Abstract
The regulation of angiogenesis by hypoxia is an essential homeostatic mechanism that depends on a precise balance between positive and negative angiogenic regulatory molecules. Pro-angiogenic factors are well characterized; however, several in vivo and in vitro studies indicate that there are feedback mechanisms in place to inhibit angiogenesis during hypoxia. Understanding the signaling pathways leading to the negative feedback of angiogenesis will undoubtedly provide important tools to develop novel therapeutic strategies not only to enhance the angiogenic response in coronary artery disease but also to hinder deregulated angiogenesis in tumorigenesis.
Introduction
Oxygen homeostasis is critical for the survival of all multicellular organisms. The response to low oxygen levels (hypoxia) stimulates the oxygen-delivery system and provides mechanisms to activate cell death or survival pathways, depending on the context of the hypoxia signal. Systemically, highly sensitive tissues detect acute hypoxia and in response increase respiration and cardiac output. Extended hypoxia is sensed at the cellular level, which leads to the induction of angiogenesis, to increase delivery of oxygen and nutrients to tissues. This is accomplished by the de novo sprouting of capillaries from post-capillary venules, and in adults is stimulated mainly via the induction of hypoxia-inducible factor (HIF-1) expression.
The hypoxic response is significantly controlled in most cells by HIF-1, a heterodimeric transcription factor composed of the nearly ubiquitous HIF-1α and its dimerization partner HIF-1β. HIF-1 activates approximately 200 genes encoding proteins that regulate cellular metabolism, proliferation, motility, hematopoiesis, and angiogenesis (Semenza 2000). Upon initiation of the hypoxic signal, HIF1-α translocates to the nucleus, dimerizes with HIF1-β to form the HIF-1 complex and induces the expression of its transcriptional targets via binding to hypoxia-responsive elements (HREs) (Chilov et al. 1999). HREs are present in many angiogenic genes, such as VEGF, angiopoietin-2, VEGF receptors (Flt1 and KDR), and neuropilin-1 (Hickey and Simon 2006; Simons 2005). Hypoxia can up-regulate these angiogenic molecules by several mechanisms, including direct transcriptional activation by HIFs or indirect up-regulation by HIF-induced molecules. In addition, other transcription factors induced by hypoxia, such as Related Transcription Enhancer Factor-1 (RTEF-1) and early growth response 1 (EGR-1), can both target VEGF to enhance angiogenesis (Shie et al. 2004; Yan et al. 2000). Additional angiogenic growth factors such as IGF are also induced by hypoxia, but can signal through a HIF-1-independent pathway (Slomiany and Rosenzweig 2006).
Angiogenesis is important in physiological conditions such as embryogenic development and wound healing, as well as pathological conditions including tumorigenesis, diabetic retinopathy, rheumatoid arthritis, and atherosclerosis (Fong 2008). Moreover, hypoxia is associated with virtually all forms of vascular disorders, such as coronary and peripheral arterial diseases, including stroke, myocardial and limb ischemia; lung disorders; and diabetes (Fong 2008). Severe hypoxia is also found in solid tumors, where capillary networks are insufficiently organized (Folkman 2006). Physiological stresses such as hypoxia are regulated by a complex balance of both stimulatory and inhibitory signals that promote or inhibit angiogenesis. Specifically, understanding the role and regulation of genes during angiogenesis is becoming increasingly important to elucidate the compensatory hypoxic response. In the present review, we will mainly discuss the anti-angiogenic feedback mechanisms in the HIF-1- and VEGF-related angiogenic pathways.
HIF-1-related anti-angiogenesis
As a hypoxia-induced transcription factor, HIF-1 both stimulates and represses a multitude of genes important for adaptation to the low oxygen environment. Regulator of G protein Signaling 5 (RGS5) is a HIF-1-dependent, hypoxia-induced angiogenic inhibitor (Jin et al. 2009) that functions as a negative regulator of G protein-mediated signaling (Adams et al. 2000; Bell et al. 2001).
One of our previous reports showed that hypoxia specifically increased RGS5 expression in endothelial cells, which is confirmed in the DNA microarray data in Table 1 (Jin et al. 2009). RGS5 mRNA expression was induced by hypoxia while two other family members, RGS2 and RGS4, were not impacted. In addition to changes in oxygen levels, HIF-1α played a key role in hypoxia-induced RGS5 expression by stimulating RGS-5 promoter activity in endothelial cells. RGS5 slowed endothelial cell growth and significantly enhanced the apoptotic protein Bax, which led to increased apoptosis due to the change in the Bcl-2/Bax ratio (Jin et al. 2009; Yang and Korsmeyer 1996). Furthermore, RGS5 inhibited VEGF-induced angiogenesis through the p38 MAPK-dependent pathway in vitro and in vivo.
