Abstract
The ability to extend the elbow following stroke depends on the magnitude and direction of torques acting at the shoulder. The mechanisms underlying this link remain unclear. The purpose of this study was to evaluate whether the effects of shoulder loading on elbow function were related to weakness or its distribution in the paretic limb. Ten subjects with longstanding hemiparesis performed movements with the arm either passively supported against gravity by an air bearing, or by activation of shoulder muscles. Isometric maximum voluntary torques at the elbow and shoulder were measured using a load cell. The speed and range of elbow extension movements were negatively impacted by actively supporting the paretic limb against gravity. However, the effects of gravity loading were not related to proximal weakness or abnormalities in the elbow flexor–extensor strength balance. The findings support the existence of abnormal descending motor commands that constrain the ability of stroke survivors to generate elbow extension torque in combination with abduction torque at the shoulder.
Keywords: gravity, kinematics, muscle weakness, reaching, stroke
The ability to adapt arm movements to external loads is critical for many activities of daily living. However, reaching movements performed by stroke survivors exhibit an extreme sensitivity to mechanical loading.2,30 With the weight of the arm supported by an external device, many stroke survivors are able to perform reaching movements with surprising skill.2,3 Yet gravity-loaded reaching movements are uncoordinated and severely limited in speed and range.2,19,20,25,27 Even a small force applied at the hand greatly impacts reaching distance in a parasagittal plane.30 Identification of the mechanisms that contribute to the load-dependence of reaching performance is central to the development of therapeutic approaches to decrease poststroke disability.
We recently compared rapid arm movements performed in the horizontal plane with the limb either supported against gravity by a low-friction air-bearing (passive support condition) or by volitional generation of abduction and external rotation torques at the shoulder (active support condition).2 The ability to extend the elbow was severely degraded with active limb support, even though there was no gravity torque about the elbow axis of rotation. This finding was consistent with our earlier isometric studies4,12 showing: (1) stroke survivors generated abnormally large elbow flexion torques during maximum voluntary shoulder abduction or external rotation tasks, and (2) elbow extension torque generation was facilitated by shoulder adduction and inhibited by shoulder abduction. We hypothesized that abnormal neural coupling between shoulder and elbow motoneuron pools was the primary mechanism underlying the effects of gravity loading on poststroke reaching.
Other studies have suggested that the effects of shoulder loading on extension movements of the elbow are related to muscle weakness rather than abnormal neural coupling. As the elbow is extended in the horizontal plane, the shoulder abduction torque required to support the arm against gravity increases progressively. Therefore, stroke survivors may limit the range of elbow extension as a compensatory response to weakness of the antigravity shoulder musculature.10,24 Differential weakness of the elbow musculature may also contribute to disturbances of actively supported reaching. Lum et al.23 found that the abnormal generation of elbow flexion torque during isometric shoulder tasks was related to an imbalance of strength between the paretic elbow flexors and extensors. Accordingly, coactivation of the elbow musculature during active limb support may diminish the speed and range of elbow extension, particularly in subjects with substantially greater weakness of elbow extensors than flexors.
Several studies have shown a significant correlation between the strength of upper-limb muscle groups and motor function.6,9,24 However, whether the load-dependence of reaching performance is related to weakness of specific muscle groups has not been previously addressed. The present study combined kinematic analysis of planar arm movements with isometric measurements of shoulder and elbow muscle strength in 10 subjects with longstanding hemiparesis. Our goal was to evaluate the impact of gravity loading on elbow extension movements and its relationship to weakness of the shoulder antigravity muscles and the balance of strength at the elbow.
MATERIALS AND METHODS
Subjects
Ten subjects with longstanding hemiparesis participated in this study. Demographic and clinical data for each subject are summarized in Table 1. The primary inclusion criterion was paresis of the upper extremity resulting from a first unilateral lesion of the cortex or subcortical white matter with an onset at least 1 year prior to data collection. Exclusion criteria were: (1) sensory deficits, atrophy, or contractures of the paretic upper limb; (2) cognitive or affective dysfunction that interfered with comprehension or completion of the protocols; (3) visual or visuoperceptual deficits that precluded torque feedback using a computer monitor or interfered with target localization for arm movements; and (4) severe concurrent medical problems (e.g., cardiorespiratory dysfunction). All subjects provided informed consent in accordance with the Declaration of Helsinki prior to participation in this study, which was approved by the Institutional Review Board of Northwestern University.
TABLE 1.
Clinical data for hemiparetic stroke subjects.
| Subject | Gender | Age | Lesioned hemisphere |
Years since onset |
Functional evaluation* |
EF/EE tone† |
|---|---|---|---|---|---|---|
| 1 | M | 80 | Left | 3.5 | 37 | 2/1 + |
| 2 | F | 51 | Left | 5.5 | 36 | 3/1 + |
| 3 | M | 61 | Right | 2 | 34 | 1/1 + |
| 4 | F | 43 | Right | 16 | 30 | 3/3 |
| 5 | M | 46 | Right | 13 | 27 | 3/2 |
| 6 | M | 59 | Right | 1.5 | 26 | 1/0 |
| 7 | M | 55 | Right | 5.5 | 23 | 3/0 |
| 8 | M | 41 | Left | 2 | 21 | 2/0 |
| 9 | M | 63 | Left | 4 | 18 | 2/1 |
| 10 | M | 55 | Right | 1 | 15 | 3/2 |
Based on arm portion of the Fugl-Meyer Motor Assessment (maximum score = 66).
Based on Modified Ashworth Scale. EF, elbow flexion; EE, elbow extension (0 = normal tone, 4 = rigid limb).
Clinical Evaluation
Motor function of the paretic upper extremity was evaluated using the arm motor portion of the Fugl–Meyer Motor Assessment.15 This assessment included the evaluation of tendon reflexes and voluntary movements performed within and out of the pathologic limb synergies.8,31 Possible scores range from 0 to a maximum of 66, which indicates no observable deficit. Muscle tone at the elbow was evaluated using the modified Ashworth scale,5 with 0 representing normal tone and 4 representing a rigid limb. The results of these clinical evaluations are summarized in Table 1.
