Abstract
Although hippocampal infusions of glucose enhance memory, we have found repeatedly that septal glucose infusions impair memory when γ-aminobutyric acid (GABA) receptors are activated. For instance, hippocampal glucose infusions reverse the memory-impairing effects of co-infusions of the GABA agonist muscimol, whereas septal glucose infusions exacerbate memory deficits produced by muscimol. One potential explanation for these deleterious effects of glucose in the septum is that there are higher levels of endogenous extracellular fluid glucose concentrations in the septum than in the hippocampus. Another hypothesis is that septal glucose infusions impair memory by increasing septal GABA synthesis or release, which is possible because elevating glucose increases GABA levels in other brain regions. To test these hypotheses, Experiment 1 quantified extracellular fluid glucose levels in the septum and hippocampus using zero net flux in vivo microdialysis procedures in conscious, freely moving rats. Experiment 2 determined whether septal infusions of glucose would increase GABA concentrations in dialysates obtained from the septum. The results of Experiment 1 indicated that extracellular fluid glucose levels in the hippocampus and septum are comparable. The results of Experiment 2 showed that co-infusions of glucose with muscimol, at doses that did not affect memory on their own, decreased percent alternation memory scores. However, none of the infusions significantly affected GABA levels. Collectively, these findings suggest that the memory-impairing effects of septal infusions of glucose are not likely due to regional differences in basal extracellular fluid glucose concentrations and are not mediated via an increase in septal GABA release.
Keywords: Muscimol, GABA, Glucose, Spontaneous alternation, Septum, Memory, Zero net flux, in vivo microdialysis
1. Introduction
Glucose typically has a positive effect on memory (Korol, 2002; Korol and Gold, 2008; Messier, 2004); however, evidence is accumulating that under some conditions, elevations in glucose can be deleterious to memory in rodents and humans (Awad et al., 2002; Craft et al., 1993,1994; Gold et al., 1986; Gradman et al.,1993; Meneilly et al., 1993; Messier, 2004; Naor et al., 1997; Rodriguez et al., 1994; Vanhanen et al., 1997). The memory-modulating effects of peripheral elevations in glucose are mediated, at least in part, via an effect on the brain (Krebs and Parent, 2005; Lee et al., 1988; McNay et al., 2000; Parent et al., 1997; Ragozzino et al., 1998; Schroeder and Packard, 2003; Shah and Parent, 2003; Stefani and Gold, 1998). One method that has been used extensively to understand the neural mechanisms underlying the mnemonic effects of glucose is to infuse small volumes of glucose into specific brain areas. The results of such studies indicate that glucose can have positive or negative effects on memory, that the effects of glucose vary by brain region, and that glucose does not interact with all memory-modulating substances in the same way (Parent et al., 1997; Parent and Gold, 1997; Ragozzino and Gold, 1995; Ragozzino et al., 1998; Shah and Parent, 2003, 2004; Stefani and Gold, 1998; Stefani et al., 1999). For instance, in the septum, infusions of glucose reverse the memory-impairing effects produced by co-infusions of morphine (McNay et al., 2006; Ragozzino and Gold, 1994, 1995; Ragozzino et al., 1995, 1992), but exacerbate the memory deficits produced by GABA receptor agonists and interact with sub-effective doses of these agonists to impair memory (Parent et al.,1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004).
Interestingly, this memory-impairing interaction between glucose and GABAergic drugs that is seen in the septum is not observed in the hippocampus. Hippocampal infusions of glucose do not exacerbate, but rather reverse memory deficits produced by hippocampal co-infusions of the GABA receptor agonist muscimol (Krebs-Kraft and Parent, 2008). This finding is consistent with other evidence suggesting that many characteristics of glucose are not uniform throughout the brain. Specifically, there are brain region differences in glucose utilization (Gage et al., 1984; Gerber et al., 1983; Kimbrell et al., 2002) and in glucose transporter distribution, activation, and expression (Barros et al., 2005; Choeiri et al., 2005; Khandelwal et al., 2004; McNay et al., 2001). In addition, the effects of glucose on electrochemical signaling vary by neuronal phenotype, brain area, and glucose concentration (Fioramonti et al., 2004; Song et al., 2001; Wang et al., 2004).
Importantly, recent evidence indicates that there are brain region-dependent differences in basal and memory-training induced changes in brain extracellular fluid glucose levels (Fray et al., 1997; McNay and Gold, 1999; McNay et al., 2001). Extracellular glucose levels in the hippocampus are estimated to be 1.0–1.2 mM, whereas striatal levels are 0.71 mM (McNay and Gold, 1999; McNay et al., 2001). This raises the possibility that the brain region-dependent effects of glucose on memory are related to regional differences in extracellular fluid glucose concentrations. That is, the finding that glucose reverses muscimol-induced deficits in the hippocampus, but exacerbates the effects of muscimol in the septum may be due to differences in basal glucose levels in each brain region. If basal septal extracellular fluid glucose levels are higher than those of the hippocampus, then smaller increases in glucose may be required to produce memory deficits in the septum than in the hippocampus. Presently, the extracellular fluid glucose concentration of the septum is unknown. As a result, the purpose of the present experiments was to use zero net flux in vivo microdialysis procedures (Lonnroth et al., 1987) to determine whether basal extracellular fluid glucose concentrations differ between the septum and hippocampus.
The memory-impairing interaction between glucose and muscimol in the septum occurs with multiple GABAergic drugs: the GABAA/C agonist muscimol (Parent et al., 1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004), the benzodiazepine GABAA receptor modulator chlordiazepoxide (Krebs and Parent, unpublished findings), and the GABAB agonist baclofen (Erickson et al., 2006). Importantly, these negative effects of glucose and GABAergic drugs on memory are not likely due to extracellular fluid hyperosmolarity, because equiosmolar concentrations of other sugars, such as fructose, do not produce memory deficits when combined with muscimol injections into the septum (Shah and Parent, 2003, 2004). The interaction between glucose and GABA agonists in the septum is synergistic, such that doses of glucose and GABAergic drugs that individually have no effect produce memory deficits when the two are co-infused (Parent and Gold, 1997; Shah and Parent, 2003, 2004). This synergistic interaction suggests that both glucose and the GABA agonists are acting on a common mechanism to impair memory (Seeley and Moran, 2002); we hypothesize that this mechanism is the GABA neurotransmitter system. The fact that glucose impairs memory when it is combined with agonists that act at GABA receptor subtypes with very different properties suggests that it is unlikely that the effects of glucose involve an influence on GABA receptor function. Rather, a more parsimonious hypothesis is that glucose influences the neurotransmitter that binds to these different receptor subtypes. Consequently, another hypothesis for the mechanism underlying the impairing effects of glucose in the septum is that glucose increases septal GABA synthesis or release, and that this increase in extracellular fluid GABA summates with the effects of the sub-effective doses of the GABA agonists to produce memory deficits. This hypothesis is supported by the fact that acute administration of large amounts of glucose and experimentally-induced chronic hyperglycemia increase brain GABA levels (Amoroso et al., 1990; Fink and Gothert, 1993; Fink et al., 1994; Ohtani et al., 1997; Schmid-Antomarchi et al., 1990). Consequently, the second set of experiments used in vivo microdialysis procedures in rats performing in a memory task to determine whether administration of glucose, at a dose that produces memory deficits in the septum, would increase septal extracellular fluid GABA levels.
