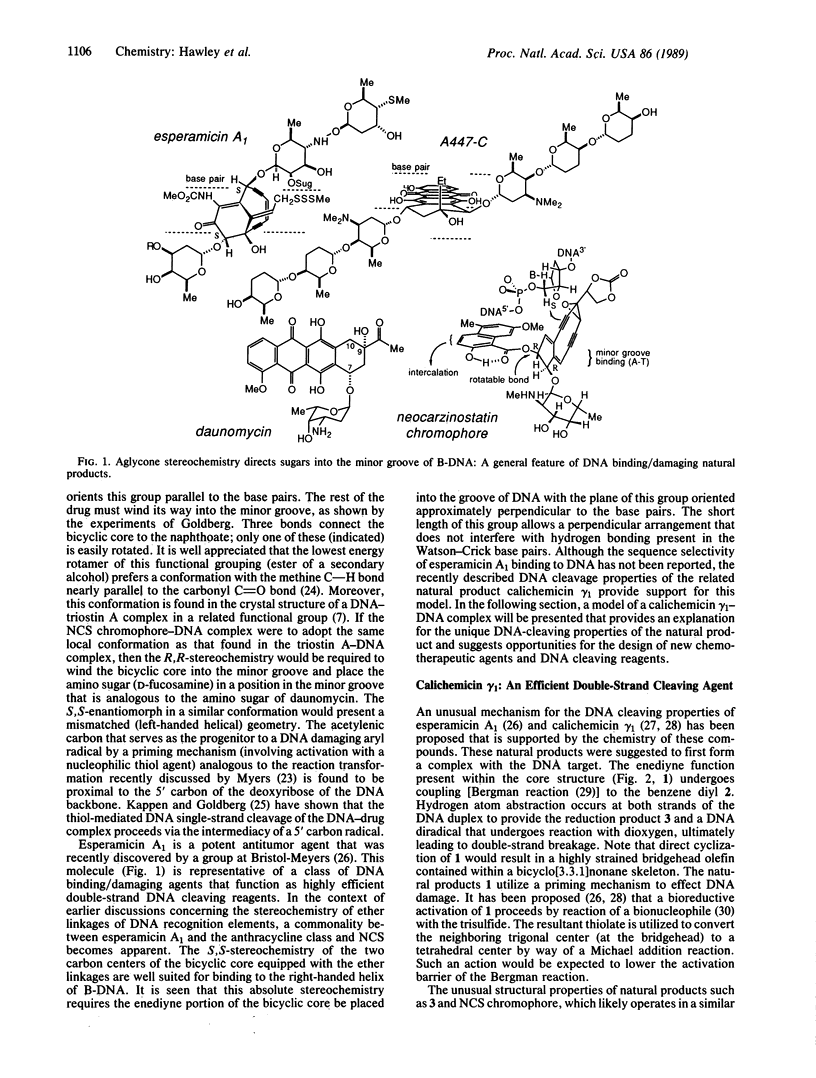
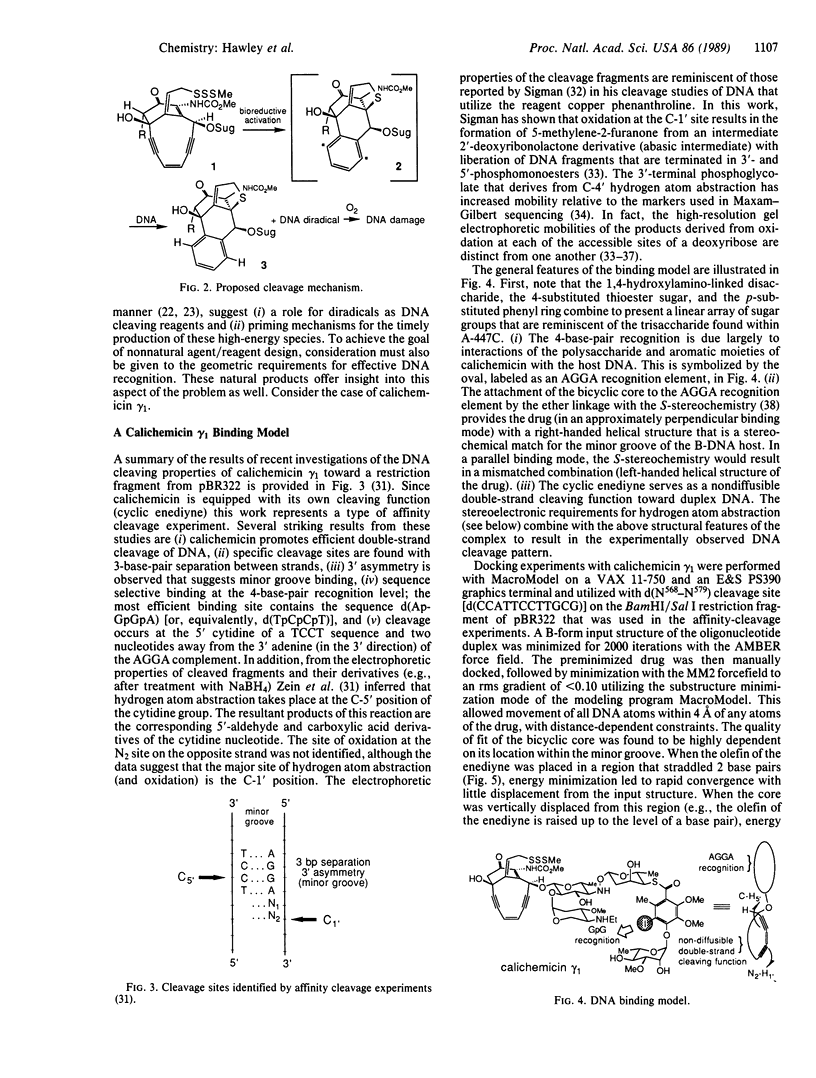
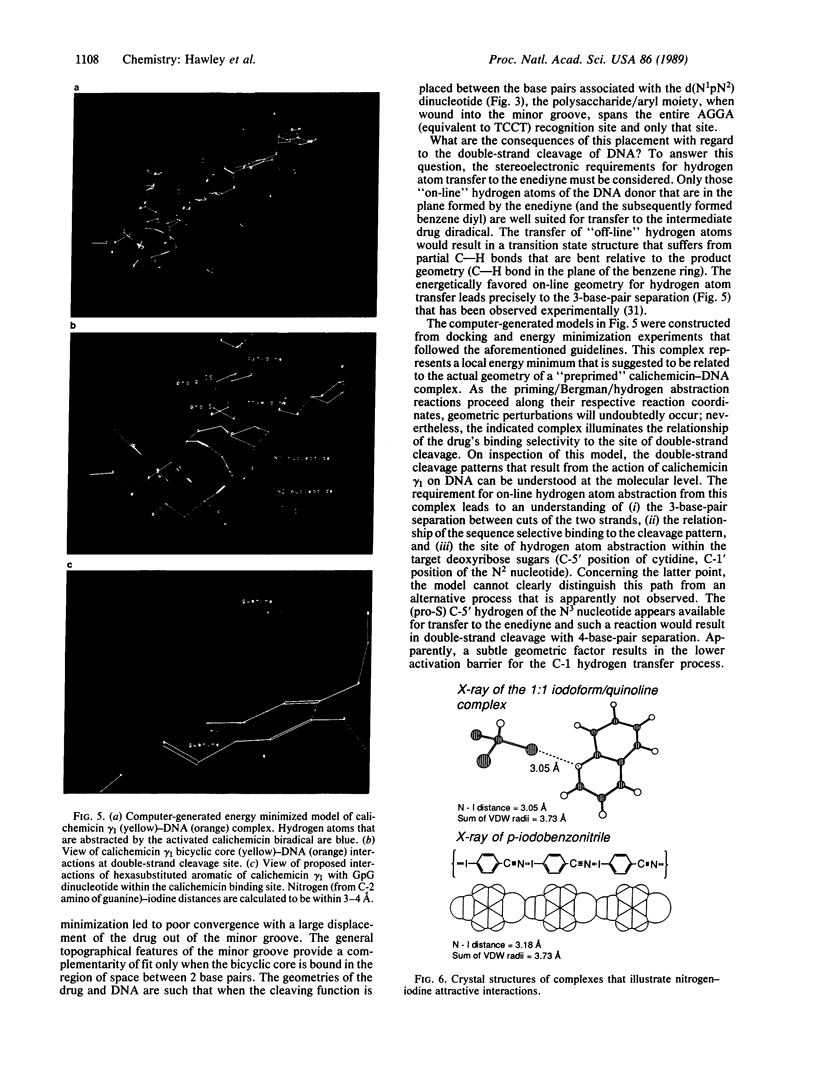
Abstract
An analysis of the binding interactions of several DNA-drug complexes that utilize carbohydrates for DNA recognition has been undertaken. It is proposed that the carbohydrate residues function as general minor groove binding elements, and the stereochemistry of aglycone attachment sites is generally disposed to promote a right-handed helical geometry that is complementary to right-handed DNA. The constitution and stereochemistry of the DNA double-strand cleaving agent calichemicin gamma 1 is consistent with this analysis. Docking experiments with computer-generated models of this drug and a dodecamer duplex that was found to serve as a calichemicin cleavage site were performed to gain insight into the origin of the drug's sequence-selective binding and cutting properties. A model is presented that provides a molecular level understanding of the double-strand cleavage patterns that result from the action of calichemicin gamma 1 on DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Schulte-Frohlinde D., von Sonntag C. gamma-Radiolyses of DNA in oxygenated aqueous solution. Structure of an alkali-labile site. Z Naturforsch C. 1977 Nov-Dec;32(11-12):1021–1022. doi: 10.1515/znc-1977-11-1226. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Chen C. Q., Goldberg I. H. Atypical abasic sites generated by neocarzinostatin at sequence-specific cytidylate residues in oligodeoxynucleotides. Biochemistry. 