Abstract
Methyl-CpG-binding protein 2 is a transcription factor that is involved in gene silencing. It is mutated in the majority of cases of Rett syndrome. This X-linked neurodevelopmental disorder is reported to involve abnormalities in autonomic cardiovascular regulation. As an initial step in understanding the basis for these abnormalities we have characterized autonomic cardiovascular function in Mecp2 deficient mice. Arterial pressure waves were recorded in freely moving animals using telemetry. Baseline blood pressure and pulse interval (PI) as well as indices of heart rate variability (HRV): standard deviation of PI (SDNN), range encompassing 90% of PIs (PI90) and standard deviation of adjacent PIs (SDSD) were similar in Mecp2+/+ and Mecp2+/− animals. Spectral analysis of mean arterial pressure (MAP) and PI in the frequency domain showed similar relative power in low frequency 1 (LF1, 08–0.4 Hz), low frequency 2 (LF2, 0.4–1.0 Hz), middle frequency (MF, 1–3 Hz) and high frequency (HF, 3.0–10.0 Hz) bands. Autonomic blockade with atropine or propranolol as well as elevation in ambient temperature to 32 °C resulted in changes in blood pressure, PI and HRV that did not differ between the strains. Atropine, propranolol and elevated temperature resulted in similar changes in both MAP and PI spectral power. Baroreceptor function was tested using intravenous injections of nitroprusside followed by phenylephrine. Maximum gain was not different. These results do not reveal any disturbance of autonomic cardiovascular regulation in the Mecp2 deficient mouse genotype.
Keywords: Atropine, Ambient temperature, Heart rate variability, Propranolol, Rett syndrome
1. Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder caused by mutations in the gene that encodes the transcription factor methyl-CpG-binding protein 2 (Mecp2) (Amir et al., 1999; Hagberg et al., 1983; Rett, 1972). Mecp2 is involved in transcriptional silencing, however, specific genes that are affected by its deficiency are generally not known. MECP2 is X-linked and affected females are heterozygous for the mutations. In most cases X inactivation does not favor either the wild type or mutated allele (Amir et al., 2000; Hoffbuhr et al., 2002). Included in the phenotype of this disorder are abnormalities in autonomic regulations of heart rate that may contribute to the observation that 26% of deaths occur suddenly (Glaze, 2005; Kerr et al., 1997). Subjects with RTT have been reported to have shorter pulse intervals than age matched controls (Guideri et al., 2001; Weese-Mayer et al., 2006) although this was not observed in other studies (Johnsrude et al., 1995; Julu et al., 2001). RTT individuals have decreased heart rate variability reflected by a lower standard deviation of R–R intervals and root mean square of successive differences (Johnsrude et al., 1995). Spectral analyses of R–R intervals in the frequency domain have reported that subjects with Rett syndrome have decreased total power especially in the high frequency component (Guideri et al., 2001; Johnsrude et al., 1995). In addition decreased baroreceptor sensitivity determined during spontaneous fluctuations in blood pressure has been reported (Julu et al., 2001). Taken together these observations have suggested that individuals with Rett syndrome have an increase in sympathetic activity that is not counter balanced by adequate vagal tone.
The generation of mice with either deficient (Chen et al., 2001, Guy et al., 2001) or truncated (Shahbazian et al., 2002) Mecp2 has opened the potential to determine mechanisms underlying autonomic dysfunction in RTT. The goal of the present study was to characterize autonomic cardiovascular regulation in a mouse model for RTT by examining indices of heart rate variability and spectral analysis of mean arterial pressure (MAP) and pulse interval (PI) in the frequency domain under basal conditions and after either muscarinic parasympathetic or beta-adrenergic sympathetic blockade. In addition since elevation of ambient temperature in rodents results in withdrawal of sympathetic and augmentation of vagal tone we used this treatment to compare Mecp2 deficient to wild type mice. In contrast to human subjects with Rett syndrome no striking differences were seen in this mouse model.
2. Materials and methods
2.1. Animals
The protocols used were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee and were in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. B6.129P22tml.l Bird (stock number :003890) heterozygous females and wild type females from the colony were obtained from the Jackson Laboratory, Bar Harbor, ME, at two months of age and were used for the initial experiments. Additional Mecp2+/− females and wild type littermates were obtained by crossing Mecp2+/− females with C57BL/6J males. Animals were genotyped by polymerase chain reaction (PCR) according to the protocol of the supplier (http://aretha.jax.org/pub-cgi/protocols/protocols.sh?obitype=protocol&protocol_id=468). This strain was originally generated by insertion of loxP sites around exons 3 and 4, and crossing homozygous floxed females to male CMVCre mice (6).
2.2. Telemetry monitoring
Under general anesthesia (1.5% isoflurane in oxygen) a catheter attached to a transmitter (model PA-C20, Data Sciences International, St. Paul MN) was placed in the carotid artery for telemetric monitoring of blood pressure. Animals were allowed to recover for 5 to 7 days before initiating study protocols. On the day of experiment the animals in their cages were placed on a telemetry receiver (model RBC-1, Data Sciences).