2. Materials and methods
2.1. Experiment 1A
The purpose of Experiment 1A was to use zero-net-flux in vivo microdialysis procedures in conscious, freely-moving rats to estimate basal extracellular fluid glucose levels in the septum and hippocampus in order to test the hypothesis that the different effects of glucose on memory in the septum and hippocampus are related, at least in part, to differences in basal glucose levels in each brain region. A dual-probe design was used so that extracellular fluid glucose levels in both brain areas could be assayed simultaneously in the same rat. Thus, any differences we might observe could not be due to the fact that the septum and hippocampus were somehow assayed in a different manner.
2.1.1. Subjects
Five male Sprague-Dawley derived rats weighing 200–250 g upon arrival (Charles River, Wilmington, MA) were used. The rats were housed individually in polycarbonate cages (20×40×20 cm) with corncob bedding on a 12 h light-dark cycle (lights on at 7:00 a.m.) in a temperature-controlled colony room (70–74 °F). Animals had free access to food and water. The rats were acclimated to laboratory conditions for approximately 1 week prior to surgery. The Georgia State University Institutional Animal Care and Use Committee (IACUC) approved all procedures involving the rats.
2.1.2. Surgery
Prior to surgery, rats were placed in a clear, plastic gas induction chamber and anesthetized with 5% isoflurane (Baxter, Deerfield, IL) delivered in 1000 ml/min medical grade oxygen. After the rat was no longer ambulatory, it was removed from the chamber and placed on a face–mask that delivered 3% isoflurane in 1000 ml/min of oxygen. Rats were then given injections of atropine sulfate (0.4 mg/kg, i.p., Baxter, Deerfield, IL) and penicillin (1500 U, i.m., Hanfords US Vet, Syracuse, NY). The future incision site was shaved with a #50 electric clipper blade (Oster) and betadine solution was applied to the surgical area. The percentage of isoflurane given to the rats was adjusted from 1–3% to maintain a surgical plane of anesthesia as determined by the toe pinch test. A stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) that was equipped with an anesthesia mask was used to implant one 15 mm inert, biocompatible plastic dialysis probe guide cannulae (Bioanalytical Systems, Inc., Roanoke, VA) aimed at the septum (0.5 mm anterior to bregma and 4.9 mm ventral to dura; Paxinos and Watson, 1998) and one aimed at the dorsal hippocampus (−5.0 mm; AP, ±4.5 from the interaural line, and −1.6 mm; DV; Paxinos and Watson, 1998). The hemisphere in which the unilateral hippocampal dialysis guide cannulae were implanted was counterbalanced across rats. The incision site was anesthetized with a 2% lidocaine/.001% epinephrine cocktail (0.5–2.0 cc, s.c., Abbott Labs, Chicago, IL). After the incision, the 2% lidocaine/.001% epinephrine cocktail solution (.05–1.0 cc) was applied topically to the skull to facilitate seeing lambda and bregma. The cannula was secured to the skull with three jeweler’s screws and cranioplastic cement and a stylet was inserted to keep the cannula free of debris. Immediately after surgery, the rats were given an injection of 0.9% sterile saline (3.0 cc, s.c.) and the non-steroidal anti-inflammatory flunixin meglumine (2.5 mg/kg, i.p., Fort Dodge Animal Health, Fort Dodge, IA), and then wrapped with a paper towel and kept under a warm lamp until recovery from anesthesia. Two days following surgery, the patency of each cannula was checked and betadine was applied to the surgical wound. If signs of infection were evident, the rats were anesthetized with isoflurane (5%) gas delivered in 1000 ml/min of oxygen and given an additional injection of penicillin (1500 U, i.m.).
2.1.3. In vivo microdialysis procedures
Each rat was handled for 2 min on two separate occasions prior to microdialysis procedures. Each rat participated in two microdialysis sessions separated by at least 4 days. Before and after all handling and in vivo microdialysis, the rats were allowed a minimum of 30 min to habituate to the laboratory environment. In vivo microdialysis procedures were conducted between 7:00 a. m. and 7:00 p.m. On the day of microdialysis, the rat was placed in a round Plexiglas containment bowl (BAS) and attached to a tether (BAS) that permitted him to move in the microdialysis containment bowl. Following a 1 h habituation period, a polyacrylonitrile microdialysis probe (320 μm OD; BAS) that extended 1 mm (septum) and or 3 mm (hippocampus) beyond the guide cannula was inserted into the guide cannula of both brain areas at the same time. The 1 mm probe length was selected because it would restrict the probe to the septal area and allow for sampling of the dorsal-ventral extent of the septum. The 3 mm probe length was selected for the hippocampus based on previous research using this length to investigate extracellular fluid glucose levels in the hippocampus (Canal et al., 2005; McNay et al., 2000, 2001, 2006; McNay and Gold, 2001). Furthermore, this length would permit sampling from a more representative extent of the dorsal-ventral range of the hippocampus. Using a microinfusion pump (BAS), the probe was perfused at the rate of 2 μl/min with artificial cerebrospinal fluid (aCSF; mM: NaCl 145.0, KCl 3.0, CaCl2 1.5, MgCl2 1.0, NaH2PO4, 2.0, NaH2PO4 2.0, dextrose 0.0; pH 7.3; filtered [0.2 μm] and degassed). The flow rate of 2 μl/min was chosen to permit the collection of sample volumes (10 μl) needed to assay glucose at 5 min sample periods. This is the sample period used in previous research estimating hippocampal extracellular fluid glucose levels (McNay and Gold, 1999; McNay et al., 2001). After a 2 h stabilization period, the two brain areas were perfused simultaneously with seven different quasi-randomly assigned concentrations of glucose (0–3 mM) each for 6 sample periods. The range of glucose concentrations was based on the range of brain extracellular glucose values observed in previous research (Fellows et al., 1992; Forsyth et al., 1996; Fray et al., 1997; Lund-Anderson, 1979; McNay et al., 2000; McNay and Gold, 1999). The perfusates were kept on dry ice during the experiment and then transferred to a −80 °C freezer for storage until analysis.
2.1.4. Glucose assay
To increase the likelihood that the extracellular fluid glucose levels had reached steady state, only the last three of the six samples from each glucose concentration were assayed. The amount of glucose in each sample was measured using a glucose hexokinase kit (Pointe Scientific, Inc. Canton, MI) and a spectrophotometer (Molecular Devices; Spectra Max 340PC) with SOFTmax Pro software (absorbance read at 340 nm). Samples were run in triplicate with fresh standards (0–10 mM) that showed a linear response to glucose concentration.
2.1.5. Histology
At the completion of the experiment, the rats were euthanized with an overdose of sodium pentobarbital (400 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 10% formalin. Their brains were stored in a 10% formalin solution for at least 2 days before sectioning. All brains were sectioned on a cryostat (Leica CM 30510 S) and 45–60 μm sections were taken through the septal cannulae tracts. The brain sections were stained with thionin and an unbiased observer determined the cannulae placement using a light microscope (Olympus BX41). Acceptable septal cannula placement was defined as septal injection and dialysis probe sites located within the septum and dorsal portions of the ventral diagonal band of Broca, but not within the lateral septum. Moreover, the cannula must not have penetrated the fimbria. Acceptable placement for hippocampal cannula was defined as dialysis probe membranes located within hippocampal fields CA1, CA2, CA3, or dentate gyrus. Only rats with acceptable cannulae placements in both brain regions were included in the statistical analyses.
