Abstract
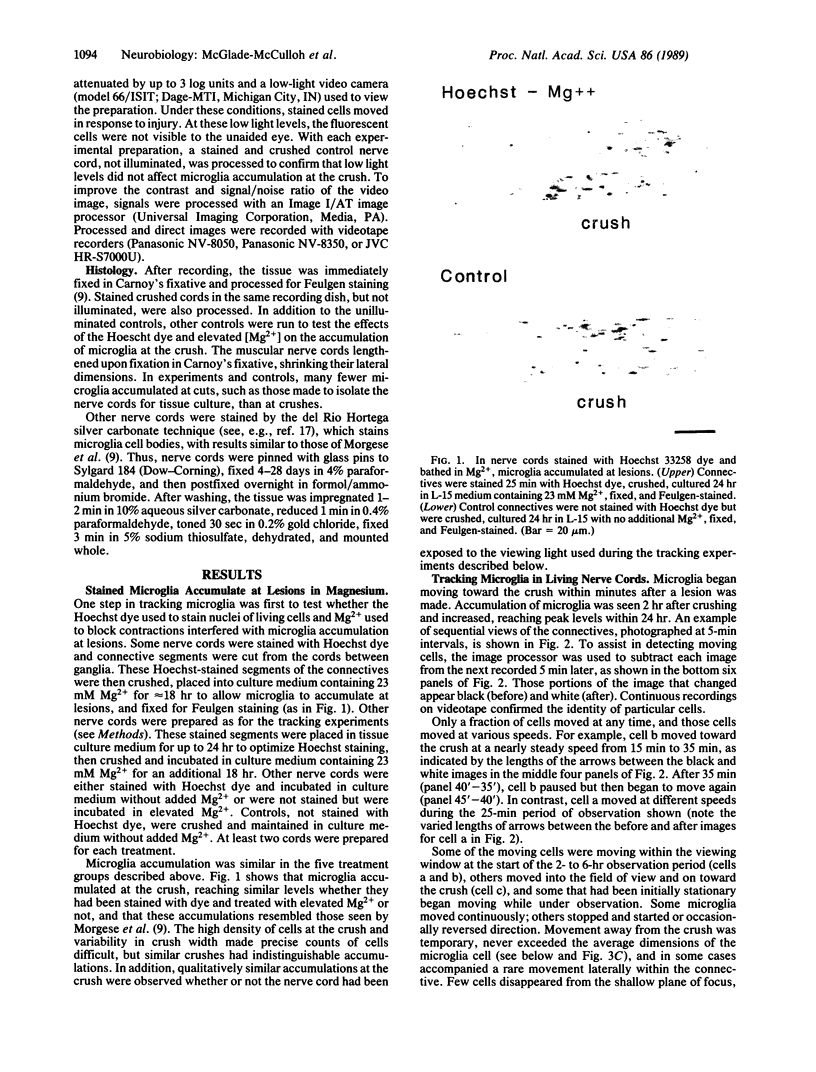
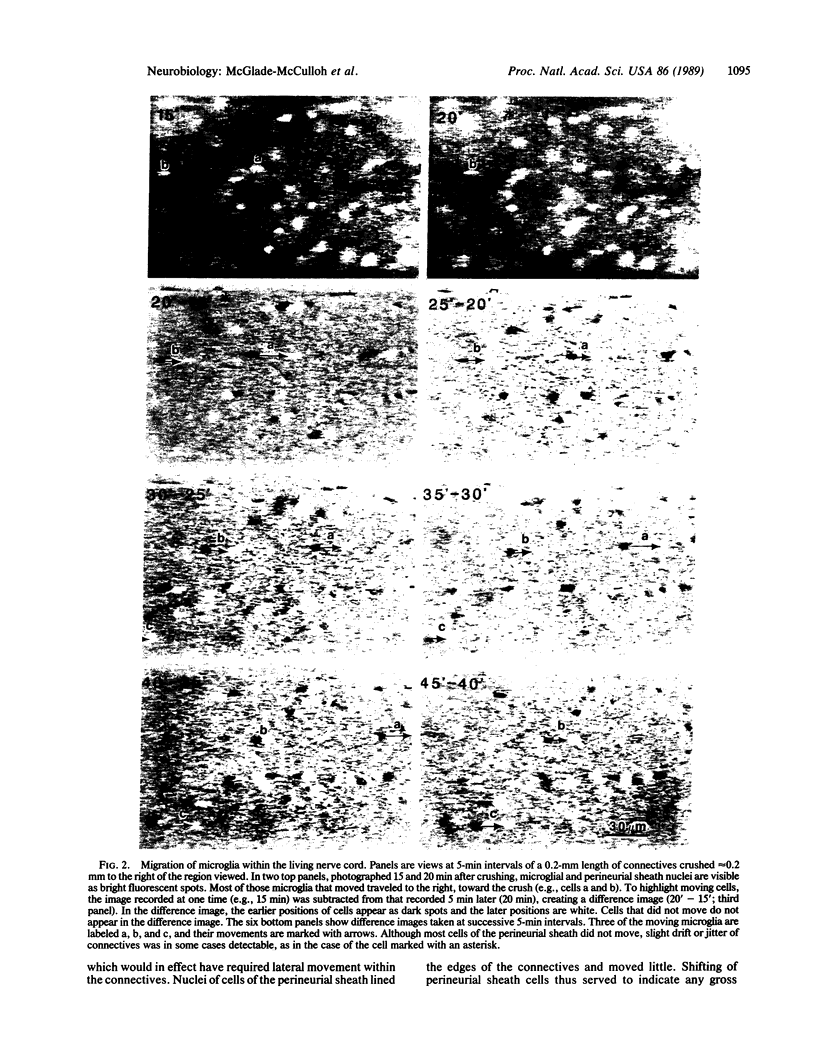
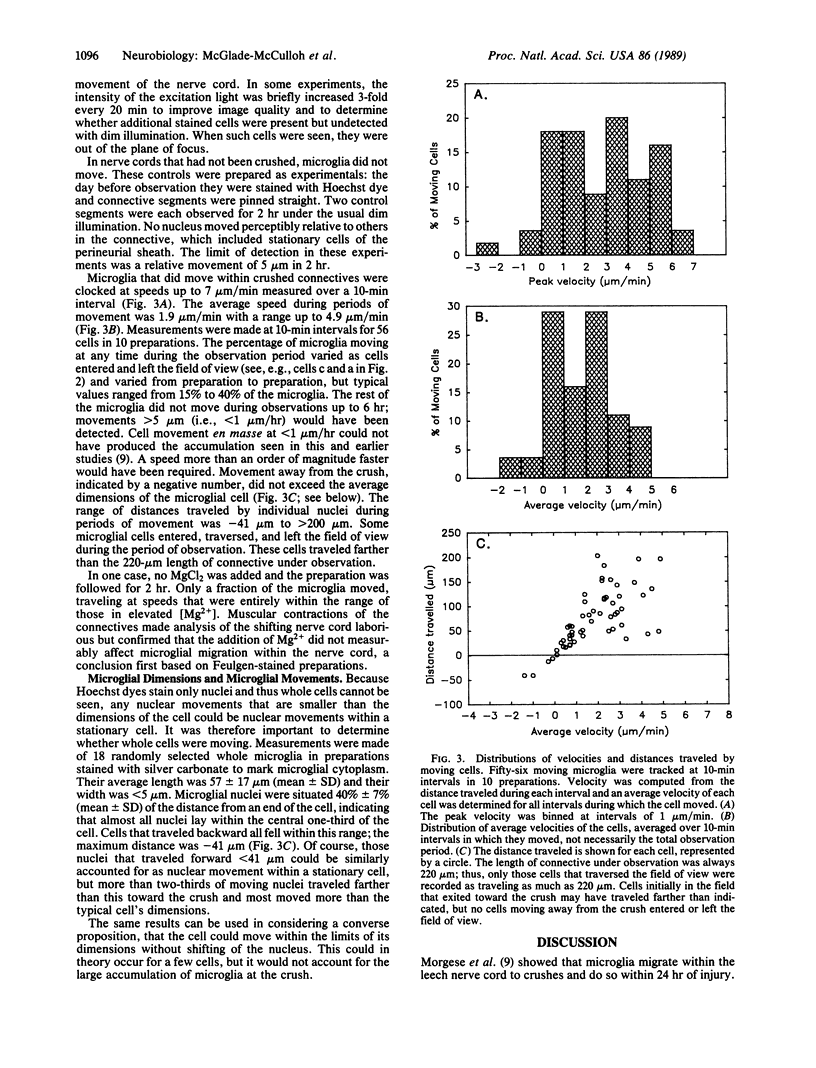
Small cells called microglia, which collect at nerve lesions, were tracked as they moved within the leech nerve cord to crushes made minutes or hours before. The aim of this study was to determine whether microglia respond as a group and move en masse or instead move individually, at different rates, and whether they move along axons directly to the lesion or take another route, such as along the edges of the nerve cord. Cell nuclei in living nerve cords were stained with Hoechst 33258 dye and observed under dim ultraviolet illumination using fluorescence optics, a low-light video camera, and computer-assisted signal enhancement. Muscular movements of the cord were selectively reduced by bathing in 23 mM MgCl2. Regions of nerve cord within 300 microns of the crush were observed for 2-6 hr. Only a fraction of microglia, typically less than 50%, moved at any time, traveling toward the lesion at speeds up to 7 microns/min. Cells were moving as soon as observation began, within 15 min of crushing, and traveled directly toward the lesion along axons or axon tracts. Movements and roles of leech microglia are compared with their vertebrate