2.3. Experimental protocols
Pharmacological experiments: Administrations of drugs or vehicle were performed in random order between 09:00 and 14:00 h. Only one study was done per day. A minimum of one hour was allowed before acquiring data. Following 15 min recording of baseline data either atropine (1 mg/kg), propranolol (4 mg/kg) or vehicle (sterile water) was given intraperitoneally. The injection volumes were 80 to 100 µl depending on animal weight. Data was acquired for the following 45 min. Vehicle injections resulted in a significant increase in heart rate in Mecp2+/+ mice that lasted 15 min. Heart rate also went up in Mecp2+/− following vehicle, although not significantly. Therefore data for the effects of atropine and propranolol was taken from 15 to 45 min after injection. The changes in heart rate were sustained for these 30 min.
Baroreceptor sensitivity. For these experiments catheters were placed in a jugular vein. Blood pressure was lowered with injections of nitroprusside (5–10 µg/kg). When a plateau was reached phenylephrine (2–4 µg/kg) was administered. Injection volumes were 5–10 µl. The relationship between systolic arterial pressure and heart rate was acquired over a 20 to 40 second period.
Effect of increased ambient temperature. Animal cages were placed inside a clear plastic box (80 L volume) that contained a fan and heat panel. Baseline data was acquired for 30 min and then ambient temperature (Ta) was increased to 32 °C from 23 °C. Data was not taken for the first 45 minutes of increased Ta and then was acquired for 30 minutes.
2.4. Data analysis
Arterial pressure waveforms were sampled at 5 KHz and pulse intervals calculated form the peaks of the pressure record. Data for analysis was averaged in 5 min blocks. Spectral power of mean arterial pressure and pulse interval in the frequency domain was determined using custom written functions in Matlab software (Math Works, Natick, MA). Arterial pressure waveforms were sampled at 5 Ks/s. 8192 point Fourier transforms with 512 point overlap and a Hamming window were computed for a time frequency display, or spectrogram. An average spectrum using 16,384 point Fourier transforms with 25% overlap was then computed for a compact spectrum display. Pulse intervals were then computed using a peak finding algorithm. Manual corrections were performed for dropout or over sensing. The intervals were then linearly interpolated to a regular 10 ms grid. 4096 point spectrograms with 50% overlap were computed. The results were also averaged for a compact spectrum. A histogram of pulse intervals was also generated.
Spectra were divided into four frequency ranges as reported by Janssen et al. (2000): low frequency 1 (LF1) 0.08–0.4 Hz; low frequency 2 (LF2) 0.4–1.0 Hz; mid frequency (MF) 1–3 Hz; and high frequency (HF) 3–10 Hz. The α1-adrenergic antagonist prazosin significantly lowered MAP power in the LF1 and LF2 ranges indicating that these domains are suitable for defining sympathetic outflow in mice (Janssen et al., 2000). Similarly the effects of the muscarinic blocker atropine were evident in these ranges. Spectral power for MAP and PI are expressed for each frequency relative to that for the four frequencies combined. Baroreceptor sensitivity was determined by fitting the heart rate response to changes in blood pressure to a sigmoid logistic function (Kent et al., 1972). The maximum slope was calculated from: maximum − minimum heart rate × slope coefficient /4.
2.5. Statistics
Values are reported as means ± S.E. The effects of pharmacological agents or ambient temperature were analyzed by two way ANOVA with strain and before and after treatment as the two factors. Newman–Keuls was used for post hoc tests. Difference were considered significant when p<0.05.
3. Results
3.1. Animal characteristics
FemaleMecp2+/− mice were grossly indistinguishable from Mecp+/+. At the time of study (8.7 ± 0.7 months) their weights were the same (Mecp2+/− 21.8 ± 0.9; Mecp2+/+ 22.2 ± 0.2 g). Six of seven Mecp2+/− animals showed clasping of their hind legs when elevated by the tail. At autopsy Mecp2+/− brain weights (418 ± 8 mg) were significantly less than Mecp2+/+ (472 ± 5 mg) p = <0.001) Heart weights in Mecp2+/− (106 ± 6 mg) were not different than Mecp2+/+ (112 ± 7 mg).
3.2. Heart rate and blood pressure
Baseline heart rate and blood pressure together with indices of heart rate variability (standard deviation of pulse interval, (SDNN) range which encompassed 90% of pulse intervals (PI90) and standard deviation of differences between adjacent pulse intervals (SDSD), were the same for Mecp2+/− and wild type mice (Tables 1–3).
Table 1.
Effect of atropine on heart rate, mean arterial pressure and indices of heart rate variability
| Strain | Control heart rate (bpm) |
Atropine heart rate (bpm) |
Control MAPa (mm Hg) |
Atropine MAP (mm Hg) |
Control SDNNb (ms) |
Atropine SDNN (ms) |
Control SDSDc (ms) |
Atropine SDSD (ms) |
Control PI90d (ms) |
Atropine PI90 (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mecp2+/+ | 620 ± 26 | 669 ± 16 | 115 ± 4 | 111 ± 4 | 7.7 ± 0.6 | 3.9* ± 0.5 | 7.8 ± 1.0 | 4.6* ± 0.6 | 25.1 ± 2.1 | 11.8* ± 1.8 |
| Mecp2+/− | 586 ± 26 | 672* ± 11 | 101 ± 5 | 104 ± 3 | 7.6 ± 0.5 | 3.7* ± 0.9 | 7.3 ± 1.0 | 5.3 ± 0.6 | 24.3 ± 1.4 | 11.4* ± 1.3 |
Values are mean ± S.E. N = 6 for both strains.
significantly different from control, p values between 0.024 and <0.0001. There are no differences between strains.
mean arterial pressure;
standard deviation of pulse interval;
standard deviation of adjacent pulse intervals;
range encompassing 90% of pulse intervals.