2.1.6. Statistical analysis
The mean change in concentration between the samples and perfusate in the last three samples of each glucose concentration (y-axis) was plotted against the perfusate concentration alone (x-axis) for each individual rat. Using regression analyses, the point at which the concentration of glucose perfused into the brain matched the glucose concentration in the extracellular fluid (i.e., point of zero net flux) was determined (x-intercept when y=0) and used as a measure of extracellular fluid glucose levels. The zero net flux data were expressed as mean intercept±S.E.M. The data from each concentration of glucose were also expressed as mean (±S.E.M.) glucose gain or loss. The resultant zero net flux data from the septum and hippocampus were analyzed using paired t-tests to detect differences between group zero-net flux intercept means. An alpha level of 0.05 was used as the criterion for statistical significance.
2.2. Experiment 1B
The septum and hippocampus are highly connected brain regions that interact as a system to influence memory (Borisyuk et al., 1999; Gold, 2003a; Izquierdo and Medina, 1997; Parent and Baxter, 2004). This connectivity raises the possibility that manipulations of the glucose concentration in one brain area during zero-net flux procedures in Experiment 1A could potentially influence the glucose concentration in the other. As a result, the goal of Experiment 1B was to determine whether basal extracellular fluid glucose concentrations would differ between the septum and hippocampus when in vivo microdialysis procedures were used to sample each brain area from the same rat on separate days. The same procedures were used as in Experiment 1A, with the exception that two microdialysis sessions were separated by 4 days and the order of the brain areas was counterbalanced across rats. Fourteen male Sprague-Dawley rats were used in this experiment.
2.3. Experiment 2
The goal of Experiment 2 was to test the hypothesis that the memory-impairing interaction between glucose and muscimol in the septum is mediated, at least in part, via a glucose-induced increase in septal GABA release. To do so, in vivo microdialysis procedures were used in rats performing in a spontaneous alternation memory task to determine whether septal administration of glucose would increase septal extracellular fluid GABA levels. To date, the memory-impairing effects of septal infusions of glucose have been observed only when glucose is co-infused with muscimol or other GABA agonists or modulators (Krebs and Parent, unpublished findings; Parent et al., 1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004). That is, septal infusions of glucose alone do not produce any deficits. This raises the possibility that the effects of glucose on memory and extracellular fluid GABA levels depend on the co-occurrence of GABA receptor activation. Therefore, we also tested whether the effects of septal infusions of glucose on septal extracellular fluid GABA levels would vary as a function of the presence or absence of muscimol. In order to assess the effects of the infusions on memory and extracellular fluid GABA levels simultaneously, the drug infusions were combined with in vivo microdialysis procedures while rats were performing in a spontaneous alternation task. Given that our goal was to understand the mechanisms underlying glucose and muscimol-induced deficits, we selected a dose of glucose that has been shown previously to impair memory when combined with the GABA agonist muscimol (Parent et al., 1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004). Similarly, the dose of muscimol was selected based on previous findings showing that this dose does not significantly impair memory when infused alone into the septum, but does significantly impair memory when combined with glucose (Parent and Krebs, unpublished findings). We reasoned that one dose of each was sufficient as long as a dose that produced deficits was employed. Importantly, the fact that memory and GABA levels were assessed simultaneously in the same rats would provide confirmation that a deficit was present at the time that GABA was sampled. The same procedures as those used in Experiments 1A and B were used in the present experiment with the following exceptions.
2.3.1. Subjects
Twenty-four male Sprague-Dawley rats (n=5–8 per group) were used.
2.3.2. In vivo microdialysis procedures
Following a 1-h habituation period, a polyacrylonitrile microdialysis probe (320 μm OD; BAS) that extended 2 mm beyond the guide cannula was inserted into the guide cannula. The dialysis probe was equipped with an injection guide, which allowed for both injections into and sampling from the septal brain region. The probe length allowed for sampling from the septal area and possibly from the ventral diagonal band of Broca. The concentration of glucose was selected based on our zero net flux dialysis data, suggesting that the extracellular fluid glucose concentrations in the septum are approximately 1 mM (Experiment 1A and 1B). The flow rate of 2 μl/min was chosen to permit the collection of sample volumes needed to assay amino acids (i.e.,15 μl), while also allowing for sample periods that approximate the duration used to test the rats in previous behavioural experiments (i.e., 8 min; Parent et al., 1997; Parent and Gold, 1997). After a 2-h stabilization period, four baseline samples were collected every 8 min. Then the rats were given septal infusions of vehicle (0.5 μl, 0.5 μl/min; phosphate buffered-saline [PBS]), glucose (33 nmol), muscimol (0.1 nmol), or co-infusions of glucose with muscimol combined in one solution. The drugs that were combined in the same solution were prepared at double the desired concentration and then combined, reducing the concentration of each by half. To approximate our prior behavioural experiments, spontaneous alternationwas assessed after 2 sample periods (i.e.,16 min) following the drug infusions. The rats were placed in a 3-arm maze at the onset of the third sample period after the injection and remained in the maze for the duration of the sample period (i.e., 8 min). Three more samples were collected following the manipulations. The samples were kept on dry ice during the experiment and then transferred to a −80 °C freezer for storage until analysis with high-performance-liquid-chromatography (HPLC).
2.3.3. Spontaneous alternation
Spontaneous alternation is assumed to be a hippocampal-dependent measure of spatial working memory (Johnson et al., 1977; Lalonde, 2002; Richman et al., 1987; Stevens and Cowey, 1973). The underlying assumption is that in order to alternate successfully between locations the rats must remember their visits to previous places. This interpretation is supported by the finding that spontaneous alternation is impaired by removing directional cues or by increasing the interval between arm choices (Lalonde, 2002; Richman et al., 1987). Sixteen min after the drug injections, spontaneous alternation performance was assessed by placing each rat in a Y-maze composed of three equally spaced arms (60°; 61 cm long×20 cm high) separated by a circular center with a diameter of 43.5 cm. The 3-arm maze was used for the microdialysis experiments in order to parallel our previous research (Parent et al., 1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004). Each arm was composed of black plexiglass (15.5 cm wide) and the top was left uncovered. All rats were placed in center of the Y-maze and allowed to explore the maze for 8 min. The experimenter recorded the sequence and number of arms the rats entered during the 8-min period. The maze was cleaned with 70% ethanol after each rat. The number of arms each rat entered was used as a measure of activity. A percent alternation score was computed for all rats that entered at least 10 arms. An alternation was defined as entering three different arms consecutively. The percent alternation score was computed by dividing the number of alternations each rat made by the number of arms entered minus two (i.e., the number of alternations possible) and then multiplying that resulting quotient by 100.
2.3.4. High performance liquid chromatography (HPLC)
The amount of GABA in each sample of perfusate was assayed using reverse-phase HPLC (Waters 2695 Separations Module; Waters Corporation) using a mBondapak C18 precolumn connected to a Waters Spherisorb ODSD2 C18 column (4.6×250 mm, 5 mm) and fluorescence detection (Waters 474; Waters Corporation)as previously published (see Parent et al., 2001). Briefly, the amino acids were derivatized with o-phthaldialdehyde (OPA, Fluoraldehyde reagent ® Pierce). The products of the reaction, fluorescent isoindoles, were measured using excitation and emission wavelengths of 260 and 455 nm, respectively. A calibration curve consisting of a series of concentrations of GABA (.0078–1 ng/5 μL) was constructed with each assay run.
2.3.5. Statistical analysis
The neurochemical data were expressed as a percentage of the mean of the baseline samples and were analyzed with a mixed ANOVA. The spontaneous alternation data were expressed as means and standard errors of the mean (SEM) and analyzed using a one-way analysis of variance (ANOVA) and Tukey post hoc tests.
