bonds. It has been reported that human VEGI gene codes for a type II transmembrane protein with 20–30% sequence homology with human tumor necrosis factor α (TNF-α).3 Recently, the crystal structure of human VEGI-251 was reported by Jin et al. VEGI-251 forms a homotrimer with each monomer in the asymmetric unit, assuming a jellyroll β-sandwich fold, which resembles the trimer structure of other TNF family members.13 Since the trimeric structure of TNF-α is required for its biologic activity,14 we propose that VEGI-192 may require the formation of stable disulfide bonds to fold properly into a native and active oligomer conformation so as to function as an inhibitor of angiogenesis. As expected, nonreducing SDS-PAGE and Western blotting analysis performed in this study confirmed the ability of rhVEGI-192 to oligomerize following the refolding procedure, which also exhibited a high degree of bioactivity, suggesting a possible relationship between oligomerization and function of the protein. Further investigation on such a relationship, and its potential significance in further improving the bioactivity of rhVEGI-192, is underway in the laboratory.
Materials and Methods
Reagents, bacterial strains, and plasmids
Unless otherwise stated, laboratory chemicals, reagents, and disposable labware were purchased from Sigma-Aldrich (St. Louis, MO), Sangon Biotech (Shanghai, China), or Oxoid (Basingstoke, Hampshire, UK). rhVEGI-192 expression plasmid pET-19b-rhVEGI-192 was generated as previously described 8, using BL21 (DE3) pLysS or Origami B (DE3) (Transgen) as host E. coli strains. Media for bacteria culture included Luria-Bertani broth for the IPTG- induction system and autoinduction media for the autoinduction system.10
Expression and purification of rhVEGI-192
Briefly, expression of rhVEGI-192 was induced with IPTG at various concentrations for additional 4 h according to standard methods.10 For the autoinduction system, expression plasmid-containing E. coli cells were grown in autoinduction growth medium supplemented with 100 μg/mL carbenicillin at 25°C or other designated tested temperatures (e.g., 10, 25, and 37°C) for 24 h (OD600 ≈ 20).
To purify recombinant proteins, the cultures were collected by centrifugation at 10,000g for 10 min. And the pellets were dissolved in a lysis buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8M urea, pH 8.0) at a concentration of 10 mg (wet weight) per ml, and subsequently subjected to freeze-and-thaw cycles. Then cells were disrupted by sonication, followed by centrifugation of the lysates at 10,000g for 30 min to remove the cellular debris pellet. The supernatant containing His-tagged protein was purified with Ni-NTA resin (QIAGEN) according to the manufacturer's instructions using acidic elution condition (pH 4.0). Eluted fractions were collected and subjected to subsequent SDS-PAGE followed by staining with Coomassie brilliant blue R-250, and/or Western blotting analysis. Methods for reducing and non-reducing SDS-PAGE (12% separation gel), that is, with or without dithiothreitol (DTT) applied in the sampling buffer as the reducing agent, respectively, as well as the Western blotting assay, were performed according to a standard method.15 Intensities of the bands of interest on the SDS-PAGE gels were quantitatively measured with the Quantity One 4.6.3 measurement software (Bio-Rad), using the intensity of 2-μg BSA band as a standard.16 A goat anti-human VEGI (F-15) antibody (Santa Cruz Biotechnology) was employed at a 1:1000 dilution in Western blotting to detect VEGI-192.
Refolding and freeze-drying of rhVEGI-192 protein
The eluents collected from Ni-NTA column were diluted with deionized water to a concentration of 3 mg protein/mL. To refold rhVEGI-192, firstly the diluted eluents were dialyzed against 100× volume of buffer A (50 mM Tris-HCl, pH 6.5, 0.1 mM of DTT) containing 4M urea, followed by dialyses against 100× volume of dialysis buffer A without urea at 4°C for 3 h twice. Subsequently, further dialyses was performed twice against 100× volume of dialysis buffer B (50 mM Tris-HCl, pH 6.5) at 4°C for 3 h/time. Following the fifth dialysis, cleavage of the fusion protein with enterokinase was performed using the Tag off™ rEK Cleavage/Capture Kit (Novagen), according to the manufacturer's instructions. A sixth dialysis against buffer C (50 mM Tris-HCl, pH 7.2, 1 mmol/L of GSH, 0.2 mmol/L of GSSH) in a 50× volume was then performed at 4°C overnight, followed by a final dialysis against buffer D (Deionized water, pH 7.2, 100× volume) at 4°C for 3 h. The dialyzed product was centrifuged and collected. For long-term preservation, the final purification product was lyophilized using the Freeze Dry Systems (Labconco Corporation).
Solubility assay
The lyophilized recombinant protein was resuspended at a final concentration of 20 mg/mL in deionized water. Samples were incubated on ice for 1 h with frequent vortex and subsequently subjected to 1 h centrifugation at 15,000g at 4°C, followed by collection of the supernatants. The yield of purified rhVEGI-192 was quantified using the Bicinchoninic Acid Protein Assay Kit (Pierce Biotech) by following manufacturer's instruction. Concentration of endotoxin in the rhVEGI-192 protein preparation was measured by the Quantitative Chromogenic Tachypleus Amebocyte Lysate Endpoint assay Kit (Chinese Horseshoe Crab Reagent Manufactory) according to the instruction provided by the manufacturer.
Cell viability assay
Effects of rhVEGI-192 on the growth of human umbilical endothelial cells (HUVEC) and adult bovine aortic endothelial cells (ABAE) were determined with MTT assay by exposing the cells to various amounts of rhVEGI-192 for 48 h as previously described.6 Cell viability obtained from the vector control group, in which endothelial cells were exposed to the product obtained by lysing autoinduced host bacteria containing only the pET-19b vector, without rhVEGI-192, using a same purification procedure as described earlier, was defined as 100%. All experiments were performed three times, and the half maximal inhibitory concentration (IC50) was calculated with the Bliss's software.