Experimental Arrangement
A schematic of the arrangement used to examine voluntary arm movements is shown in Figure 1. The subject was seated in front of a large table with the trunk restrained by a set of straps and the wrist and finger joints immobilized using a fiberglass cast. The starting point for all movements was aligned with the mid-sagittal plane of the subject and located at a distance from the body that yielded an elbow angle of 80° (straight arm = 180°). Chair height was adjusted to yield a 75° shoulder abduction angle (humerus parallel to ground = 90°). Gravitational loads on the arm were resisted either passively by supporting the forearm on an air bearing that floated over the table surface (passive support condition) or actively by volitional generation of abduction and external rotation torques at the shoulder (active support condition). In the latter case the air bearing remained attached to the forearm to preserve mass properties across support conditions and the chair height was increased by 3 cm.
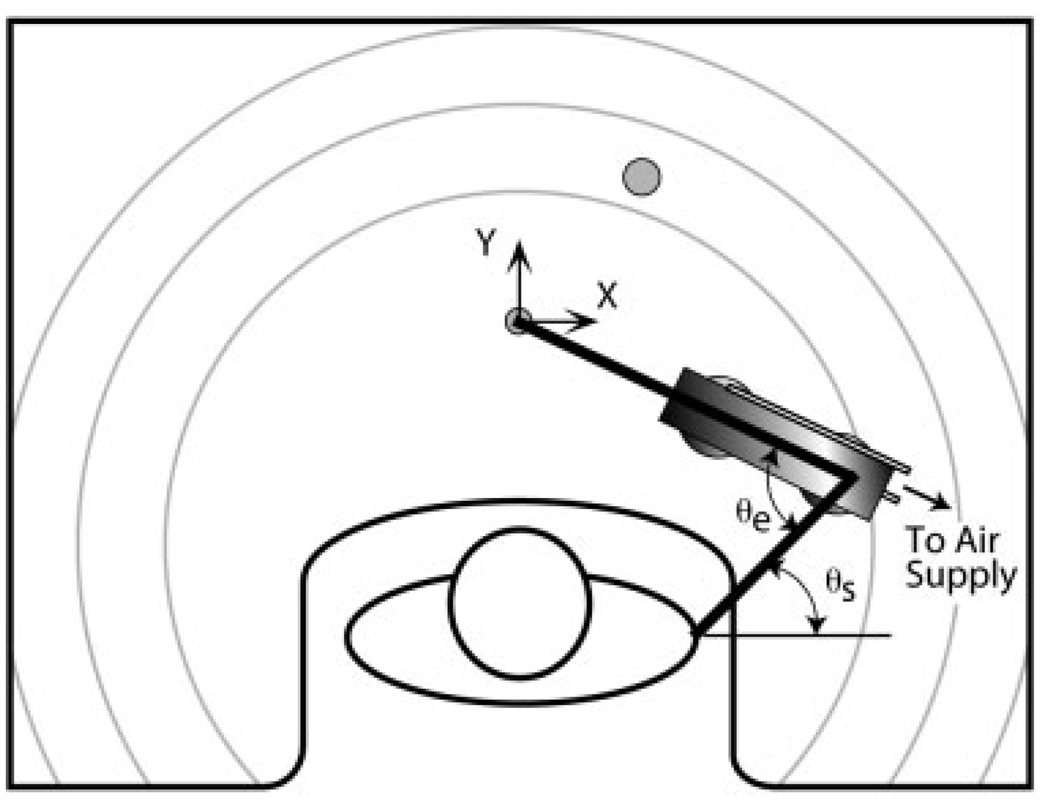
FIGURE 1.
Schematic of the experimental arrangement (viewed from above). Movements were performed with the forearm in a horizontal plane and the arm abducted to 75°. Subjects completed point-to-point and active range of motion protocols, with the arm either passively supported on a lightweight air-bearing device or actively supported by the shoulder musculature. The target (shaded circle) for point-to-point movements was located to require a 30° extension rotation at the elbow, without change in the elbow Cartesian position. The active range of motion protocol required subjects to trace out the largest possible area in front of their body. Circular arcs on the table surface were used to encourage achievement of maximum reaching distance. The positions of the hand, elbow, and shoulder were measured in Cartesian coordinates using an optoelectric system and converted off-line into the corresponding shoulder horizontal flexion/extension (θs) and elbow (θe) angles.
Kinematic data were collected using the Optotrak/3020 motion analysis system (Northern Digital Inc., Waterloo, Ontario, Canada). Infrared light-emitting diodes (IREDs) were placed above the acromial process of the scapula, in-line with the flexion/extension axis of rotation of the elbow,29 and on the cast above the tip of the ring finger. The position of each IRED was sampled at 300 Hz for point-to-point movements and at 100 Hz for the active range of motion protocol.
Movement Protocols
Subjects completed point-to-point and active range of motion (AROM) protocols. The protocols were completed with each arm in separate sessions spaced a few days apart, beginning with the nonparetic limb. A short training session was provided prior to data collection to familiarize the subject with the apparatus and procedures.
For the point-to-point protocol, circles designating the starting position and target were displayed on a computer monitor and projected onto the tabletop (see Fig. 1). The target was located to require a 30° extension rotation at the elbow, without change in the elbow Cartesian position. However, given the initial shoulder abduction angle of 75°, ∼8° of external rotation was required to maintain the forearm in a horizontal plane. Target diameter was set equal to 20% of the movement amplitude to emphasize open-loop control of the trajectory.33 At the beginning of each trial the subject aligned the distal IRED over the starting position. The appearance of the target circle served as a cue for the initiation of movement. The subject was instructed to move the distal marker to the target as rapidly as possible and to maintain the final position until disappearance of the target, which indicated the end of data collection. Movements in the two support conditions were performed in randomly ordered blocks of 10 trials. Actively supported trials were repeated if the arm contacted the table during movement.