2.4. Experiment 3
Acute drug infusions were used in Experiment 2 to mimic the procedures used in our previous behavioral experiments. It is possible, however, that the microdialysis and assay procedures could not detect changes in extracellular fluid GABA levels produced by the brief infusions of glucose. As a result, the goal of Experiment 3 was to determine whether elevating glucose in the septum, over multiple sample periods, would increase extracellular fluid GABA levels. Also, as a positive control, we determined whether increasing potassium levels in the dialysate, which is a manipulation known to increase GABA levels, including GABA levels in the septum (Parent et al., 2001), would increase septal extracellular fluid GABA levels using our procedures. The same procedures as those used in Experiment 2 were used in the present experiment with the following exceptions.
2.4.1. Subjects
Six (6.6 mM glucose) and 12 (50 mM potassium) male Sprague-Dawley-derived rats were used.
2.4.2. In vivo microdialysis
After three baseline samples were collected, the aCSF was changed to one that contained a higher concentration of glucose (from 1 to 6.6 mM) for two sample periods. For other rats, the concentration of potassium was increased to from 3 to 50 mM for these two sample periods and the Na+ concentration was decreased accordingly (i.e., from 145 to 95 mM) to correct for the increase in osmolarity. For the remaining samples, the aCSF was changed back to the one used at baseline. The concentration of glucose was selected based on previous research showing that it increased hippocampal extracellular fluid acetylcholine levels in rats performing in the spontaneous alternation task (Ragozzino et al., 1998; Stefani and Gold, 2001). For the potassium manipulation, a concentration of potassium was chosen based on previous data showing it significantly increased extracellular fluid GABA levels in the septum and other brain regions (Campbell et al., 1993; Herbison et al., 1990; Hu et al., 2006; Parent et al., 2001; Takeda et al., 2003; Tossman and Ungerstedt, 1986).
2.4.3. Statistics
To increase statistical power, the data for the different sampling periods were pooled. Specifically, the GABA values for the baseline samples (i.e., samples 1–3) were collapsed, as were the GABA values for the two post-baseline samples in which glucose or potassium were elevated (i.e., samples 4–5). To correct for non-normality and non-heterogeneity of variance, the data were transformed to the log (base10) of the percent of baseline. Paired t-tests were used to detect differences between the average baseline and post-baseline values for glucose or potassium.
3. Results
3.1. Experiment 1A
Fig. 1 shows the approximate locations of septal and hippocampal dialysis probes for Experiment 1A. The results show that extra-cellular fluid glucose levels in the septum and hippocampus are similar [t(1,16)=.326, P > .05; see Fig. 2]. The extracellular fluid glucose concentrations obtained when the data were expressed as the means of the individual zero net flux regression values (see Fig. 2 inset) were slightly different from the extracellular fluid glucose concentrations estimated using zero net flux linear regression of all the rat’s mean values at each concentration of glucose (see Fig. 2).
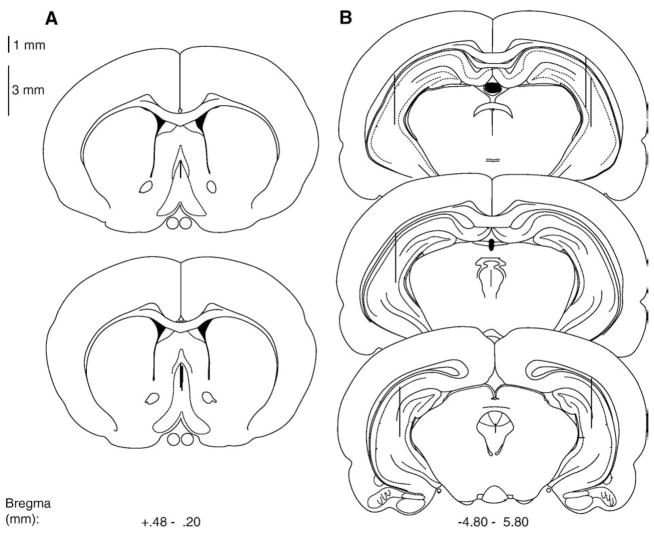
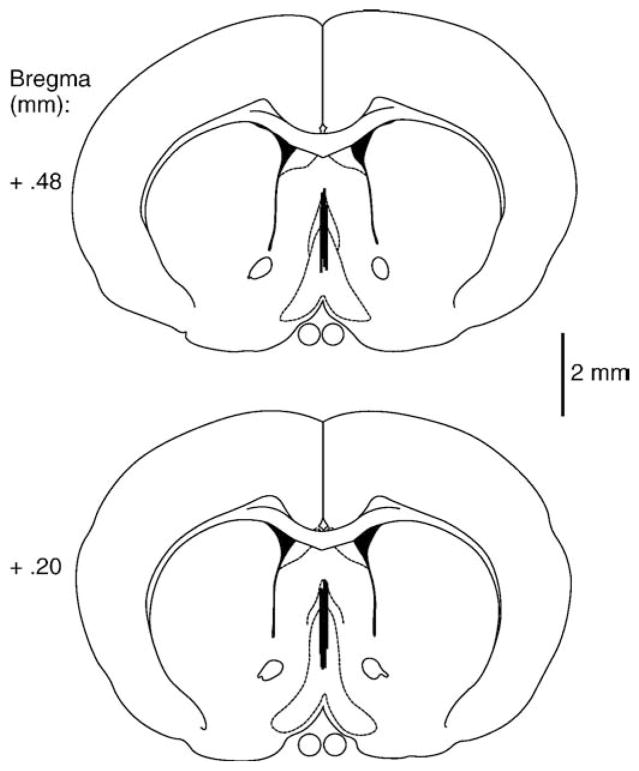
Fig. 1.
Schematic illustration of coronal sections of the rat brain showing the approximate locations of (A) septal and (B) hippocampal dialysis probe and infusion sites. Atlas plates were adapted from Paxinos and Watson (1998).
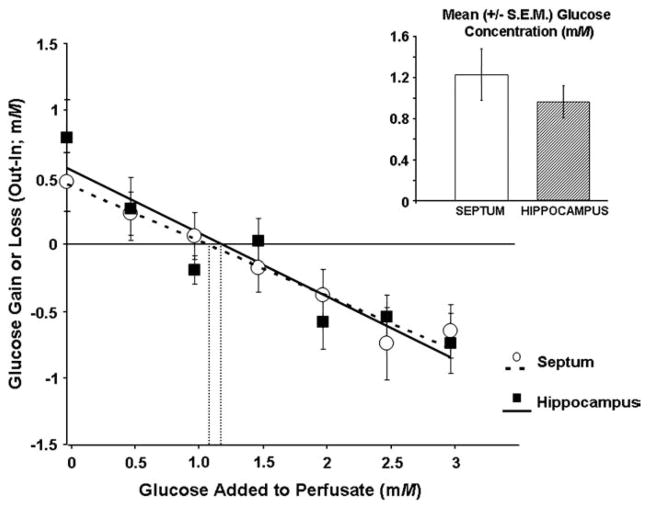
Fig. 2.
Glucose gain or loss to the brain as a function of perfusate concentration (0–3 mM) in the septum or hippocampus of rats using dual-probe microdialysis procedures. The point of zero net flux is the estimated extracellular fluid concentration of glucose. The solid (hippocampus) and dashed (septum) lines are the line of best fit for the point of zero net flux determination. The insert shows the mean (±S.E.M.) estimated glucose concentrations based on the individual regression analyses of each rat. The extracellular fluid glucose concentration in the septum (1.22±.25 mM) and the hippocampus (0.96±.16 mM) do not differ significantly (P>.05).