Annexin V-FITC/PI staining assay
Induction of endothelial apoptosis was tested using Annexin V-FITC/PI staining assay following exposure of 500 ng/mL HUVEC to rhVEGI-192 for 8 h according to a standard protocol.17 The percentage of stained cells was measured by flow cytometric analysis.
Chicken chorioallantoic membrane assay
To determine antiangiogenic activity in vivo, CAM assays were performed at the 8th day of development of fertilized chicken eggs as previously described.18 Two independent observers scored the angiogenic response at a 0–3 scale (0 = none, 1 = medium, 2 = medium/high, and 3 = high), and angiogenic score was indicated as the mean number from the two observers. The angiogenesis property of the vehicle-treated group was defined as 100%, and the angiogenic inhibition of the tested groups were expressed as a percentage of mean angiogenic score value of the vehicle group. Statistical analysis was carried out using two-tailed student's t-test.
Statistical analysis
For recombinant protein yield assessment, solubility assay, MTT test and CAM assay, student's t-tests were performed to analyze significance of the observed differences between groups, with 0.05 as the cutoff P value. Data applied to statistical analyses were obtained from at least three independent experiments and presented as means ± standard deviation (SD).
Glossary
Abbreviations
- ABAE
adult bovine aortic endothelial cell
- CAM
chorioallantoic membrane
- HUVEC
human umbilical endothelial cells
- rhVEGI
recombinant human vascular endothelial growth inhibitor.
References
- 1.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Tan KB, Harrop J, Reddy M, Young P, Terrett J, Emery J, Moore G, Truneh A. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene. 1997;204:35–46. doi: 10.1016/s0378-1119(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhai Y, Ni J, Jiang GW, Lu J, Xing L, Lincoln C, Carter KC, Janat F, Kozak D, Xu S, Rojas L, Aggarwal BB, Ruben S, Li LY, Gentz R, Yu GL. VEGI, a novel cytokine of the tumor necrosis factor family, is an angiogenesis inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J. 1999;13:181–189. doi: 10.1096/fasebj.13.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Zhai Y, Yu J, Iruela-Arispe L, Huang WQ, Wang Z, Hayes AJ, Lu J, Jiang G, Rojas L, Lippman ME, Ni J, Yu GL, Li LY. Inhibition of angiogenesis and breast cancer xenograft tumor growth by VEGI, a novel cytokine of the TNF superfamily. Int J Cancer. 1999;82:131–136. doi: 10.1002/(sici)1097-0215(19990702)82:1<131::aid-ijc22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Tian S, Metheny-Barlow L, Chew LJ, Hayes AJ, Pan H, Yu GL, Li LY. Modulation of endothelial cell growth arrest and apoptosis by vascular endothelial growth inhibitor. Circ Res. 2001;89:1161–1167. doi: 10.1161/hh2401.101909. [DOI] [PubMed] [Google Scholar]
- 7.Chew LJ, Pan H, Yu J, Tian S, Huang WQ, Zhang JY, Pang S, Li LY. A novel secreted splice variant of vascular endothelial cell growth inhibitor. FASEB J. 2002;16:742–744. doi: 10.1096/fj.01-0757fje. [DOI] [PubMed] [Google Scholar]
- 8.Hou W, Medynski D, Wu S, Lin X, Li LY. VEGI-192, a new isoform of TNFSF15, specifically eliminates tumor vascular endothelial cells and suppresses tumor growth. Clin Cancer Res. 2005;11:5595–5602. doi: 10.1158/1078-0432.CCR-05-0384. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatan endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 10.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 12.Marshak DR, Kadonaga JT, Burgess RR, Knuth MW, Brennan WA, Lin SH. Strategies for protein purification and characterization: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 13.Jin T, Guo F, Kim S, Howard A, Zhang YZ. X-ray crystal structure of TNF ligand family member TL1A at 2.1A. Biochem Biophys Res Commun. 2007;364:1–6. doi: 10.1016/j.bbrc.2007.09.097. [DOI] [PubMed] [Google Scholar]
- 14.Tang P, Hung MC, Klostergaard J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry. 1996;35:8216–8225. doi: 10.1021/bi952182t. [DOI] [PubMed] [Google Scholar]
- 15.Stern BD, Wilson M, Jagus R. Use of nonreducing SDS-PAGE for monitoring renaturation of recombinant protein synthesis initiation factor, eIF-4 alpha. Protein Expr Purif. 1993;4:320–327. doi: 10.1006/prep.1993.1041. [DOI] [PubMed] [Google Scholar]
- 16.Schnepf HE, Lee S, Dojillo J, Burmeister P, Fencil K, Morera L, Nygaard L, Narva KE, Wolt JD. Characterization of Cry34/Cry35 binary insecticidal proteins from diverse Bacillus thuringiensis strain collections. Appl Environ Microbiol. 2005;71:1765–1774. doi: 10.1128/AEM.71.4.1765-1774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10:353–363. doi: 10.1080/14653240802035926. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg L, Shinkaruk S, Lequin O, Rousseau B, Hagedorn M, Costa F, Caronzolo D, Balke M, Canron X, Convert O, Lain G, Gionnet K, Goncalves M, Bayle M, Bello L, Chassaing G, Deleris G, Bikfalvi A. Structure and inhibitory effects on angiogenesis and tumor development of a new vascular endothelial growth inhibitor. J Biol Chem. 2003;278:35564–35573. doi: 10.1074/jbc.M304435200. [DOI] [PubMed] [Google Scholar]