The AROM protocol required the subject to trace out slowly the largest possible area in a horizontal plane in front of their body. To encourage subjects to achieve their maximum reaching distance, circular arcs were indicated on the table surface using tape. Data collection time ranged from 10 to 30 s, depending on the subject’s capability. Beginning with the passive support condition, 10 trials were performed for each support condition in randomized blocks consisting of five clockwise and five counterclockwise trials.
Measurement of Maximum Voluntary Torques
Maximum voluntary torques (MVTs) at the elbow and shoulder were measured isometrically with the limb in the starting configuration used for voluntary movements. Additionally, to investigate the sensitivity of MVTs to slight changes in limb configuration maximum torques were also measured with the tip of the ring finger aligned with the mid-sagittal plane and the elbow extended to 100°. The 100° elbow configuration resulted in an ∼10° increase in shoulder horizontal flexion, relative to the 80° elbow configuration, and a slight increase in shoulder external rotation. MVTs were measured in the 80° elbow configuration first, followed by a 10-min rest period during which the limb was repositioned in the 100° elbow configuration.
The measurement of MVTs followed the methods described in Dewald and Beer.12 The subject’s wrist and fingers were immobilized with a fiberglass cast. A cuff was placed around the cast at wrist level and mounted inside a metal ring attached to a 6 degrees of freedom (DOF) load cell (JR3 Inc., Woodland, California; Model 45E15A). Forces and moments measured at the wrist were converted online to respective elbow (flexion/extension) and shoulder (flexion/extension, abduction/adduction, and external/internal rotation) torques. MVTs were generated isometrically in four randomly ordered blocks consisting of elbow flexion/extension, shoulder flexion/extension, shoulder abduction/adduction, and shoulder external/internal rotation. Realtime feedback of the torque was provided by a dial gauge displayed on a computer monitor. Three successive MVTs were performed for each of the eight torque directions, with a 1-min rest period between trials to minimize fatigue. Additional trials were performed if the smallest peak torque for the three trials was less than 90% of the largest peak torque, until no increase in peak torque was observed. Verbal encouragement was provided throughout the protocol.
Subjects completed the isometric protocol with both upper limbs on consecutive days, beginning with the nonparetic limb. Typically, the movement and MVT protocols were completed within a 1-week period.
Data Analysis
Kinematics
The Cartesian positional data were digitally filtered using a fourth-order Butterworth filter with a forward and reverse pass to eliminate phase delays. Cutoff frequencies for the point-to-point data (typically 15–25 Hz) were determined using a residual analysis.34 A cutoff frequency of 5 Hz was used for the AROM data. To facilitate comparisons of left and right limbs, the sign of the x component of the positional data was reversed for movements performed with the left limb. The elbow extension angle (θe) and the shoulder motion in the horizontal plane (shoulder horizontal flexion/extension, θs) were calculated from the filtered Cartesian data. Velocities and accelerations in Cartesian and joint coordinates were obtained using numerical three-point differentiation.
Customized interactive software was used to identify the peak elbow angular acceleration and velocity and the initial and final elbow and shoulder angles for each point-to-point trial. Joint excursion was calculated as the difference between final and initial joint angles. For the AROM protocol, the 10 trials for each support condition were used to generate envelopes bounding angle–angle plots of elbow extension and shoulder horizontal flexion/extension. Visual inspection of the envelopes for the paretic limb suggested that elbow AROM was dependent on the shoulder horizontal flexion angle. To quantify this dependency, the envelopes were used to determine the maximum elbow extension AROM at shoulder horizontal flexion angles of 30° and 70°.
To characterize the impact of active limb support on elbow extension (for correlation with results of the MVT protocol) we used the following variables: (1) the ratio of mean peak elbow angular accelerations for actively and passively supported point-to-point movements, (2) the difference between elbow extension AROMs in the active and passive support conditions, and (3) the elbow extension AROM in the active support condition. The latter variable was equivalent to considering the difference between the elbow extension AROM in the active support condition and a straight arm configuration. The elbow extension AROMs used for correlation analysis corresponded to the shoulder horizontal flexion angle measured during isometric testing with the elbow extended to 100°.
Maximum Voluntary Torques
Torque signals were smoothed with a 250-ms moving-average filter. For each trial the peak torque in each of the four DOFs was identified. MVT for each direction was taken as the maximum peak torque across all trials.
Residual strength (RS, the ratio of MVTs for the paretic and nonparetic limbs for a given torque direction) was used as a relative measure of weakness in elbow extension, shoulder abduction, and external rotation. Additionally, the strength balance (SB) in the elbow flexion/extension DOF was quantified as the difference between elbow extension and flexion MVTs, normalized by the torque range (sum of elbow extension and flexion MVTs). The latter parameter, based on Lum et al.,23 ranged between 1 and −1, with a positive value indicating greater MVT in the elbow extension direction.
The magnitude of the shoulder postural torque required to support the arm against gravity, relative to MVT, may also be an important factor in post-stroke upper-limb function.10 For each subject the shoulder abduction and external rotation torques required for postural support were determined as a function of elbow angle based on the estimated mass properties of the limb.37 The mass properties of the forearm were adjusted to include the air bearing and cast. Normalized MVT (in abduction and external rotation) was calculated as the ratio of the MVT and the torque required to support the limb in a given configuration.
Statistical Analysis
Differences in elbow peak angular acceleration, elbow peak angular velocity, and shoulder and elbow joint excursions due to Limb (paretic vs. nonparetic) and Support Condition (active vs. passive support) were evaluated using separate repeated measures analyses of variance (ANOVAs). Similarly, the isometric MVT and SB data were analyzed using two-factor repeated measures ANOVA, with Limb and Configuration (80 vs. 100° elbow angle) as independent variables. To improve sphericity, peak angular acceleration, peak angular velocity, and MVTs were transformed logarithmically prior to analysis. A three-factor (Limb, Support Condition, Shoulder Angle [30° vs. 70° horizontal flexion]) repeated measures ANOVA was performed for the elbow extension AROM data. Post-hoc comparisons were made using the Scheffe test.