Table 1. Hypoxia-induced anti-angiogenic factors.
DNA Microarray data from hypoxia-treated human umbilical vein endothelial cells (HUVECs) analyzed by Ingenuity Pathway Analysis software. This table shows molecules associated with inhibition of angiogenesis that were enhanced upon hypoxia in comparison with normoxia.
| Symbol | Entrez Gene Name | p value |
|---|---|---|
| TIE1 | tyrosine kinase with immunoglobulin-like and EGF-like domains 1 | 2.00E-05 |
| RGS5 | Regulator of G protein signaling 5 | 2.00E-05 |
| COL181A | collagen, type XVIII, alpha 1/endostatin | 2.00E-05 |
| HHEX | hematopoietically expressed homeobox | 2.00E-05 |
| LAMA5 | laminin, alpha 5 | 2.00E-05 |
| SEMA3F | sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3F | 2.10E-05 |
| STAB1 | stabilin 1 | 4.00E-05 |
| THDS1 | thrombospondin, type I domain containing I | 4.60E-05 |
| ANGPT2 | angiopoietin 2 | 1.89E-04 |
| PLAT | plasminogen activator, tissue | 2.19E-04 |
| PGK1 | phosphoglycerate kinase 1 | 2.19E-04 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 motif, 1 | 3.46E-04 |
| SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | 3.88E-04 |
| PDE4B | Homo sapiens phosphodiesterase 4B (cyclic nucleotide phosphodiesterases) | 3.89E-04 |
| NOTCH1 | Notch homolog 1 (Drosophila) | 3.89E-04 |
| WARS | tryptophanyl-tRNA synthetase | 3.89E-04 |
| BMPR1A | bone morphogenetic protein receptor, type 1A | 4.68E-04 |
| PTX3 | pentraxin-related gene, rapidly induced by IL-1 beta | 5.52E-04 |
| PDCD5 | programmed cell death 5 | 6.18E-04 |
| SERPINE1 | serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | 6.92E-04 |
| TGFB2 | transforming growth factor, beta 2 | 1.32E-03 |
| PAWR | PRKC, apoptosis, WT1, regulator | 2.25E-03 |
| BNIP3 | BCL2/adenovirus E1B 19kDa interacting protein 3 | 4.48E-03 |
| PTRPM | protein tyrosine phosphatase, receptor type, M | 5.64E-03 |
Apoptosis occurring in endothelial cells causes an inhibitory effect on cell proliferation, which has a similar impact to that of anti-angiogenic factors. Hypoxia promotes apoptosis in endothelial cells, as demonstrated by changes in p53 protein levels (Hammond and Giaccia 2005; Stempien-Otero et al. 1999). Several studies suggest that HIF-1α stabilizes p53 and contributes to hypoxia-induced p53-dependent apoptosis (Xenaki et al. 2008). A recent report proposed that P300/CBP-associated factor (PCAF), an HIF-1α cofactor, regulates the balance between cell cycle arrest and apoptosis in hypoxia by modulating the activity and protein stability of both p53 and HIF-1α (Xenaki et al. 2008). Previous reports demonstrated that p53 can also inhibit angiogenesis by the following mechanisms: elevating thrombospondin-1 levels, repressing both VEGF and FGF-2, and by inducing degradation of HIF-1 (Folkman 2006).
HIF-1α mediates hypoxia-induced apoptosis through the up-regulation of genes as well as suppression of genes such as Bcl-2 (Carmeliet et al. 1998; Jin et al. 2009). One member of the Bcl-2 family, Bcl-2/E1B interacting protein (BNIP3) is up-regulated under hypoxic conditions (Chen et al. 1997; Kothari et al. 2003). Kothari and colleagues showed that blocking hypoxia-induced BNIP3 expression using siRNA or a mutant BNIP3 inhibits hypoxia-induced cell death. Additionally, hypoxia-mediated BNIP3 expression occurs by direct binding to an HRE in the human BNIP3 promoter, and mutation of this HRE site eliminates the hypoxic responsiveness of the promoter. Furthermore, hypoxia-induced BNIP3 expression was detectable 24 h after initial up-regulation of HIF-1α, indicating that BNIP3 expression occurs much later in the hypoxic response than that of other genes such as angiogenesis factor VEGF (Kothari et al. 2003).