counterparts, which are also active and respond to nerve injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COGGESHALL R. E., FAWCETT D. W. THE FINE STRUCTURE OF THE CENTRAL NERVOUS SYSTEM OF THE LEECH, HIRUDO MEDICINALIS. J Neurophysiol. 1964 Mar;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- Chiquet M., Nicholls J. G. Neurite outgrowth and synapse formation by identified leech neurones in culture. J Exp Biol. 1987 Sep;132:191–206. doi: 10.1242/jeb.132.1.191. [DOI] [PubMed] [Google Scholar]
- Elliot E. J., Muller K. J. Long-term survival of glial segments during nerve regeneration in the leech. Brain Res. 1981 Aug 10;218(1-2):99–113. doi: 10.1016/0006-8993(81)90991-4. [DOI] [PubMed] [Google Scholar]
- Elliott E. J., Muller K. J. Sprouting and regeneration of sensory axons after destruction of ensheathing glial cells in the leech central nervous system. J Neurosci. 1983 Oct;3(10):1994–2006. doi: 10.1523/JNEUROSCI.03-10-01994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Peptides released by ameboid microglia regulate astroglial proliferation. J Cell Biol. 1985 Dec;101(6):2411–2415. doi: 10.1083/jcb.101.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Kai-Kai M. A., Pentreath V. W. The structure, distribution, and quantitative relationships of the glia in the abdominal ganglia of the horse leech, Haemopis sanguisuga. J Comp Neurol. 1981 Oct 20;202(2):193–210. doi: 10.1002/cne.902020206. [DOI] [PubMed] [Google Scholar]
- Macagno E. R. Number and distribution of neurons in leech segmental ganglia. J Comp Neurol. 1980 Mar 15;190(2):283–302. doi: 10.1002/cne.901900206. [DOI] [PubMed] [Google Scholar]
- Morgese V. J., Elliott E. J., Muller K. J. Microglial movement to sites of nerve lesion in the leech CNS. Brain Res. 1983 Aug 1;272(1):166–170. doi: 10.1016/0006-8993(83)90375-x. [DOI] [PubMed] [Google Scholar]
- Muller K. J., Carbonetto S. The morphological and physiological properties of a regenerating synapse in the C.N.S. of the leech. J Comp Neurol. 1979 Jun 1;185(3):485–516. doi: 10.1002/cne.901850305. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Howes E. A., Treherne J. E. Mechanisms of glial regeneration in an insect central nervous system. J Exp Biol. 1987 Sep;132:59–78. doi: 10.1242/jeb.132.1.59. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Adal M. N., Nicholls J. G. Regeneration of synaptic connections by sensory neurons in leech ganglia maintained in culture. Proc R Soc Lond B Biol Sci. 1977 Dec 30;199(1137):567–585. doi: 10.1098/rspb.1977.0164. [DOI] [PubMed] [Google Scholar]