Relationships between movement variables and strength-related parameters were evaluated using the Pearson product-moment correlation coefficient. Peak elbow acceleration typically occurred within the first 5° of movement. Therefore, correlations involving this parameter were based on MVT data for the 80° elbow position. With active support of the paretic limb, the group mean elbow extension AROM was 115°. Therefore, correlations between elbow extension AROM (or change in AROM) and strength parameters were based on MVT data for the more extended (100°) elbow position.
The significance level was set at P < 0.05 for all statistical analyses, which were performed using DataDesk (Data Description, Ithaca, New York).
RESULTS
Impact of Gravity on Reaching Performance
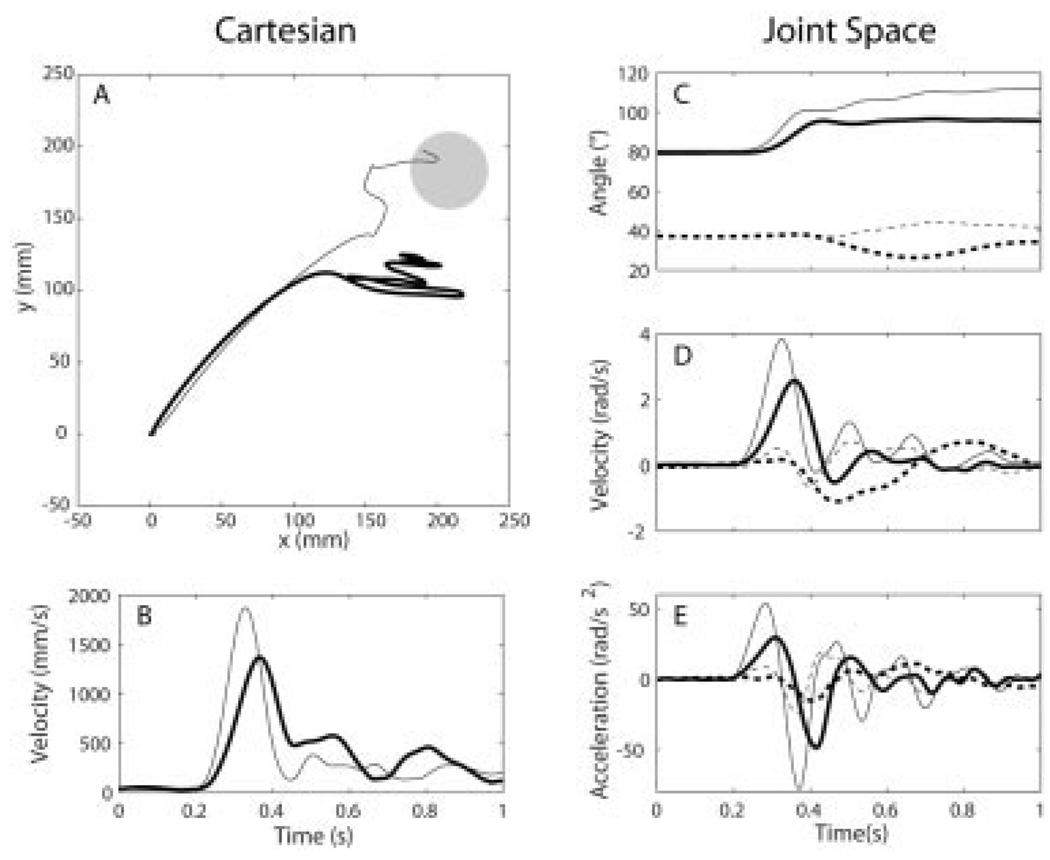
Figure 2 provides a representative comparison of point-to-point movements of the paretic limb performed in the active and passive support conditions. Group results for the dependent variables (elbow peak angular acceleration and velocity and excursions at the elbow and shoulder) are summarized in Table 2. With the exception of the shoulder excursion, statistical analysis (ANOVA) indicated a significant main effect of limb on each variable, which reflected reduced values of the parameters for the paretic elbow. Additionally, for each variable there was a significant interaction of limb and support conditions, which reflected the support-dependent movement performance of the paretic limb.
FIGURE 2.
Cartesian and joint kinematics for actively (thick lines) and passively (thin lines) supported point-to-point movements of the paretic limb. Mean hand paths and tangential velocity profiles are shown in (A) and (B), respectively. Mean joint angle, angular velocity, and angular acceleration profiles are shown for the elbow (solid lines) and shoulder (dashed lines) in (C–E). Trials were aligned at movement onset (at 0.2 s in (B–E)) for averaging. Positive ordinates correspond to elbow extension and shoulder horizontal flexion. Data is for subject 5 (see Table 1).
TABLE 2.
Mean (SEM) values of kinematic variables as a function of limb and support condition.
| Nonparetic |
Paretic |
|||
|---|---|---|---|---|
| Passive | Active | Passive | Active | |
| Point-to-Point Protocol | ||||
| Elbow peak velocity (rad/s) | 6.1(0.5) | 5.7(0.4) | 2.8(0.5) | 1.2 (0.3)† |
| Elbow peak acceleration (rad/s2) | 89.8(10.6) | 94.2(11.0) | 35.3(7.0) | 14.6(3.7)† |
| Elbow excursion (°) | 29.8(0.8) | 30.7(0.8) | 25.8(2.0) | 20.4(3.1)* |
| Shoulder excursion (°) | 0.3(0.4) | 0.7(0.3) | 2.2(1.6) | −1.4(1.6)* |
| AROM Protocol | ||||
| Elbow AROM, θs= 30° (°) | 170.5(1.3) | 172.9(1.7) | 131.2(6.5) | 102.9 (4.8)§ |
| Elbow AROM, θs= 70° (°) | 172.5(0.9) | 172.9(1.6) | 146.4(5.0) | 116.9 (6.1)§ |
Asterisks indicate a significant difference compared to passive support condition based on Scheffe test;
P < 0.05,
P < 0.001,
P < 0.0001
Active support of the paretic limb against gravity resulted in significant reductions in the peak angular acceleration (Scheffe, P < 0.001) and angular velocity (Scheffe, P < 0.001) of elbow extension (Table 2). On a group basis, both parameters were reduced 51% with active limb support, relative to the passively supported condition. On an individual basis, the reductions in peak angular acceleration ranged from 10% to 90%, and reductions in peak angular velocity ranged from 13% to 93%. Peak angular accelerations and angular velocities for the nonparetic elbow were not significantly different between support conditions.