3.2. Experiment 1B
The approximate locations of the septal and hippocampal dialysis probes in Experiment 1B were similar to those in Experiment 1A. As in the simultaneous dialysis experiment, the results of Experiment 1B show that extracellular fluid glucose concentrations in the septum and hippocampus were similar when each brain area was sampled on two separate occasions [t(1,25)=1.11, P>.05; see Fig. 3]. Similar to the results obtained in Experiment 1A, the extracellular fluid glucose concentrations obtained when the data were expressed as the means of the individual zero net flux regression values (see Fig. 3 inset) were slightly different from the extracellular fluid glucose concentrations estimated based on zero net flux determination with the linear regression of all the mean values for each rat at each concentration of glucose (see Fig. 3).
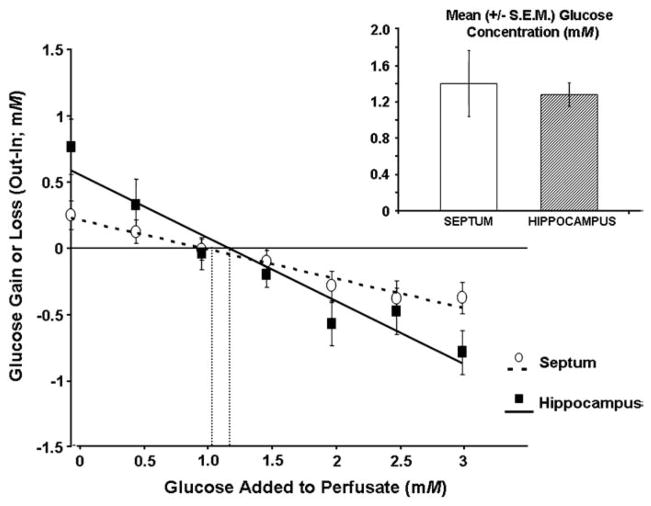
Fig. 3.
Glucose gain or loss to the brain as a function of perfusate concentration (0–3 mM) when the septum and hippocampus are sampled separately rather than simultaneously. The point of zero net flux is the estimated extracellular fluid concentration of glucose. The solid (hippocampus) and dashed (septum) lines are the line of best fit to the data for the point of zero net flux determination. The insert shows the mean (±S.E.M.) estimated glucose concentrations based on the individual regression analyses of each rat. The estimated extracellular fluid glucose concentration in the septum (1.39±.36 mM) and hippocampus (1.27±.13 mM) did not significantly differ (P>.05).
The sampling procedure did not yield significantly different mean extracellular fluid glucose concentrations between Experiment 1A and 1B [F(1,44) = .247, P >.05]. There was also no significant difference in terms of the brain region sampled in Experiment 1A and 1B [F(1,44)=.736, P>.05]. More importantly, the sampling procedure did not significantly interact with the brain region to affect the mean extracellular fluid glucose concentrations found in the septum versus hippocampus in Experiments 1A and B [F(1,44)=.192, P>.05].
3.3. Experiment 2
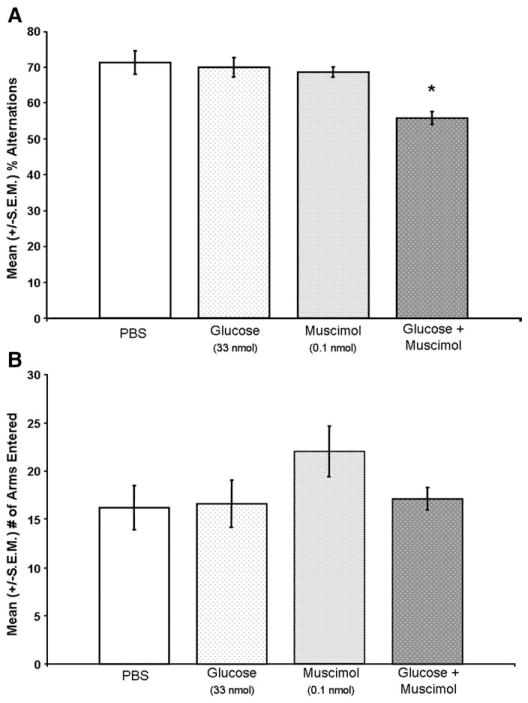
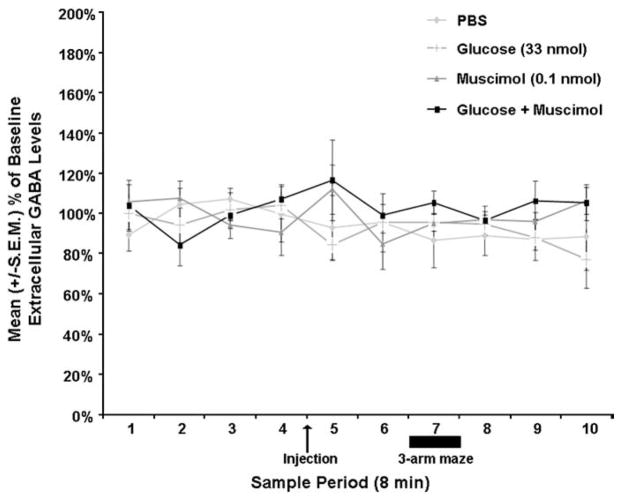
Fig. 4 shows the approximate location of the septal microdialysis probes. The 2 mm probes sampled primarily from the septum and ventral diagonal band of Broca. Drug infusions into the septum [F(3,23)=11.60; P <.05] significantly affected spontaneous alternation performance (see Fig. 5A). Septal infusions of muscimol or glucose alone did not affect spontaneous alternation performance. That is, the scores of rats given septal infusions of muscimol or glucose were not significantly different from the scores of rats given infusions of PBS (P >.05). As in previous research, co-infusions of muscimol with glucose into the septum produced memory deficits. Specifically, the percent alternation scores of rats given muscimol combined with glucose in the septum were significantly lower than those of rats given PBS (P <.05). In contrast, the drug infusions into the septum [F(3,23)= 1.59; P >.05] did not significantly affect the number of arms the rat entered in the maze (see Fig. 5B), although there was a tendency for rats given muscimol to enter more arms than rats given PBS (P=.052). More importantly, the drug infusions into the septum did not significantly affect septal extracellular fluid GABA levels [F(27, 117)= .856, P>.05; see Fig. 6].
Fig. 4.
Schematic illustration of coronal sections of the rat brain showing the approximate locations of septum dialysis probe and infusion sites. Atlas plates were adapted from Paxinos and Watson (1998).
Fig. 5.
A. Septal infusions of muscimol or glucose alone did not significantly decrease mean (±S.E.M.) percent alternation scores (P>.05 vs. control). Septal infusions of glucose with muscimol significantly decreased percent alternation scores (*P<.05 vs. PBS control). B. Septal drug treatments did not significantly affect the number of arms entered in the maze (P>.05).
Fig. 6.
Septal drug infusions did not significantly affect mean (±S.E.M.) extracellular fluid GABA levels (P>.05).