While HIF-1 mainly targets pro-angiogenic genes, there are feedback molecules to counteract this up-regulation, such as endostatin, which down-regulates HIF-1α (Abdollahi et al. 2004). Hypoxia-induced endostatin inhibits VEGF-induced angiogenesis by preventing proliferation and migration of endothelial cells (Abdollahi et al. 2004; Heljasvaara et al. 2005; Morbidelli et al. 2003) and simultaneously up-regulates anti-angiogenic genes, including thrombospondin, vasostatin, and kininogen (Abdollahi et al. 2004). Reduced oxygen supply in FVB mice exposed to a hypobaric hypoxia chamber showed enhanced MMP production leading to a subsequent increase of endostatin generation in the lung and aorta (Paddenberg et al. 2006). These results were confirmed by Suhr et. al, who reported a significant increase in the endostatin and MMP-9 plasma concentration following hypoxic conditions in human cyclists (Suhr et al. 2007).
During sustained exposure to hypoxia, vascular beds may react differently. For example, a chronically hypoxic lung has structural changes in the pulmonary vascular bed which can become the major determinant of elevated vascular resistance (Fried et al. 1983). In the previously mentioned study, Paddenberg et al. questioned whether endostatin participates in the vascular alterations occurring in the lung under chronic hypoxia (Paddenberg et al. 2006). They found a hypoxia-induced increase in endostatin generation and MMP-2 activity in the lung, which corresponds with the findings of Frisdal et al. who demonstrated enhanced MMP-2 activity in rat pulmonary vessels during hypoxia-induced pulmonary hypertension (Paddenberg et al. 2006, Frisdal, 2001 #62). In the pulmonary vasculature, hypoxia-induced angiogenesis counteracts the development of pulmonary hypertension caused by vasoconstriction and vascular remodeling, while hypoxia-induced angiogenic inhibitors, i.e. endostatin would contribute to the development of pulmonary hypertension.
VEGF-associated anti-angiogenic regulation
While hypoxia-induced VEGF initiates angiogenesis, it also up-regulates expression of molecules within the endothelium that act as negative feedback regulators to modulate excessive vascular sprouting and endothelial cell proliferation. In addition to the aforementioned molecules, our previous report by An et al. demonstrated that Response Gene to Complement (RGC)-32 is another important VEGF-inducible gene that serves as a negative regulator of hypoxia-induced angiogenesis. RGC-32 expression was significantly increased under hypoxic conditions. Hypoxic induction of RGC-32 was also mediated by HIF-1α at both the transcriptional and posttranscriptional levels (An et al. 2009). While HIF-1-induced RGS5 was not VEGF-mediated (Jin et al. 2009), RGC-32 expression was significantly increased by VEGF but not factors including TNF-α, FGF2 and IL-1β (An et al. 2009). Unlike genes such as COX-2 (Wu et al. 2003), RGC-32 did not follow the canonical VEGF-induced angiogenic pathway. Overexpression of RGC-32 destabilized vascular structure formation in vitro by down-regulating FGF-2 and cyclin E, which caused negative feedback to VEGF activation. Additionally, when angiogenesis was examined in vivo in a mouse model, RGC-32 drastically inhibited VEGF-induced angiogenesis in matrigel, attenuated the recovery rate in hindlimb ischemia and reduced tumor size. (An et al. 2009).
Similarly, Delta-like ligand 4 (Dll4) is induced by VEGF (Lobov et al. 2007) yet has anti-angiogenic properties. Dll4 is part of the Notch signaling pathway (Liu et al. 2003) and highly expressed in the vascular endothelium, largely in angiogenic blood vessels (Lobov et al. 2007). Lobov, et al. demonstrated that Dll4 is an antagonistic regulator of angiogenesis by injecting a soluble version of Dll4 (Dll4-Fc) that blocks Dll4/Notch interactions into the vitreous membrane of oxygen-induced ischemic retinopathic (OIR) mice. Dll4 blockade markedly enhanced angiogenic sprouting while suppressing ectopic pathological neo-vascularization in the retinal vasculature. (Lobov et al. 2007). Therefore, Dll4 plays a role as a negative regulator of sprouting angiogenesis in response to the release of hypoxia-induced factors such as VEGF (Lobov et al. 2007).