The excursion for the paretic elbow (Table 2) was reduced with active support of the limb (Scheffe, P < 0.05). Although 9 of 10 subjects acquired the target in the passive support condition, only 4 subjects acquired the target during actively supported movement. Additionally, there was a small (3.6°), but significant (Scheffe, P < 0.01), shift in shoulder excursion toward horizontal extension with active limb support. However, group mean shoulder excursions for the paretic limb were not significantly different from zero (t-test) in either support condition. Inspection of hand paths indicated that the shift toward horizontal extension may have been a compensatory response to inadequate elbow extension in the active support condition. Shoulder horizontal extension resulted in an approximately lateral movement of the hand that served to decrease the distance between the hand and target (see Fig. 2A). Joint excursions for the nonparetic limb were not significantly different in the actively and passively supported conditions and were consistent with the target excursion values of 30° and 0° at the elbow and shoulder, respectively.
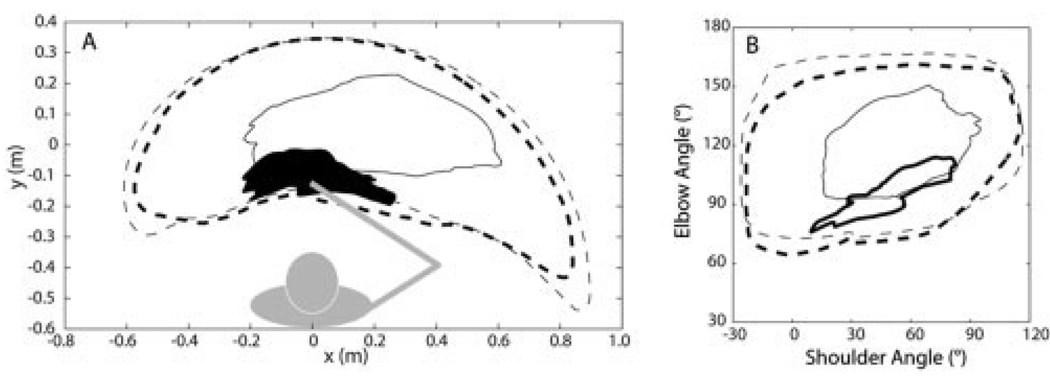
Figure 3 shows typical envelopes for the active range of motion protocol. In contrast to the nonparetic limb, the work area for the paretic limb was severely contracted and support-dependent (Fig. 3A). AROM was reduced at both joints (Fig. 3B). Group results for elbow extension AROM are summarized in Table 2. Relative to the nonparetic elbow, AROM for the paretic elbow was reduced across the conditions examined (mean difference = 47.8°, limb, F(1,9) = 100.18, P < 0.0001). This deficit was exacerbated by active support of the paretic limb against gravity (mean effect = 28.9°, limb*support condition, F(1,9) = 52.5, P < 0.0001), and partially mitigated by positioning of the shoulder in a more flexed position (mean effect = 14.6°, limb*shoulder angle, F(1,9) = 16.2, P = 0.003).
FIGURE 3.
Typical results for the range of motion protocol for the paretic (solid lines) and nonparetic (dotted lines) limbs. (A) Work area of the hand with active (thick lines) and passive (thin lines) support of the arm against gravity. The work area of the paretic limb with active support is shaded for clarity. Underlying shoulder and elbow motions are shown in (B), with a 180° elbow angle representing a straight limb and positive values of shoulder angle indicating a flexed position of the upper arm segment in the horizontal plane. The plotted data represent envelopes derived from 10 trials performed by subject 7.