Table 3.
Effect of elevated ambient temperature (Ta 32 °C) on heart rate, mean arterial pressure and indices of heart rate variability
| Strain | Control heart rate (bpm) |
Ta 32 °C heart rate (bpm) |
Control MAPa (mm Hg) |
Ta 32 °C MAP (mm Hg) |
Control SDNNb (ms) |
Ta 32 °C SDNN (ms) |
Control SDSDc (ms) |
Ta 32 °C SDSD (ms) |
Control PI90d (ms) |
Ta 32 °C PI90 (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mecp2+/+ | 598 ± 24 | 484* ± 26 | 105 ± 3 | 96 ± 6 | 7.1 ± 1.0 | 9.1* ± 0.9 | 6.3 ± 0.8 | 9.7* ± 1.2 | 22.8 ± 3.2 | 29.5* ± 2.7 |
| Mecp2+/− | 617 ± 30 | 473* ± 22 | 119 ± 3 | 99* ± 6 | 7.7 ± 0.5 | 10.6* ± 1.3 | 8.7 ± 1.1 | 11.6* ± 1.2 | 24.6 ± 1.9 | 34.5* ± 4.5 |
Values are mean ± S.E. N = 7 for Mecp2+/+ and 6 for Mecp2+/−.
significantly different from control, p values between 0.043 and 0.007. There are no differences between strains.
mean arterial pressure;
standard deviation of pulse interval;
standard deviation of adjacent pulse intervals;
range encompassing 90% of pulse intervals.
3.3. Spectral power of MAP and PI
It may appear that there was a difference between strains under baseline conditions in some bands, for example MAP relative power in LF1 of Fig. 1. Analysis, however, did not reveal any significant differences between Mecp2+/− and Mecp2+/+ mice. This was the case for basal conditions prior to the three protocols individually (atropine, propranolol and increased ambient temperature) and when basal values for all three were combined (p values between 0.229 and 0.571).
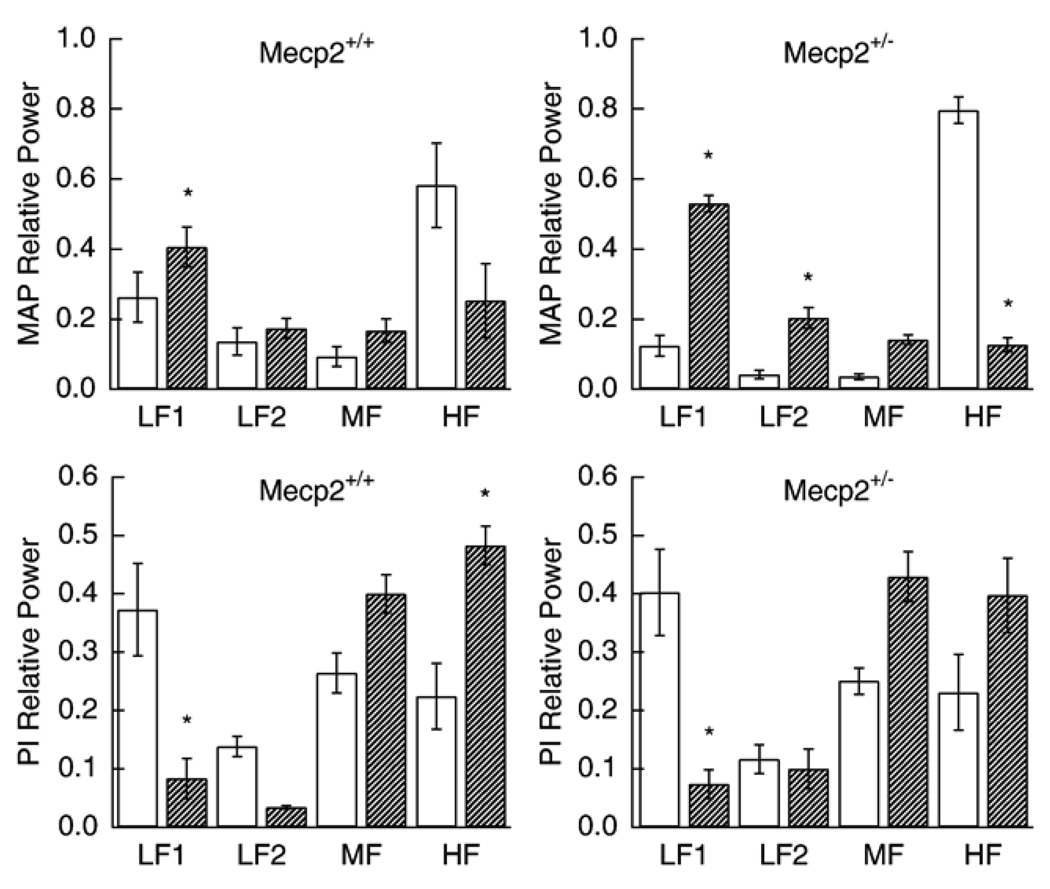
Fig. 1.
Effect of atropine on relative MAP spectral power (upper panels) for Mecp2+/+ and Mecp2+/− and relative PI spectral power (lower panels). Open bars are baseline and hatched bars are after administration of atropine. * significantly different from corresponding control (p values between <0.0001and 0.021).