3.4. Experiment 3
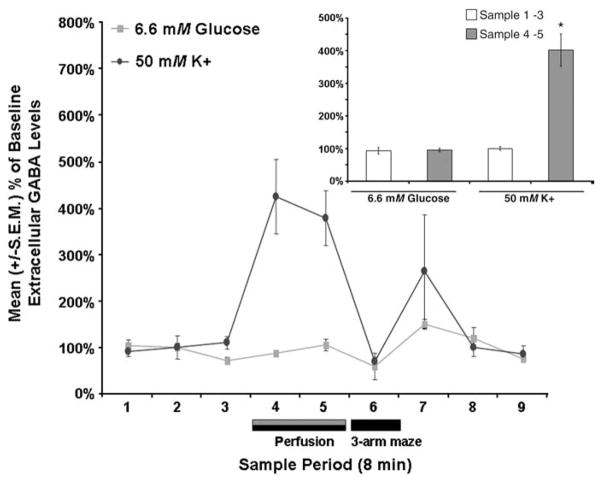
Increasing the concentration of glucose in the septal perfusate did not affect septal extracellular fluid GABA levels [t(1,5)=.45, P>.05; see Fig. 7]. In contrast, increasing the concentration of potassium did significantly increase septal extracellular fluid GABA levels [t(1,11)= 6.98, P<.05; see Fig. 7]. Specifically, compared to baseline levels the mean extracellular fluid GABA levels of rats were significantly higher during the sample periods when potassium was elevated (P<.05).
Fig. 7.
Septal perfusions of 6.6 mM glucose did not significantly affect mean (±S.E.M.) extracellular fluid GABA levels (P>.05) whereas septal perfusions of 50 mM potassium did (P<.05). Inset shows the pooled mean (±S.E.M.) extracellular fluid GABA values for the baseline samples (i.e., samples 1–3) and the extracellular fluid GABA values for the two post-baseline samples in which glucose or potassium were elevated (i.e., samples 4–5). Septal perfusion of glucose (6.6 mM) did not significantly affect mean septal extracellular fluid GABA levels (P>.05 vs. baseline). The mean septal extracellular fluid GABA levels in the samples in which potassium was elevated were significantly higher than the extracellular fluid GABA values of the baseline samples (P<.05).
4. Discussion
The findings from the present experiments demonstrate that basal extracellular glucose concentrations do not significantly differ between the septum and hippocampus. Specifically, extracellular fluid glucose concentrations are estimated to be ~1.0–1.2 mM in both the septum and the hippocampus. This finding was observed under conditions when both brain areas were studied simultaneously or on separate occasions in the same rats. These findings are of methodological importance, because they suggest that using a dual-probe dialysis design to sample extracellular fluid neurochemicals may not create an artifactual result. These data replicate previous findings showing that the basal extracellular fluid glucose levels in the hippocampus are approximately 1.0 mM (McNay and Gold, 1999; McNay et al., 2001). This 1.0 mM concentration is not uniform across the brain. Specifically, striatal extracellular fluid glucose levels are approximately 30% lower than hippocampal glucose levels (Fray et al., 1997; McNay et al., 2001), although it may be worth noting that striatum and hippocampus were assayed in different rats in those experiments. The present findings extend previous results by showing that the extracellular fluid glucose concentration of the septum is similar to the hippocampus rather than to levels observed in the striatum. These findings are also consistent with the observation that glucose utilization in the septum and hippocampus is similar (Huang et al., 1999; Wree et al., 1995). Collectively, these findings do not support the hypothesis that the different effects of glucose in the septum versus hippocampus on memory are due to differences in basal extracellular fluid concentrations of glucose in these two brain areas.
Knowing that basal extracellular fluid glucose levels are approximately 1 mM in the septum is important because it will permit researchers to use the appropriate amount of glucose in their perfusate when performing microdialysis procedures in the septum. In the past, some studies involving microdialysis procedures have omitted glucose from the perfusate, which is a problem because the concentration gradient that is created may cause the glucose to be drawn out of the extracellular fluid and into the probe, potentially impairing neural activity. Some researchers have included as high as 7–10 mM glucose in the perfusate, which is a concern because similar amounts (6.6 mM) have a profound influence on extracellular levels of various neurotransmitters (Anderson et al., unpublished findings; Ragozzino et al., 1998).
In addition, the present results demonstrate that septal co-infusions of the GABA receptor agonist muscimol with glucose, at doses that have no effect alone, impair spatial working memory. These data are consistent with previous research indicating that glucose interacts synergistically with muscimol to produce memory deficits (Parent et al., 1997; Parent and Gold, 1997; Shah and Parent, 2003, 2004). More importantly, the present experiments also show that the memory deficits produced by co-infusions of glucose and muscimol are not due to glucose-induced increases in septal extracellular fluid GABA levels, because these manipulations do not increase septal extracellular fluid GABA levels at the time memory is impaired in these animals. Furthermore, these data show that perfusing the septal area with glucose for longer durations, at a concentration that increases hippocampal extracellular fluid acetylcholine levels (Ragozzino et al., 1998; Stefani and Gold, 2001), did not increase septal extracellular fluid GABA levels. In contrast, septal perfusion with aCSF containing elevated potassium did increase septal extracellular fluid GABA levels, indicating that our measures are sensitive to changes in extracellular fluid GABA levels. These data are in agreement with previous results showing that elevating potassium within the septum increases extracellular fluid GABA levels (Parent et al., 2001), but are inconsistent with previous research showing that elevating glucose levels increases GABA release in the cortex and substantia nigra (Amoroso et al., 1990; During et al., 1995; Fink and Gothert, 1993; Fink et al., 1994; Ohtani et al., 1997; Schmid-Antomarchi et al., 1990). Collectively, the findings of the present experiments suggest that co-infusions of glucose with muscimol do not produce memory deficits through a process that involves elevated extracellular fluid glucose levels or increases in extracellular fluid GABA levels.
Thus, it remains to be determined how glucose produces memory deficits in the context of GABA receptor activation. One possibility is that the impairing effects of glucose on memory may involve a product of glycolytic metabolism, such as pyruvate (Shah and Parent, 2003, 2004). Alternatively, glucose may impair memory by increasing acetylcholine release in the septum as it does in the hippocampus (Degroot et al., 2003; Ragozzino et al., 1998). Although increasing acetylcholine function in the brain, including the septum, typically has positive consequences for learning and memory (Gold, 2003a,b), there are several cases where septal administration of cholinergic agonists can produce memory deficits (Bunce et al., 2003, 2004a,b; Elvander et al., 2004; Pang and Nocera, 1999; Sabolek et al., 2005). Alternatively, elevated septal glucose levels may impair memory via increases in septal glutamate activity, because glucose administration also elevates glutamate levels in the brain (Burke and Nadler, 1989; Gruetter, 2002; Hamberger et al., 1979). This latter possibility is supported by some limited evidence that suggests septal infusions of glutamate may have negative consequences for memory (Marighetto et al., 1994; Parent et al., 1997); however, our data do not support this possibility, because septal infusions of glucose and/or muscimol do not increase septal extracellular glutamate levels (Krebs-Kraft and Parent, unpublished findings). Finally, the opposite effects of glucose on memory in different brain regions could also involve interactions between glucose transporters (GLUT transporters) and different neurotransmitter groups (Apelt et al., 1999).
In summary, extracellular fluid glucose concentrations are estimated to be ~1.0–1.2 mM in both the septum and the hippocampus. This finding was shown under conditions when both brain areas were sampled simultaneously or separately. Furthermore, septal infusions of glucose with muscimol impair spatial working memory. More importantly, these same memory-impairing infusions do not concurrently increase septal extracellular fluid GABA levels during the time of memory testing. Collectively, these findings indicate that regional differences in extracellular fluid glucose levels do not contribute to the different effects of glucose on memory in the septum and hippocampus. Further, the findings suggest that the ability of glucose in the septum to produce memory deficits in the context of GABA receptor activation does not involve an increase in septal GABA release.