Relationships with Shoulder Weakness
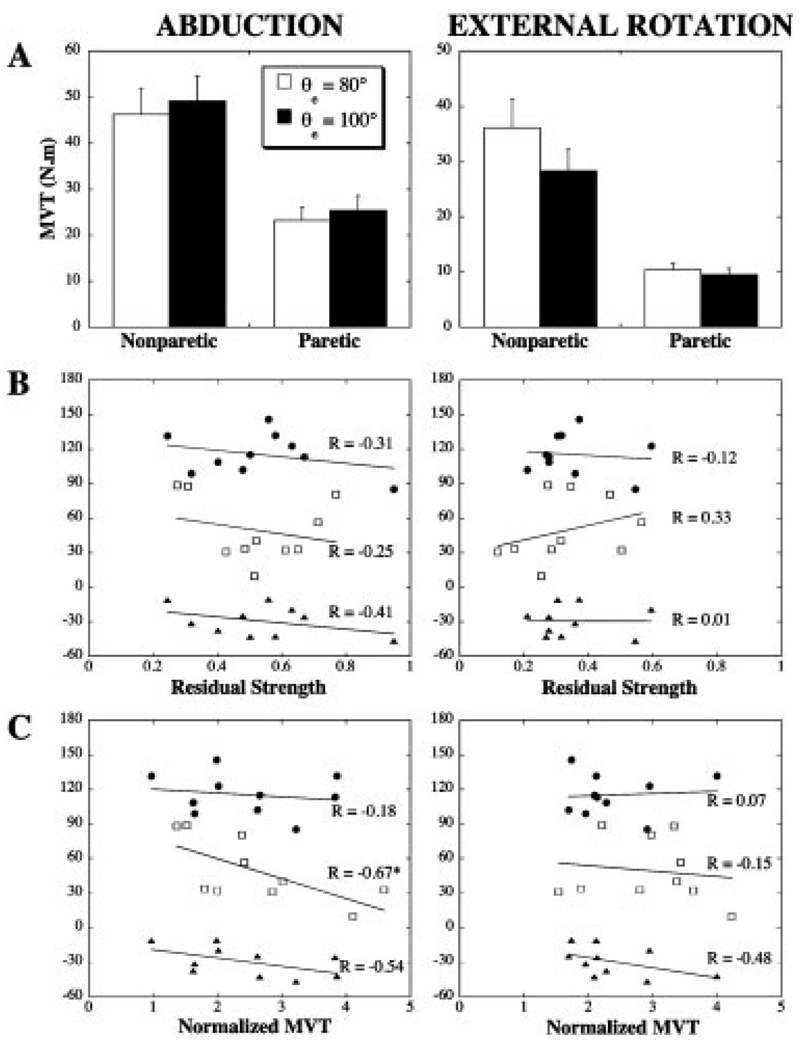
Abduction and external rotation MVTs for the two limb configurations are summarized in Figure 4A. Significant weakness was present in both paretic muscle groups (limb: F(1,9) = 38.6, P = 0.0002 and F(1,9) = 87.8, P < 0.0001, for shoulder abduction and external rotation, respectively). These results were associated with abduction and external rotation RSs of 0.53 and 0.33, respectively. For both limbs, there was a trend toward increased abduction MVT (Configuration: F(1,9) = 3.9, P = 0.08) and decreased external rotation MVT (F(1, 9) = 20.4, P = 0.002) for the more extended elbow position. However, group differences in MVTs for the two limb configurations were generally less than 10% and the Limb*Configuration interaction was not significant.
FIGURE 4.
Proximal weakness and its relationship to deficits in actively supported elbow extension. (A) Maximum voluntary abduction and external rotation torques (group mean with SEM). (B) Correlational analyses between movement parameters and residual strength. (C) Correlational analyses between movement parameters and normalized abduction and external rotation MVTs (quotient of the MVT and the corresponding torque required to support the arm against gravity). *Significant correlation, P< 0.05. ● Extension AROM, active support condition (°); □ normalized peak elbow acceleration (%); ▲ change in AROM with active support (°).
There was no evidence that the effects of active limb support on elbow extension were related to residual abduction or external rotation strength (Fig. 4B). Active support of the paretic limb required a higher percentage of MVT, relative to the nonparetic limb. However, as illustrated by Figure 4C there was no evidence that the effects of active limb support on elbow extension were more severe in subjects with lower abduction or external rotation MVTs, relative to the required postural torques. To the contrary, there was a significant (P < 0.05) negative relationship between normalized acceleration and normalized abduction MVT. Furthermore, all but one subject appeared to have sufficient abduction strength (based on the MVT measured for θe = 100°) to support the arm in a straight configuration (group mean normalized abduction torque at full elbow extension = 1.71; range: 0.69–2.72).
Relationships with Elbow Weakness
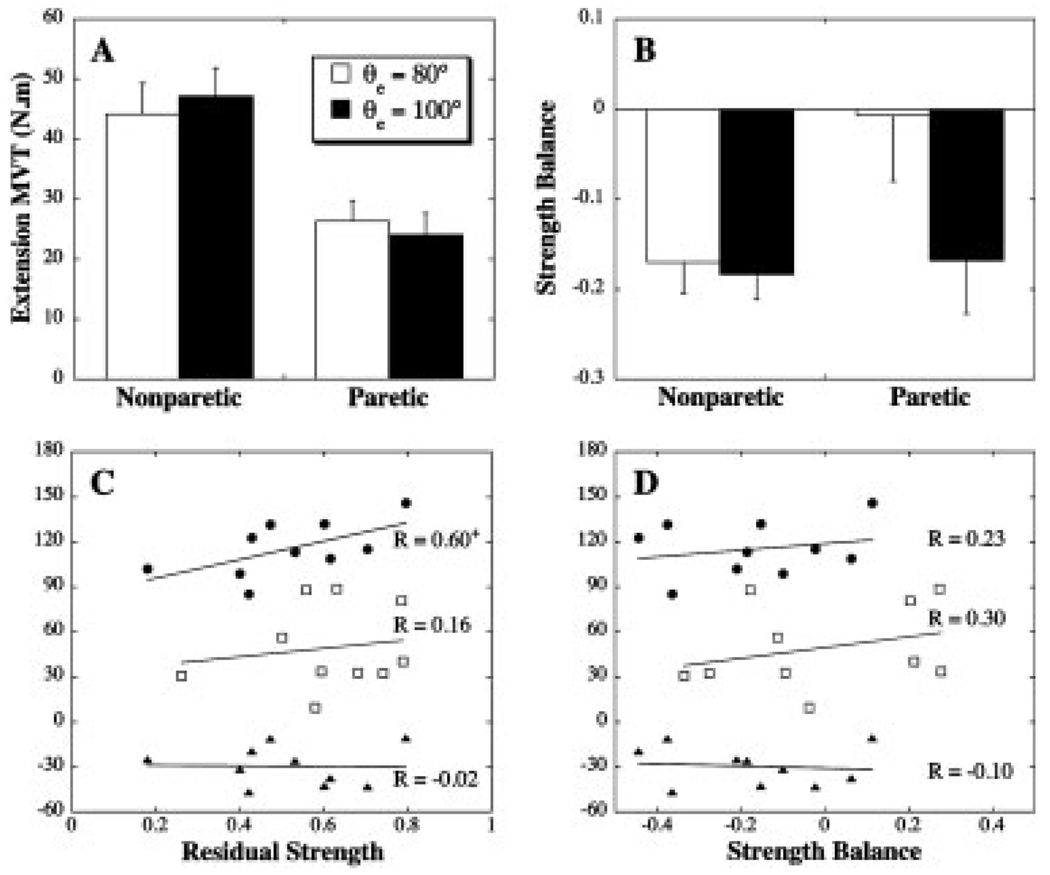
Elbow extension MVTs for the two limb configurations are summarized in Figure 5A. Significant weakness (mean RS = 0.56) was present in the elbow extensors (limb: F(1,9) = 31.08, P = 0.0003). Additionally, there was a significant interaction of limb and configuration (F(1,9) = 9.3, P = 0.0003). The more extended elbow position was associated with a slight (<10%) increase in elbow extension MVT for the nonparetic limb, whereas a slight decrease was evident for the paretic limb. As illustrated in Figure 5C, there was a marginally significant (P = 0.07) relationship between elbow extension RS and AROM in the active support condition. Relationships between elbow extension RS and normalized acceleration or change in AROM were not significant.