3.4. Effects of atropine
Atropine caused an increase in heart rate in Mecp2+/− animals (Table 1). Heart rate was elevated by muscarinic blockade in Mecp2+/+ but not to a significant level. The indices of heart rate variability declined similarly in both groups. Muscarinic blockade did not significantly affect mean arterial pressure in either strain. Spectral power of MAP increased in the 0.08–0.4 Hz range and declined in the 3–10 range in both strains after atropine while that for PI fell in the 0.08–0.4 HZ and rose in 1–3 and 3–10 Hz ranges (Fig. 1).
3.5. Effects of propranolol
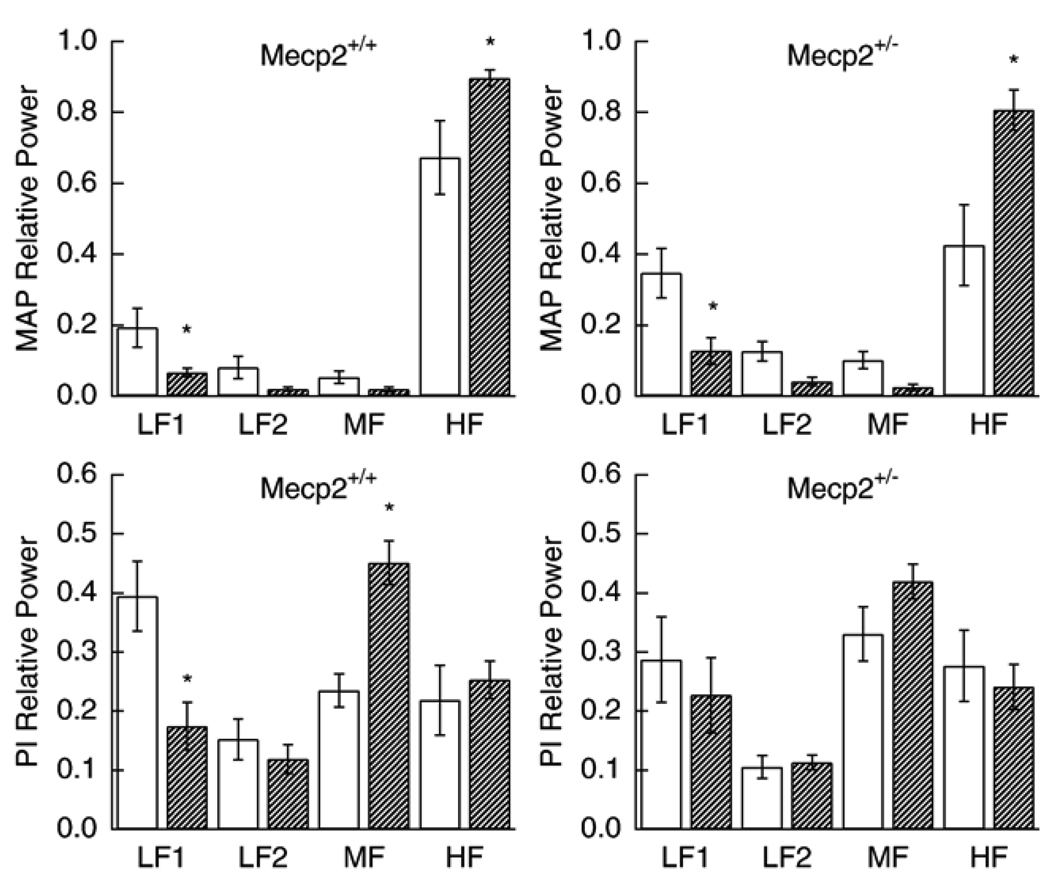
Propranolol lowered heart rate in both wild type and Mecp2 deficient mice but did not affect blood pressure. It caused a decrease in SDNN and PI90 while SDSD was not significantly lowered (Table 2). Adrenergic blockade lowered MAP spectral power in the 0.08–0.4 Hz frequency and increased it in the 3–10 Hz range. Propranolol decreased relative PI spectral power in the 0.08–0.4 Hz range and increased it in the 1–3 Hz frequency for Mecp2+/+ mice. The results were qualitatively the same in Mecp2+/− animals although the differences from control were not significant (Fig. 2).
Table 2.
Effect of propranolol on heart rate, mean arterial pressure and indices of heart rate variability
| Strain | Control heart rate (bpm) |
Propranolol heart rate (bpm) |
Control MAPa (mm Hg) |
Propranolol MAP (mm Hg) |
Control SDNNb (ms) |
Propranolol SDNN (ms) |
Control SDSDc (ms) |
Propranolol SDSD (ms) |
Control PI90d (ms) |
Propranolol PI90 (ms) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mecp2+/+ | 596 ± 24 | 506* ± 13 | 108 ± 3 | 111 ± 2 | 9.6 ± 1.7 | 4.0* ± 0.4 | 8.9 ± 2.1 | 5.1 ± 0.7 | 26.5 ± 2.1 | 12.1 ± 0.9* |
| Mecp2+/− | 620 ± 22 | 504* ± 25 | 114 ± 6 | 119 ± 5 | 7.4 ± 0.6 | 5.0* ± 0.9 | 8.5 ± 1.5 | 6.6 ± 1.5 | 24.3 ± 2.2 | 15.2 ± 2.6* |
Values are mean ± S.E. N = 7 for both strains.
significantly different from control, p values between 0.043 and 0.0002. There are no differences between strains.
mean arterial pressure;
standard deviation of pulse interval;
standard deviation of adjacent pulse intervals;
range encompassing 90% of pulse intervals.