Acknowledgments
This research was supported in part by grants from NINDS-NIDDK-JDF (RO1NS41173-02), the STC Program of the National Science Foundation under Agreement No. IBN-9876754, and the Brains and Behavior Program at GSU. GBB is supported by a grant from the Canadian Institutes of Health Research (CIHR).
References
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- Awad N, Gagnon M, Desrochers A, Tsiakas M, Messier C. Impact of peripheral glucoregulation on memory. Behav Neurosci. 2002;116:691–702. doi: 10.1037/0735-7044.116.4.691. [DOI] [PubMed] [Google Scholar]
- Barros LF, Porras OH, Bittner CX. Why glucose transport in the brain matters for PET. Trends Neurosci. 2005;28:117–119. doi: 10.1016/j.tins.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Borisyuk R, Denham M, Denham S, Hoppensteadt F. Computational models of predictive and memory-related functions of the hippocampus. Rev Neurosci. 1999;10:213–232. doi: 10.1515/revneuro.1999.10.3-4.213. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal infusion of oxotremorine impairs memory in a delayed-non-match-to-sample radial maze task. Neuroscience. 2003;121:259–267. doi: 10.1016/s0306-4522(03)00462-7. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal infusion of the cholinergic agonist carbachol impairs delayed-non-match-to-sample radial arm maze performance in the rat. Hippocampus. 2004a;14:450–459. doi: 10.1002/hipo.10200. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Timing of administration mediates the memory effects of intraseptal carbachol infusion. Neuroscience. 2004b;127:593–600. doi: 10.1016/j.neuroscience.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Burke SP, Nadler JV. Effects of glucose deficiency on glutamate/aspartate release and excitatory synaptic responses in the hippocampal CA1 area in vitro. Brain Res. 1989;500:333–342. doi: 10.1016/0006-8993(89)90329-6. [DOI] [PubMed] [Google Scholar]
- Campbell K, Kalen P, Lundberg C, Wictorin K, Rosengren E, Bjorklund A. Extracellular gamma-aminobutyric acid levels in the rat caudate-putamen: monitoring the neuronal and glial contribution by intracerebral microdialysis. Brain Res. 1993;614:241–250. doi: 10.1016/0006-8993(93)91041-p. [DOI] [PubMed] [Google Scholar]
- Canal CE, McNay EC, Gold PE. Increases in extracellular fluid glucose levels in the rat hippocampus following an anesthetic dose of pentobarbital or ketamine–xylazine: an in vivo microdialysis study. Physiol Behav. 2005;84:245–250. doi: 10.1016/j.physbeh.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Choeiri C, Staines W, Miki T, Seino S, Messier C. Glucose transporter plasticity during memory processing. Neuroscience. 2005;130:591–600. doi: 10.1016/j.neuroscience.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Craft S, Dagogo-Jack SE, Wiethop BV, Murphy C, Nevins RT, Fleischman S, Rice V, Newcomer JW, Cryer PE. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci. 1993;107:926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- Craft S, Murphy C, Wemstrom J. Glucose effects and glucose regulation in memory and nonmemory tasks: the influence of age, sex, regulation, and glucoregulatory response. Psychobiology. 1994;22:95–105. [Google Scholar]
- Degroot A, Kornecook T, Quirion R, DeBow S, Parent M. Glucose increases hippocampal extracellular acetylcholine levels upon activation of septal GABA receptors. Learn Mem. 2003;979:71–77. doi: 10.1016/s0006-8993(03)02868-3. [DOI] [PubMed] [Google Scholar]
- During MJ, Leone P, Davis KE, Kerr D, Sherwin RS. Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. J Clin Invest. 1995;95:2403–2408. doi: 10.1172/JCI117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvander E, Schott P, Sandin J, Bjelke B, Kehr J, Yoshitake T, Ogren S. Intraseptal muscarinic ligands and galanin: influence on hippocampal acetylcholine and cognition. Neuroscience. 2004;126:541–557. doi: 10.1016/j.neuroscience.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Erickson EJ, Watts KD, Parent MB. Septal co-infusions of glucose with a GABAB agonist impair memory. Neurobiol Learn Mem. 2006;85:66–70. doi: 10.1016/j.nlm.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, Fillenz M. Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J Neurochem. 1992;59:2141–2147. doi: 10.1111/j.1471-4159.1992.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fink K, Gothert M. High D-glucose concentrations increase GABA release but inhibit release of norepinephrine and 5-hydroxytryptamine in rat cerebral cortex. Brain Res. 1993;618:220–226. doi: 10.1016/0006-8993(93)91269-x. [DOI] [PubMed] [Google Scholar]
- Fink K, Zentner J, Gothert M. Increased GABA release in the human brain cortex as a potential pathogenetic basis of hyperosmolar diabetic coma. J Neurochem. 1994;62:1476–1481. doi: 10.1046/j.1471-4159.1994.62041476.x. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Lorsignol A, Taupignon A, Penicaud L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes. 2004;53:2767–2775. doi: 10.2337/diabetes.53.11.2767. [DOI] [PubMed] [Google Scholar]
- Forsyth R, Fray A, Boutelle M, Fillenz M, Middleditch C, Burchell A. A role for astrocytes in glucose delivery to neurons? Dev Neurosci. 1996;18:360–370. doi: 10.1159/000111429. [DOI] [PubMed] [Google Scholar]
- Fray AE, Boutelle M, Fillenz M. Extracellular glucose turnover in the striatum of unanaesthetized rats measured by quantitative microdialysis. J Physiol. 1997;504 (Pt 3):721–726. doi: 10.1111/j.1469-7793.1997.721bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kelly PA, Bjorklund A. Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci. 1984;4:2856–2865. doi: 10.1523/JNEUROSCI.04-11-02856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JC, III, Choki J, Brunswick DJ, Reivich M, Frazer A. The effect of antidepressant drugs on regional cerebral glucose utilization in the rat. Brain Res. 1983;269:319–325. doi: 10.1016/0006-8993(83)90142-7. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003a;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine: cognitive and brain functions. Neurobiol Learn Mem. 2003b;80:177. doi: 10.1016/j.nlm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Gold PE, Vogt J, Hall JL. Glucose effects on memory: behavioral and pharmacological characteristics. Behav Neural Biol. 1986;46:145–155. doi: 10.1016/s0163-1047(86)90626-6. [DOI] [PubMed] [Google Scholar]
- Gradman TJ, Laws A, Thompson LW, Reaven GM. Verbal learning and/or memory improves with glycemic control in older subjects with non-insulin-dependent diabetes mellitus. J Am Geriatr Soc. 1993;41:1305–1312. doi: 10.1111/j.1532-5415.1993.tb06480.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int. 2002;41:143–154. doi: 10.1016/s0197-0186(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Hamberger AC, Chiang GH, Nylen ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dyer RG. Endogenous release of gamma-aminobutyric acid from the medial preoptic area measured by microdialysis in the anaesthetised rat. J Neurochem. 1990;55:1617–1623. doi: 10.1111/j.1471-4159.1990.tb04947.x. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Huang YH, Tsai SJ, Huang HJ, Sim CB. The effect of acute administration of risperidone on local cerebral glucose utilization in the rat. Eur J Pharmacol. 1999;370:257–261. doi: 10.1016/s0014-2999(99)00160-0. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Johnson CT, Olton DS, Gage FH, III, Jenko PG. Damage to hippocampus and hippocampal connections: effects on DRL and spontaneous alternation. J Comp Physiol Psychol. 1977;91:508–522. doi: 10.1037/h0077346. [DOI] [PubMed] [Google Scholar]