FIGURE 5.
(A) Elbow extension MVT (group mean with SEM). (B) Elbow strength balance (SB) (group mean with SEM). A positive SB represents a larger MVT in extension, compared to flexion. Correlational analyses between movement parameters and elbow extension residual strength (C) and elbow strength balance (D)+Marginally significant correlation, P= 0.07. ● Extension AROM, active support condition (°); □ normalized peak elbow acceleration (%); ▲ change in AROM with active support (°).
Figure 5B summarizes the strength balance at the elbow, with a positive value representing greater strength in extension than flexion. Torque generation was generally biased toward the flexion direction. However, the variation of SB with elbow angle was limb-dependent (limb*configuration, (F(1,9) = 6.88; P = 0.03). Post-hoc comparisons (Scheffe tests) indicated that relative to the nonparetic elbow the mean SB for the paretic elbow was shifted toward extension for the starting configuration used for movements (θe= 80°). Interlimb differences in SB for the more extended elbow position were not significant. Gravity-related deficits in elbow extension acceleration or AROM were not related to the strength balance at the elbow (Fig. 5D).
DISCUSSION
We found no evidence that the negative impact of external (gravity) loading on poststroke reaching was related to proximal weakness or the balance of strength at the elbow. Although elbow extension RS and actively supported AROM were positively correlated, our kinematic findings are also not attributable to extensor weakness. Movements were performed with the arm abducted to 75° so that gravitational forces provided a slight (<1 Nm) assistive torque to elbow extension. Yet active support of the limb against gravity diminished the range and speed of elbow extension movements.
The influence of external loads on poststroke arm function has received surprisingly little attention in the literature. Takahashi and Reinkensmeyer30 found that maximum reaching distance in a parasagittal plane was greater when an external force applied at the hand was directed laterally, rather than medially. In an earlier study2 we found that gravity loading of the paretic shoulder had a greater impact on reaching than on movements made toward the body (e.g., elbow flexion).
Our findings point to the existence of abnormal neural constraints on upper-limb muscle activation patterns following stroke. Obligatory coupling between the activations of shoulder and elbow motor neuron pools may result from upregulation of diffusely projecting cortico-bulbospinal pathways following damage to the corticospinal tract. In particular, there appears to be a strong coupling of elbow flexor and extensor activation with activation of the shoulder abductors–external rotators and adductors–internal rotators, respectively.4,7,8,12,13,31 A limited capacity to activate muscles outside of these patterns would explain our kinematic findings as well as the differential effects of medial and lateral loads on movements performed in the parasagittal plane.30 Additional indirect support for the existence of abnormal neural constraints following stroke is provided by Zackowski et al.,36 who found that abnormal coupling between movements of the shoulder, elbow, and wrist was not related to sensation, spasticity, or strength (although a positive trend was observed for the latter factor).
There are additional mechanisms that may have contributed to the effects of gravity loading on post-stroke reaching. Multijoint muscles provide a mechanical linkage between the shoulder and elbow. The role of biceps brachii at the shoulder is unsettled,16,22,28,35 but there is some evidence that both heads contribute actively to shoulder flexion and abduction.16,28 Given that our subjects exhibited substantial deficits in elbow extension AROM in the passively supported condition, “normal” activation of the biceps in conjunction with active limb support should further reduce elbow extension AROM. Abnormal stretch reflex–mediated activation of the elbow flexors may have contributed to the limited range and speed of point-to-point elbow extension movements.21 Stretch amplitude and velocity were reduced in the actively supported condition. However, it is currently unclear how external loading affects stretch reflex threshold regulation in the paretic limb.
Even with passive support of the limb’s weight, none of our subjects were able to achieve full extension of the elbow. The reduction in AROM may be partially attributable to an increase in passive joint stiffness due to changes in the mechanical properties of muscle and tendon. However, evidence for this mechanism at the elbow is contradictory.17,26 The dependence of paretic elbow extension AROM on shoulder position was similar for both support conditions, with greater AROM observed for the more flexed shoulder position. This effect is potentially mitigated by the short head of biceps brachii and the long head of triceps brachii, both of which cross the shoulder, and are shortened and lengthened, respectively, by horizontal flexion of the shoulder.
Several limitations of the present study should be noted. The small sample size and use of convenience sampling prevent generalization of our findings to the broader population of stroke survivors. Additionally, our sample group did not include stroke survivors with severe shoulder weakness (RS < 0.2) or an extreme strength imbalance at the elbow (SB < − 0.5). Clearly, a certain level of strength is required to support the fully extended arm against gravity, and beyond a certain threshold a strength imbalance at the elbow may influence gravity-loaded reaching. However, our study demonstrates the existence of a subset of stroke survivors for which the impact of gravity loading on reaching cannot be attributed to weakness or its distribution in the paretic limb.