Fig. 2.
Effect of propranolol on relative MAP spectral power (upper panels) for Mecp2+/+ and Mecp2+/− and relative PI spectral power (lower panels). Open bars are baseline and hatched bars are after administration of propranolol. * significantly different from corresponding control (p values between 0.0006 and 0.021).
3.6. Effects of increasing ambient temperature
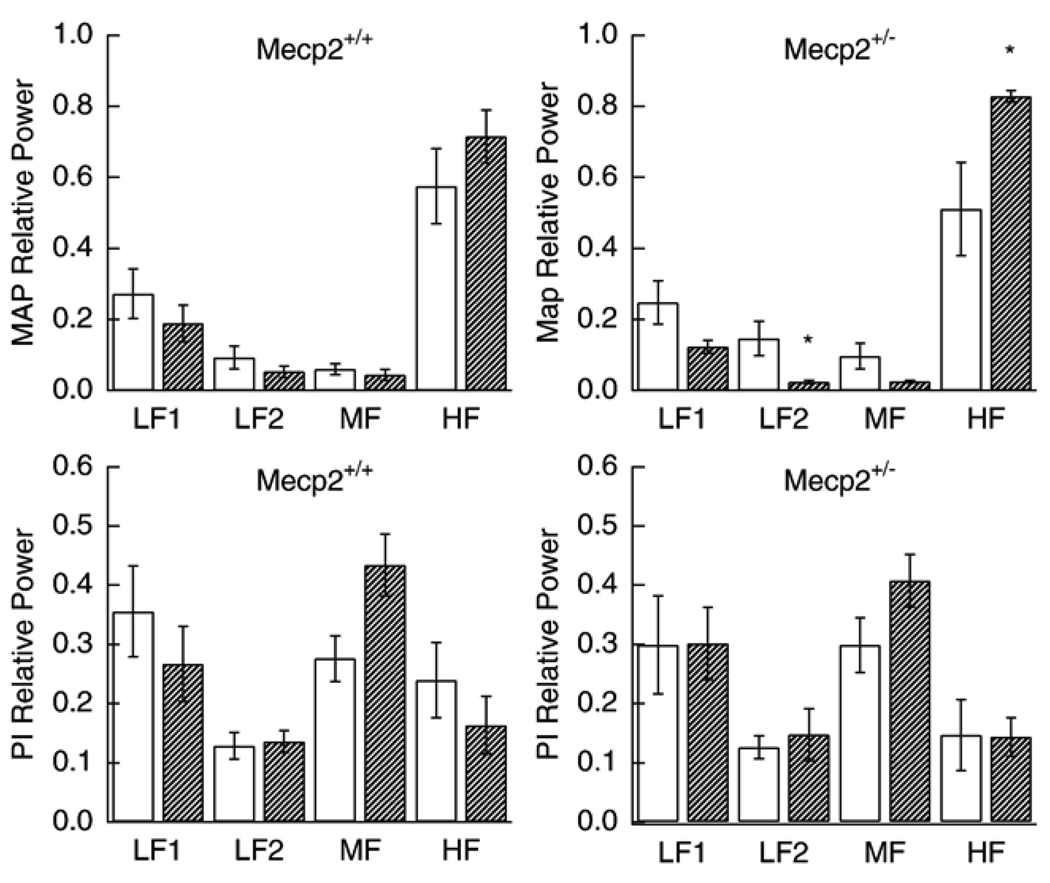
The increase in Ta from 23 °C to 32° caused a decrease in heart rate and that was similar for both strains. The fall in MAP was significant for Mecp2+/− (p = 0.013) but not for Mecp2+/+ (p = 0.203) (Table 3). Changes in spectral power associated with an increase in ambient temperature were similar to those seen with propranolol (Fig. 3). Relative power for MAP tended to decrease in the lower frequencies and increase in high frequency. The increase in the 3–10 Hz range was significant only for Mecp2 deficient animals (p = 0.005). PI spectral power tended to increase in the 1–3 Hz range and decrease in the 3–10 Hz range but these change were not significant.
Fig. 3.
Effect of elevating ambient temperature from 23 °C to 32 °C on relative MAP spectral power (upper panels) for Mecp2+/+ and Mecp2+/− and relative PI spectral power (lower panels). Open bars are baseline and hatched bars are after temperature elevation. * significantly different from corresponding control (p values between 0.005 and 0.03).