In addition to VEGF-induced anti-angiogenic genes, the hypoxia-induced VEGF, itself, is also subjected to negative regulation, such as by transcription factor E2F1 (Qin et al. 2006). Under hypoxic conditions, it is well known that VEGF is induced by HIF-1; however, another transcription factor, E2F1 down-regulates expression of VEGF by associating with p53 and specifically down-regulating VEGF expression but not other hypoxia-inducible genes. Studies of E2F1-/- mice under hypoxic conditions revealed enhanced angiogenesis, dependent on deregulated VEGF, which illustrates a novel negative regulation to VEGF.
Other hypoxia-related angiogenic inhibitors
Several hypoxia-induced angiogenesis inhibitors have been discovered. The DNA array analysis in endothelial cells under hypoxic vs. normoxic conditions shown in Table 1 suggests that angiopoietin-2 (Ang2) is also important in the inhibition of angiogenesis induced by hypoxia. Previous reports determined that hypoxia enhances Ang2 expression in vivo as well as in vitro (Oh et al. 1999). The angiopoietin ligands/Tie receptors belong to a class of ligand/receptor families that plays a critical role in angiogenesis, cell migration, proliferation, and survival. Ang1 and Ang2 are both ligands with a similar binding affinity for the Tie2 receptor, which is a member of the receptor tyrosine kinases (RTKs) predominantly expressed by vascular endothelial cells (Maisonpierre et al. 1997). Ang1 induces angiogenesis via autophosphorylation of Tie2, while Ang2 competitively inhibits this effect (Maisonpierre et al. 1997). Ang2 is an antagonist for the Ang1/Tie2 pathway, as supported by data from over-expressing Ang2 mice that showed a very similar phenotype to the Ang1 and Tie2 single knockouts (Yuan et al. 2009). Thus, hypoxia-induced Ang2 plays a role in the negative regulation of angiogenesis by competing with Ang1.
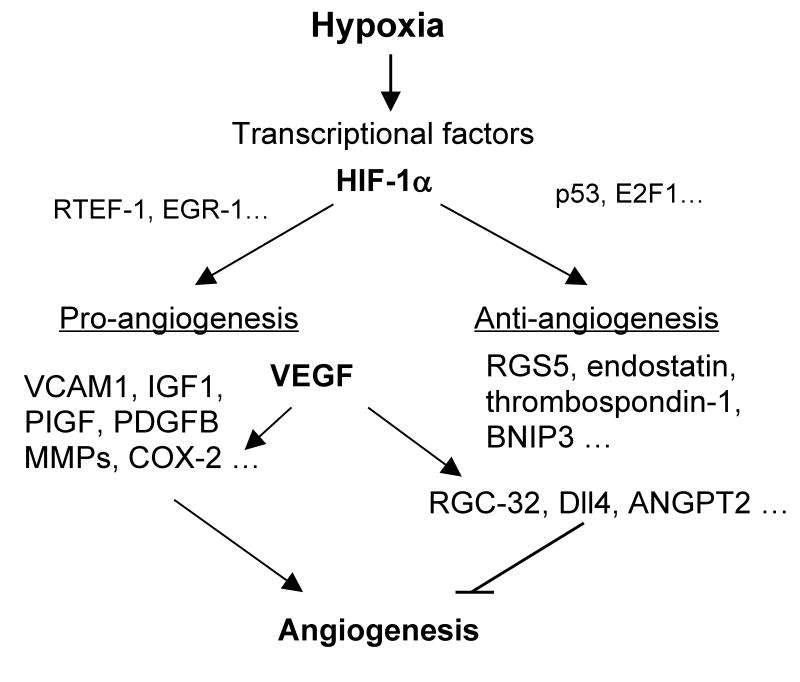
The negative feedback of angiogenesis during hypoxia, shown in the proposed schematic in Figure 1, may explain the inadequate natural collateral circulation in hypoxic heart diseases, in which up-regulated VEGF inhibits other pro-angiogenic signaling or triggers angiogenic inhibitor signaling. Therapeutic angiogenesis with either HIF-1 or VEGF resulted in a marked increase in blood flow and improved cardiac function in animal studies without apparent toxicity (Simons 2005). However, the results of clinical trials have been inconsistent and largely disappointing (Simons 2005), presumably because exogenous pro-angiogenic factors such as VEGF induce endogenous angiogenic inhibitor signaling to balance the effect; therefore, these feedback inhibitors will likely be new targets in future studies.
Figure 1. Schematic of hypoxia-mediated angiogenesis and anti-angiogenesis.