1988 Jun 14;27(12):4331–4340. doi: 10.1021/bi00412a021. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Activation of neocarzinostatin chromophore and formation of nascent DNA damage do not require molecular oxygen. Nucleic Acids Res. 1985 Mar 11;13(5):1637–1648. doi: 10.1093/nar/13.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Deoxyribonucleic acid damage by neocarzinostatin chromophore: strand breaks generated by selective oxidation of C-5' of deoxyribose. Biochemistry. 1983 Oct 11;22(21):4872–4878. doi: 10.1021/bi00290a002. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Brown S. C., Berman E., Shafer R. H. NMR studies of the interaction of chromomycin A3 with small DNA duplexes I. Biochemistry. 1987 Feb 24;26(4):1058–1067. doi: 10.1021/bi00378a012. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Peticolas W. L., Wang Y., Thomas G. A. Some rules for predicting the base-sequence dependence of DNA conformation. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2579–2583. doi: 10.1073/pnas.85.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Rao S. N., Singh U. C., Kollman P. A. Molecular mechanics simulations on covalent complexes between anthramycin and B DNA. J Med Chem. 1986 Dec;29(12):2484–2492. doi: 10.1021/jm00162a011. [DOI] [PubMed] [Google Scholar]
- Remers W. A. Natural products as probes for nucleic acid structure and sequence. J Nat Prod. 1985 Mar-Apr;48(2):173–192. doi: 10.1021/np50038a001. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Hall J. G., Denny W. A., Wakelin L. P. NMR studies of the interaction of the antibiotic nogalamycin with the hexadeoxyribonucleotide duplex d(5'-GCATGC)2. Biochemistry. 1988 Jun 14;27(12):4340–4349. doi: 10.1021/bi00412a022. [DOI] [PubMed] [Google Scholar]
- Shimosaka A., Hayakawa Y., Nakagawa M., Furihata K., Seto H., Otake N. Isolation of new anthracycline antibiotics, A447 C and D. J Antibiot (Tokyo) 1987 Jan;40(1):116–121. doi: 10.7164/antibiotics.40.116. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ughetto G., Wang A. H., Quigley G. J., van der Marel G. A., van Boom J. H., Rich A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985 Apr 11;13(7):2305–2323. doi: 10.1093/nar/13.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Rich A. Interactions between an anthracycline antibiotic and DNA: molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-A resolution. Biochemistry. 1987 Feb 24;26(4):1152–1163. doi: 10.1021/bi00378a025. [DOI] [PubMed] [Google Scholar]
- Wolberger C., Dong Y. C., Ptashne M., Harrison S. C. Structure of a phage 434 Cro/DNA complex. Nature. 1988 Oct 27;335(6193):789–795. doi: 10.1038/335789a0. [DOI] [PubMed] [Google Scholar]
- Zein N., Sinha A. M., McGahren W. J., Ellestad G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988 May 27;240(4856):1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]