3.7. Baroreceptor functions
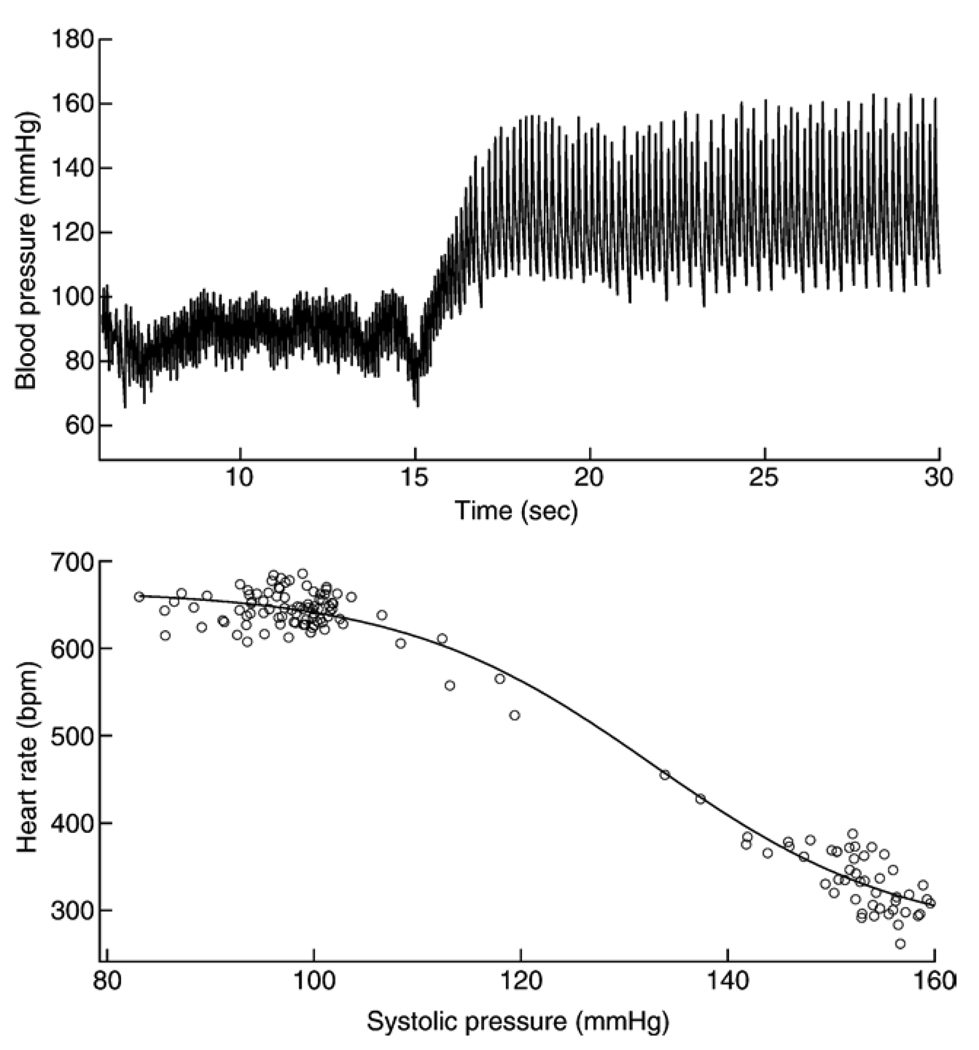
Intravenous injections of nitroprusside followed by phenylephrine resulted in rapid slowing of heart rate (Fig. 4). Maximum gain in the heart rate response to these pressure changes was the same in Mecp2+/− mice (13.3 ± 1.5 beats per minute/mm Hg) as it was in Mecp2+/+ (10.4 ± 1.6) (Table 4). The mean systolic pressure at mid-range (SBP50) was lower in Mecp2 deficient mice compared to wild type (Table 4).
Fig. 4.
Effect of phenylephrine induced increases in blood pressure on heart rate. Upper panel: representative trace of blood pressure before and following intravenous injection of phenylephrine after nitroprusside had been given to reduce pressure. Bottom panel: plot of heart rate as a function of systolic pressure obtained from the data in the trace above.
Table 4.
Baroreceptor function
| Strain | Gain (bpm/mm Hg) |
Heart rate range (bpm) |
SBP50 (mm Hg) |
Upper plateau (bpm) |
|---|---|---|---|---|
| Mecp2+/+ | 10.4 ± 1.6 | 242 ± 33* | 132 ± 5† | 560 ± 36 |
| Mecp2+/− | 13.3 ± 1.5 | 407 ± 43 | 116 ± 4 | 582 ± 31 |
Values are ± S.E. SBP = systolic pressure;
significantly less than Mecp2+/−(p = 0.016)
significantly greater than Mecp2+/− (p = 0.045).
4. Discussion
With the exception of some minor differences in spectral power following the administration of propranolol or exposure to an increase in ambient temperature these studies did not demonstrate significant differences between Mecp2+/− and Mecp2+/+ mice. The differences in power within some of the frequency bands were qualitative as the changes from baseline were always in the same direction for both strains (Figs. 1–3). They are more than offset by the observations that: 1) baseline values for mean arterial pressure, heart rate, indices of heart rate variability and spectral analysis of PI and MAP in the frequency domain were similar; 2) the response to an elevation in ambient temperature (Ta) was the same and 3) baroreceptor sensitivity was not different. Both strains increased MAP spectral power to the same extent in the lower frequencies after atropine was administered. This indicates similar sympathetic tone when parasympathetic is blocked. Since baroreceptor sensitivity was determined over a rapid time course (Fig. 4) the fall in heart rate with phenylephrine is primarily due to vagal output. Again no differences were found. Elevation in Ta is a useful tool in that both a decrease in sympathetic tone and an augmentation in vagal output are involved (Williams et al., 2003). Studies in which mice were given the β1-adrenoceptor blocker atenolol, during an elevation in Ta, found that heart rate and arterial pressure still decreased while SDNN and power in the HF band increased (Williams et al., 2003). As in those experiments the present studies found that HRV and PI spectral power in the 1–3 Hz range increased with elevated temperature, consistent with an increase in vagal tone. In addition MAP spectral power in the lower frequencies decreased indicative of a decline in sympathetic tone.