Concluding remarks
Intensive studies in the last decade have established the hypoxia signaling pathway as an important therapeutic target for various vascular diseases. Yet many questions still remain. Which pro- or anti-angiogenic factors will dominate in certain conditions? Which transcription factors regulate hypoxia-induced pro- or anti-angiogenic molecules? Understanding the signaling pathways leading to the negative feedback regulation of angiogenesis will undoubtedly provide an important means to develop therapeutic strategies that enhance neovascularization of ischemic tissue and interfere with focal, dysregulated vascular remodeling, the key mechanism for atherosclerotic disease progression as well as pulmonary hypertension. In contrast, selective stimulation of these negative feedback regulators is a promising prospect for tumor therapy.
Acknowledgments
We thank Ms. Brittany L. Cully for editing the manuscript. This work was supported by NIH grants HLR01082837 (Li), and T32 training award, HL007917 (Messmer-Blust).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin's antiangiogenic signaling network. Mol Cell. 2004;13:649–63. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- Adams LD, Geary RL, McManus B, et al. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–31. doi: 10.1161/01.res.87.7.623. [DOI] [PubMed] [Google Scholar]
- An X, Jin Y, Guo H, Foo SY, et al. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation. 2009;120:617–27. doi: 10.1161/CIRCULATIONAHA.108.841502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Chen G, Ray R, Dubik D, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186:1975–83. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilov D, Camenisch G, Kvietikova I, et al. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1alpha. J Cell Sci. 1999;112(Pt 8):1203–12. doi: 10.1242/jcs.112.8.1203. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11:121–40. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- Fried R, Meyrick B, Rabinovitch M, et al. Polycythemia and the acute hypoxic response in awake rats following chronic hypoxia. J Appl Physiol. 1983;55:1167–72. doi: 10.1152/jappl.1983.55.4.1167. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun. 2005;331:718–25. doi: 10.1016/j.bbrc.2005.03.154. [DOI] [PubMed] [Google Scholar]
- Heljasvaara R, Nyberg P, Luostarinen J, et al. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res. 2005;307:292–304. doi: 10.1016/j.yexcr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–57. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- Jin Y, An X, Ye Z, et al. RGS5, a hypoxia-inducible apoptotic stimulator in endothelial cells. J Biol Chem. 2009;284:23436–43. doi: 10.1074/jbc.M109.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari S, Cizeau J, McMillan-Ward E, et al. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene. 2003;22:4734–44. doi: 10.1038/sj.onc.1206666. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–24. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Morbidelli L, Donnini S, Chillemi F, et al. Angiosuppressive and angiostimulatory effects exerted by synthetic partial sequences of endostatin. Clin Cancer Res. 2003;9:5358–69. [PubMed] [Google Scholar]
- Oh H, Takagi H, Suzuma K, et al. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–9. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- Paddenberg R, Faulhammer P, Goldenberg A, et al. Hypoxia-induced increase of endostatin in murine aorta and lung. Histochem Cell Biol. 2006;125:497–508. doi: 10.1007/s00418-006-0158-5. [DOI] [PubMed] [Google Scholar]
- Qin G, Kishore R, Dolan CM, et al. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103:11015–20. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shie JL, Wu G, Wu J, et al. RTEF-1, a novel transcriptional stimulator of vascular endothelial growth factor in hypoxic endothelial cells. J Biol Chem. 2004;279:25010–6. doi: 10.1074/jbc.M403103200. [DOI] [PubMed] [Google Scholar]
- Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–66. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–75. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- Stempien-Otero A, Karsan A, Cornejo CJ, et al. Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J Biol Chem. 1999;274:8039–45. doi: 10.1074/jbc.274.12.8039. [DOI] [PubMed] [Google Scholar]
- Suhr F, Brixius K, de Marees M, et al. Effects of short-term vibration and hypoxia during high-intensity cycling exercise on circulating levels of angiogenic regulators in humans. J Appl Physiol. 2007;103:474–83. doi: 10.1152/japplphysiol.01160.2006. [DOI] [PubMed] [Google Scholar]
- Wu G, Mannam AP, Wu J, et al. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am J Physiol Heart Circ Physiol. 2003;285:H2420–9. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- Xenaki G, Ontikatze T, Rajendran R, et al. PCAF is an HIF-1alpha cofactor that regulates p53 transcriptional activity in hypoxia. Oncogene. 2008;27:5785–96. doi: 10.1038/onc.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Fujita T, Lu J, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–61. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- Yuan HT, Khankin EV, Karumanchi SA, et al. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–22. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]