- Khandelwal P, Beyer CE, Lin Q, McGonigle P, Schechter LE, Bach AC., II Nanoprobe NMR spectroscopy and in vivo microdialysis: new analytical methods to study brain neurochemistry. J Neurosci Methods. 2004;133:181–189. doi: 10.1016/j.jneumeth.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Korol DL. Enhancing cognitive function across the life span. Ann NY Acad Sci. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Epinephrine converts long-term potentiation from transient to durable form in awake rats. Hippocampus. 2008;18:81–91. doi: 10.1002/hipo.20372. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Parent MB. The enhancing effects of hippocampal infusions of glucose are not restricted to spatial working memory. Neurobiol Learn Mem. 2005;83:168–172. doi: 10.1016/j.nlm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Parent MB. Hippocampal infusions of glucose reverse memory deficits produced by co-infusions of a GABA receptor agonist. Neurobiol Learn Mem. 2008;89:142–152. doi: 10.1016/j.nlm.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. The neurological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lee MK, Graham SN, Gold PE. Memory enhancement with posttraining intraventricular glucose injections in rats. Behav Neurosci. 1988;102:591–595. doi: 10.1037//0735-7044.102.4.591. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lund-Anderson H. Transport of glucose from blood to brain. Physiol Rev. 1979;59:305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- Marighetto A, Micheau J, Jaffard R. Effects of intraseptally injected glutamatergic drugs on hippocampal sodium-dependent high-affinity choline uptake in “naïve” and “trained” mice. Pharmacol Biochem Behav. 1994;49:689–699. doi: 10.1016/0091-3057(94)90089-2. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. J Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Geront A Biol Sci Med Sci. 2001;56:B66–71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- McNay EC, Canal CE, Sherwin RS, Gold PE. Modulation of memory with septal injections of morphine and glucose: effects on extracellular glucose levels in the hippocampus. Physiol Behav. 2006;87:298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol. 1993;48:M117–121. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Naor M, Steingruber HJ, Westhoff K, Schottenfeld-Naor Y, Gries AF. Cognitive function in elderly non-insulin-dependent diabetic patients before and after inpatient treatment for metabolic control. J Diabetes Its Complicat. 1997;11:40–46. doi: 10.1016/1056-8727(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Ohta M, Sugano T. Microdialysis study of modification of hypothalamic neurotransmitters in streptozotocin-diabetic rats. J Neurochem. 1997;69:1622–1628. doi: 10.1046/j.1471-4159.1997.69041622.x. [DOI] [PubMed] [Google Scholar]
- Pang KC, Nocera R. Interactions between 192-IgG saporin and intraseptal cholinergic and GABAergic drugs: role of cholinergic medial septal neurons in spatial working memory. Behav Neurosci. 1999;113:265–275. doi: 10.1037//0735-7044.113.2.265. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MB, Gold PE. Intra-septal infusions of glucose potentiate inhibitory avoidance deficits when co-infused with the GABA agonist muscimol. Brain. 1997;745:317–320. doi: 10.1016/s0006-8993(96)01206-1. [DOI] [PubMed] [Google Scholar]
- Parent M, Bush D, Rauw G, Master S, Vaccarino F, Baker G. Analysis of amino acids and catecholamines, 5-hydroxytryptamine and their metabolites in brain areas in the rat using in vivo microdialysis. Methods. 2001;23:11–20. doi: 10.1006/meth.2000.1102. [DOI] [PubMed] [Google Scholar]
- Parent M, Laurey P, Wilkniss S, Gold P. Intraseptal infusions of muscimol impair spontaneous alternation performance: infusions of glucose into the hippocampus, but not the medial septum, reverse the deficit. Neurobiol Learn Mem. 1997;68:75–85. doi: 10.1006/nlme.1997.3769. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Gold PE. Task-dependent effects of intra-amygdala morphine injections: attenuation by intra-amygdala glucose injections. J Neurosci. 1994;14:7478–7485. doi: 10.1523/JNEUROSCI.14-12-07478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68:981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Hellems K, Lennartz RC, Gold PE. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci. 1995;109:1074–1080. doi: 10.1037//0735-7044.109.6.1074. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Parker ME, Gold PE. Spontaneous alternation and inhibitory avoidance impairments with morphine injections into the medial septum. Attenuation by glucose administration. Brain Res. 1992;597:241–249. doi: 10.1016/0006-8993(92)91480-3. [DOI] [PubMed] [Google Scholar]
- Richman C, Dember W, Kim P. Spontaneous alternation behavior: a review. Curr Psychol Res Rev. 1987;5:358–391. [Google Scholar]
- Rodriguez WA, Horne CA, Mondragon AN, Phelps DD. Comparable dose–response functions for the effects of glucose and fructose on memory. Behav Neural Biol. 1994;61:162–169. doi: 10.1016/s0163-1047(05)80070-6. [DOI] [PubMed] [Google Scholar]
- Sabolek HR, Bunce JG, Chrobak JJ. Intraseptal tacrine-induced disruptions of spatial memory performance. Behav Brain Res. 2005;158:1–7. doi: 10.1016/j.bbr.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H, Amoroso S, Fosset M, Lazdunski M. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc Natl Acad Sci USA. 1990;87:3489–3492. doi: 10.1073/pnas.87.9.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur J Neurosci. 2003;17:1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Moran TH. Principles for interpreting interactions among the multiple systems that influence food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R46–53. doi: 10.1152/ajpregu.00021.2002. [DOI] [PubMed] [Google Scholar]
- Shah AA, Parent MB. Septal infusions of glucose or pyruvate, but not fructose, produce avoidance deficits when co-infused with the GABA agonist muscimol. Neurobiol Learn Mem. 2003;79:243–251. doi: 10.1016/s1074-7427(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Shah AA, Parent MB. Septal infusions of glucose or pyruvate with muscimol impair spontaneous alternation. Brain Res. 2004;996:246–250. doi: 10.1016/j.brainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Res. 1998;813:50–56. doi: 10.1016/s0006-8993(98)00876-2. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intrahippocampal infusions of k-atp channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. J Neurosci. 2001;21:609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93:557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Res. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hirate M, Tamano H, Oku N. Release of glutamate and GABA in the hippocampus under zinc deficiency. J Neurosci Res. 2003;72:537–542. doi: 10.1002/jnr.10600. [DOI] [PubMed] [Google Scholar]
- Tossman U, Ungerstedt U. The effect of apomorphine and pergolide on the potassium-evoked overflow of GABA in rat striatum studied by microdialysis. Eur J Pharmacol. 1986;123:295–298. doi: 10.1016/0014-2999(86)90671-0. [DOI] [PubMed] [Google Scholar]
- Vanhanen M, Koivisto K, Karjalainen L, Helkala EL, Laakso M, Soininen H, Riekkinen P., Sr Risk for non-insulin-dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. Neuroreport. 1997;8:1527–1530. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- Wree A, Goller HJ, Beck T. Local cerebral glucose utilization in perfusion-fixed rat brains. J Neurosci Methods. 1995;58:143–149. doi: 10.1016/0165-0270(94)00168-g. [DOI] [PubMed] [Google Scholar]