The increase in heart rate after atropine in these studies failed to reach significance in the wild type mice (Table 1). It is noted that the level attained (669 bpm) is the same as that in Mecp2 deficient animals (672 bpm). The differences lies in their respective baseline values prior to giving atropine, 620 for Mecp2+/+ and 586 for Mecp2+/−. As recently pointed out atropine in mice results in a significant increase in heart rate when baseline is in the lower ranges but not when it is high (Baudrie et al., 2007).
While the mice used in these studies (Guy et al., 2001) are heterozygous for loss of Mecp2 they may not have deficits exactly like patients with RTT. Recently, however, these mice have been further characterized (Guy et al., 2007). Their loss of mobility, abnormal gait, hindlimb clasping and breathing disturbances all resemble those seen in the clinical setting. We have considered two possibilities for the failure to find differences in autonomic cardiovascular regulation between this model for RTT and wild type mice: 1) mice with their high heart rates may be unsuitable for distinguishing either an increase in sympathetic or decrease in parasympathetic tone and, 2) studies in patients with Rett syndrome have not convincingly demonstrated a defect in autonomic cardiovascular regulation. The first possibility is unlikely because previous work involving a number of transgenic mouse strains has shown autonomic imbalance. Examination of these reports shows that if autonomic dysfunction is present it can be demonstrated in transgenic mice.
Over expression of atrial β1-adrenoceptors resulted in a decrease in HRV and less power in both LF and HF bands under basal conditions (Mansier et al., 1996). In addition these mice did not respond to propranolol with the increase in PI that was seen in wild types. Similarly enhancing β-adrenergic receptor signaling by cardiac-specific Gsα over expression caused a significant reduction in PI and a marked reduction in SDNN, accompanied by reductions in frequency domain power that was more marked in the LF band (Uechi et al., 1998). Reduced vagal modulation also decreases HRV in mice. When cardiac G protein βγ-subunits, that activate muscarinic inward rectifying potassium channels, are reduced SDNN falls significantly. These mice also had a reduction in baseline LF power and a blunted response to carbachol as well as decreased baroreceptor sensitivity (Gehrmann et al., 2002). The above references involve genetic modifications confined to the heart while Rett syndrome is considered to be caused by central nervous system deficiency of Mecp2 (Chen et al., 2001; Guy et al., 2001). Thus studies in mice with a α2A-adrenoceptor null mutation that includes the brainstem are of particular interest. This is underscored by the recent report that the content of norepinephrine in the medulla, but not the pons or forebrain, is decreased in Mecp2 deficient mice. Immunolabeling studies in these mice revealed a reduction in neurons that express tyrosine hydroxylase in both the A1/C1 and A2/C2 regions of the brainstem (Viemari et al., 2005). Mice lacking α2A-adrenoceptors showed a decreased HRV as judged by the variance in heart rate and a significantly blunted response to bolus injections of phenylephrine and nitroprusside (Niederhoffer et al., 2004). In contrast we did not find any differences in the indices of HRV between wild type and Mecp2 deficient mice and their baroreceptor sensitivity was the same. Given the results summarized above we conclude that if differences in autonomic regulation of cardiovascular function existed between Mecp2+/− and Mecp2+/+ animals, they should have been demonstrated in these experiments.
There have been a limited number of observations of autonomic cardiovascular regulation in patients with Rett syndrome using the indices that have been employed in animal studies. Johnsrude et al. (Johnsrude et al., 1995) appear to have been the first to describe abnormalities in children with the disorder. They performed 24 hour monitoring in 25 patients and 25 age-matched controls. Heart rates were not different. The standard deviation of RR intervals and the root mean square of successive differences were both decreased in Rett subjects compared to control (Johnsrude et al., 1995). These are accepted criteria for reduced heart rate variability (Task Force of the European Society of Cardiology, 1996). In addition they reported that power in the high frequency range was decreased in affected individuals. This preliminary report has not been published in detail. Julu et al. (1997) initially reported decreased vagal tone in 7 subjects compared to 9 age matched controls. In this and subsequent publications they used cardiac vagal tone (CVT) as the primary measure of autonomic function. This instrument consists of integrating R–R intervals from the electrocardiogram and feeding the output in parallel into a high-pass and a low-pass filter. The output of the low-pass filter drives a voltage-controlled oscillator (VCO) with a linear negative gradient; the output of the high-pass filter is further integrated and then allowed to drive a second VCO with a positive linear gradient. The outputs of the two VCOs are fed into a phase detector, which produces voltages proportional to the phase differences in its inputs. The voltages (0 to 1.0 V) are used to generate a linear scale of vagal tone (Little et al., 1999). Using incremental doses of atropine, in dogs, it has been shown that CVT declines in a manner that mirrors the fall in SDNN (Little et al., 1999). In the initial report CVT was 3.6 ± 0.7 (SEM) units in Rett syndrome subjects and 10.5 ± 0.9 in age matched controls. A second paper reported that CVT was 4.5 ± 0.4 in 48 RTT patients compared to 9.2 ± 1.2 in 11 controls. Resting heart rate and blood pressure were not different. Baroreceptor sensitivity was also determined from spontaneous blood pressure fluctuations and was less in RTT subjects (Julu et al., 2001). More recently Julu and Engerstrom (2005) have described 72 RTT cases who’s CVT was significantly less than that of the 11 control subjects reported earlier (Julu et al., 2001). It is unfortunate that only a small number of controls have been studied in these reports.