Implications for Treatment
The potential relationship between elbow extension RS and AROM in the active support condition, if confirmed by subsequent studies, suggests that simple strength training of the elbow extensors may enhance reaching extent. However, as noted earlier, elbow extensor weakness is clearly not the primary mechanism underlying our kinematic findings. A more efficient strategy may be to target the abnormal constraints on joint torque patterns. Importantly, we have demonstrated that even severely impaired stroke survivors retain the ability to isometrically generate elbow extension torque simultaneously with low (less than required to support the limb against gravity) levels of shoulder abduction torque.4 This residual capacity can be enhanced with a targeted therapy.14 Whether gains under isometric conditions translate into improved reaching remains to be determined. It may prove more beneficial to develop dynamic training protocols that allow subjects to gradually integrate active support of the limb with control of voluntary movement, in a manner similar to partial body weight support gait training.1,18,32 Such a study involving subjects with longstanding hemiparesis is currently under way in our laboratory. However, this approach may be particularly important during the acute and subacute phases of stroke recovery to prevent patients from adopting compensatory strategies for reaching that may ultimately limit recovery of elbow extension.11
Acknowledgments
We thank the National Institutes of Health (RO1 HD39343) and the National Institute on Disability and Rehabilitation Research (H133G980063) for support of this research.
Abbreviations
- ANOVA
analysis of variance
- AROM
active range of motion
- DOF
degree of freedom
- EE
elbow extension
- IRED
infrared light-emitting diode
- MVT
maximum voluntary torque
- RS
residual strength
- SB
strength balance
REFERENCES
- 1.Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84:1458–1465. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 2.Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 3.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 4.Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- 5.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon RW, Warren ME, Cogman KA. Motor variables correlated with the hand-to-mouth maneuver in stroke patients. Arch Phys Med Rehabil. 1991;72:682–684. [PubMed] [Google Scholar]
- 7.Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- 8.Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. New York: Harper & Row; 1970. [Google Scholar]
- 9.Canning CG, Ada L, Adams R, O’Dwyer NJ. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin Rehabil. 2004;18:300–308. doi: 10.1191/0269215504cr715oa. [DOI] [PubMed] [Google Scholar]
- 10.Carr J, Sheperd R. Neurological rehabilitation: optimizing motor performance. Oxford: Butterworth-Heinemann; 1998. [Google Scholar]
- 11.Cristea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 12.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Dewald JPA, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivational patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 14.Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JPA. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32:170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 16.Furlani J. Electromyographic study of the m. biceps brachii in movements at the glenohumeral joint. Acta Anat (Basel) 1976;96:270–284. doi: 10.1159/000144679. [DOI] [PubMed] [Google Scholar]
- 17.Given JD, Dewald JPA, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Neurol Neurosurg Psychiatry. 1995;59:271–279. doi: 10.1136/jnnp.59.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesse S, Konrad M, Uhlenbrock D. Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Arch Phys Med Rehabil. 1999;80:421–427. doi: 10.1016/s0003-9993(99)90279-4. [DOI] [PubMed] [Google Scholar]
- 19.Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83:702–707. doi: 10.1053/apmr.2002.32446. [DOI] [PubMed] [Google Scholar]
- 20.Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- 21.Levin MF, Feldman AG. The role of stretch reflex threshold regulation in normal and impaired motor control. Brain Res. 1994;657:23–30. doi: 10.1016/0006-8993(94)90949-0. [DOI] [PubMed] [Google Scholar]
- 22.Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10:250–255. doi: 10.1067/mse.2001.113087. [DOI] [PubMed] [Google Scholar]
- 23.Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- 24.Mercier C, Bourbonnais D. Relative shoulder flexor and handgrip strength is related to upper limb function after stroke. Clin Rehabil. 2004;18:215–221. doi: 10.1191/0269215504cr724oa. [DOI] [PubMed] [Google Scholar]
- 25.Reinkensmeyer DJ, McKenna Cole A, Kahn LE, Kamper DG. Directional control of reaching is preserved following mild/ moderate stroke and stochastically constrained following severe stroke. Exp Brain Res. 2002;143:525–530. doi: 10.1007/s00221-002-1055-3. [DOI] [PubMed] [Google Scholar]
- 26.Reinkensmeyer DJ, Schmit BD, Rymer WZ. Assessment of active and passive restraint during guided reaching after chronic brain injury. Ann Biomed Eng. 1999;27:805–814. doi: 10.1114/1.233. [DOI] [PubMed] [Google Scholar]
- 27.Roby-Brami A, Fuchs S, Mokhtari M, Bussel B. Reaching and grasping strategies in hemiparetic patients. Mot Control. 1997;1:72–91. [Google Scholar]
- 28.Sakurai G, Ozaki J, Tomita Y, Nishimoto K, Tamai S. Electromyographic analysis of shoulder joint function of the biceps brachii muscle during isometric contraction. Clin Orthop Relat Res. 1998;354:123–131. doi: 10.1097/00003086-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HWJ. Geometry of the humeroulnar joint. J Orthop Res. 1988;6:897–906. doi: 10.1002/jor.1100060614. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi CD, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. 2003;149:131–140. doi: 10.1007/s00221-002-1340-1. [DOI] [PubMed] [Google Scholar]
- 31.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 32.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29:1122–1128. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 33.Wallace SA, Newell KM. Visual control of discrete aiming movements. Q J Exp Psychol A. 1983;35:311–321. doi: 10.1080/14640748308402136. [DOI] [PubMed] [Google Scholar]
- 34.Winter DA. Biomechanics and motor control of human movement. New York: John Wiley & Sons; 1990. [Google Scholar]
- 35.Yamaguchi K, Riew KD, Galatz LM, Syme JA, Neviaser RJ. Biceps activity during shoulder motion: an electromyographic analysis. Clin Orthop Relat Res. 1997;336:122–129. doi: 10.1097/00003086-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 37.Zatsiorsky V, Seluyanov V. Estimation of the mass and inertia characteristics of the human body by means of the best predictive regression equations. In: DA Winter, RW Norman, RP Wells, KC Hayes, AE Patla., editors. Biomechanics IX-B. Champaign, IL: Human Kinetics; 1985. pp. 233–239. [Google Scholar]