Guideri et al. (2001) have primarily used spectral analysis of R–R intervals in the frequency domain to evaluate autonomic function. 74 RTT subjects were compared to 40 age-matched controls. Individuals with RTT showed a significant decrease in total power compared to controls. Their decrease in LF power (48%) was considerably less than that seen in HF (85%). In contrast to the Julu and coauthors findings Guideri et al. (2001) report shorter R–R intervals for RTT subjects. Taken together these reports suggest that there may be some autonomic disturbances in cardiovascular function in RTT. It is unfortunate that HRV indices such as SDNN, PI90 and SDSD have not been examined in detail. Recently Weese-Meyer et al. (2006) measured heart rate in 47 RTT subjects age 2–7 years together with a like number of matched controls. The coefficient of variability for R–R intervals (10%) was the same in affected individuals as in normal subjects (Table 1 in supplemental material).
In summary, using pharmacological approaches, direct measure of baroreceptor function and the cardiovascular response to an elevation in ambient temperature we have been unable to clearly demonstrate disturbances in autonomic control in a mouse model of Rett syndrome.
Acknowledgements
The authors thank Veronique Devignot for genotyping mice and Judie McDonald for preparation of the manuscript. These studies were supported by HD 044453 from the NIH.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EO, Glaze DG, Zoghbi HY. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann. Neurol. 2000;47:670–679. [PubMed] [Google Scholar]
- Baudrie V, Laude D, Elghoz J-L. Optimal frequency ranges for extracting information on cardiovascular control from the blood pressure and pulse interval spectrograms in mice. Am. J. Physiol. 2007;292:R904–R912. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Meister M, Maguire CT, Martins DC, Hammer PE, Neer EJ, Berul CI, Mende U. Impaired parasympathetic heart rate control in mice with a reduction of functional G protein βγ-subunits. Am. J. Physiol. 2002;282:H445–H456. doi: 10.1152/ajpheart.00565.2001. [DOI] [PubMed] [Google Scholar]
- Glaze DG. Neurophysiology of Rett syndrome. J. Child Neurol. 2005;20:740–746. doi: 10.1177/08830738050200090801. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, DiPerri T, Zappella M, Hayek Y. Progressive cardiac dysautonomia observed in patients affected by classic Rett syndrome and not in the preserved speech variant. J. Child Neurol. 2001;16:370–373. doi: 10.1177/088307380101600512. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls, Rett’s syndrome, report of 35 cases. Ann. Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hoffbuhr KC, Moses LM, Jerdonek MA, Naidu S, Hoffman EP. Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:99–105. doi: 10.1002/mrdd.10026. [DOI] [PubMed] [Google Scholar]
- Janssen BJA, Leenders PJA, Smits JFM. Short-term and long-term blood pressure and heart rate variability in the mouse. Am. J. Physiol. 2000;278:R215–R225. doi: 10.1152/ajpregu.2000.278.1.R215. [DOI] [PubMed] [Google Scholar]
- Johnsrude C, Glase D, Schultz R, Friedman R. Prolonged QT intervals and diminished heart rate variability in patients with Rett syndrome. Pacing Clin. Electrophysiol. 1995;18:889. (Abstract). [Google Scholar]
- Julu PO, Engerstrom WittI. Assessment of the maturity-related brainstem functions reveals the heterogeneous phenotypes and facilitates clinical management of Rett syndrome. Brain Develop. 2005;27 Suppl 1:S43–S53. doi: 10.1016/j.braindev.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Hansen S, Apartopoulos F, Jamal GA. Functional evidence of brain stem immaturity in Rett syndrome. Eur. Child Adolesc. Psychiatry. 1997;6 Suppl 1:47–54. [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerstrom WittI, Engerstrom L, Jamal GA, Hansen S. Characterization of breathing and associated central autonomoc dysfunction in the Rett disorder. Arch. Dis. Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Armstrong DD, Prescott RJ, Doyle D, Kearney DL. Rett syndrome, analysis of deaths in the British survey. Eur. Child Adolesc. Psychiatry. 1997;6 Suppl 1:71–74. [PubMed] [Google Scholar]
- Little CJ, Julu PO, Hansen S, Reid SW. Real-time measurement of cardiac vagal tone in conscious dogs. Am. J. Physiol. 1999;276:H758–H765. doi: 10.1152/ajpheart.1999.276.2.H758. [DOI] [PubMed] [Google Scholar]
- Mansier P, Medigue C, Charlotte N, Vermeiren C, Coraboeuf E, Deroubai E, Ratner E, Chevalier B, Clairambault J, Carre F, Dahkli T, Bertin B, Briand P, Strosberg D, Swynghedauw B. Decreased heart rate variability in transgenic mice overexpressing atrial beta 1-adrenoceptors. Am. J. Physiol. 1996;271:H1465–H1472. doi: 10.1152/ajpheart.1996.271.4.H1465. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Hein L, Starke K. Modulation of the baroreceptor reflex by alpha 2A-adrenoceptors, a study in alpha 2A knockout mice. Br. J. Pharmacol. 2004;141:851–859. doi: 10.1038/sj.bjp.0705636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rett A. Cerebral atrophy associated with hyperammonaemia. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. North Holland: Amstersdam; 1977. pp. 305–329. [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Uechi M, Asai K, Osaka M, Smith A, Sato N, Wagner TE, Ishikawa Y, Hayakawa H, Vatner DE, Shannon RP, Homcy CJ, Vatner SF. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac Gsalpha. Circ. Res. 1998;82:416–423. doi: 10.1161/01.res.82.4.416. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation, breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin. Exp. Pharmacol. Physiol. 2003;30:769–778. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]