Abstract
Previous studies on cognitive dynamics showed that oscillatory responses of P300 are composed of mainly delta and theta responses. In the present study, for the first time, the long-distance intra-hemispheric event related coherence (auditory oddball paradigm) and evoked coherence (simple sound) were compared in order to evaluate the effects of cognitive tasks on the long-distance coherences. Seventeen healthy subjects (8 female, 9 male) were included in the study. The coherence was analyzed for delta (1–3.5 Hz), theta (4–7.5 Hz) and alpha (8–13 Hz) frequency ranges for (F3-P3, F4-P4, F3-T7, F4-T8, F3-O1, F4-O2) electrode pairs. The coherence to target responses were higher than the non-target and simple auditory response coherence. This difference is significant for the delta coherence for both hemispheres and for theta coherences over the left hemisphere. The highest coherences were recorded at fronto-temporal locations for all frequency bands (delta, theta, alpha). Furthermore, fronto-parietal coherences were higher than the fronto-occipital coherences for all frequency bands (delta, theta, alpha).These results show that the fronto-temporal and fronto-parietal connections are most relevant for the identification of the target signal. This analysis open the way for a new interpretation of dynamic localization results during cognitive tasks.
Keywords: Event related coherence, Brain oscillations, P300, Oddball, Delta, Theta, Alpha
Introduction
The present report aims to fill an important gap in the analysis of P300-oddball paradigm, in order to establish the effect of electrical links between cognitive networks in the whole brain.
The coherence function was first used four decades ago by Adey et al. (1960), as a pioneering work on theta rhythms of the cat limbic system during conditioning. These authors used spectral analysis and coherence functions to investigate how the rhythmic potentials of the cat brain were related to behavior. The use of the coherence function in comparing EEG activity in various nuclei of the cat brain was one of the essential steps in refuting the view that the EEG was an epiphenomenon (Adey 1989). Accordingly, the induced theta rhythm and the task-relevant increase of coherence in the limbic system is a milestone in EEG research. When carrying out a behavioral task, the cat hippocampal activity exhibits a transition from irregular activity to coherent induced rhythms. The findings of Adey et al. (1960) were the deciding factor in the choice of the hippocampus as a model for resonance processes in the brain for the Başar et al. research group (1975a, b, c, 1979, 1980), Başar (2004). Başar (1980) further developed a model for excitability in neural tissues. This model suggested that if the brain receives a sensory stimulation, and if a neural structure has an intrinsic activity, then this structure would respond with its natural frequency.
Several paradigms, including the P300-oddball paradigm, were used in order to analyze working memory and attention by means of EROs (Event Related Oscillations) (Başar and Stampfer 1985; Başar-Eroğlu et al. 1992; Fründ et al. 2008) The first studies on Brain Oscillatory Dynamics in P300 included Başar and Stampfer (1985), Başar et al. (1984), Başar-Eroğlu et al. (1992, 2001), Schürmann et al. (2001), and Stampfer and Başar (1985). Another series of studies on local oscillatory dynamics showed that the major operating rhythms of P300 are mainly the delta and theta oscillations (Başar et al. 2001; Başar-Eroğlu et al. 1992; Demiralp et al. 1999; Karakaş et al. 2000; Kolev et al. 1997; Spencer and Polich 1999; Yordanova et al. 2000). The prolongation of theta, delta and alpha oscillations were described for the target stimuli (Başar-Eroğlu et al. 1992; Demiralp and Ademoglu 2001; Stampfer and Başar 1985; Öniz and Başar 2009; Yordanova and Kolev 1998). Other research groups have also studied oddball paradigm by means of brain oscillations in EEG and MEG studies (Anokhin et al. 2001; Bernat et al. 2007; Kawamata et al. 2007; Ishii et al. 2009; Mazaheri and Picton 2005). Mazaheri and Picton (2005) reported that target stimuli increased the frontal theta activity, decreased posterior and central alpha and beta activity, and decreased central gamma activity. Gamma band activity were also studied in oddball paradigms (Başar-Eroğlu and Başar 1991; Fell et al. 1997; Gurtubay et al. 2001, 2004; Haig et al. 1999; Kang et al. 2005; Tomberg and Desmedt 1998), the late gamma activity was found to be related with target stimuli (Basar-Eroglu and Başar 1991; Gurtubay et al. 2001, 2004). The methods described in the referenced studies were mainly the amplitude and latency measures of averaged filtered responses, spectral power of target response, wavelet decomposition and phase locking factor of target and non-target responses. However, the analysis of a long-distance coherence upon application of an auditory oddball paradigm was not performed in an extended manner in most of the studies. Only a single study Kukleta et al. (2009) analyzed event-related potentials (ERP) evoked by non-target stimuli in a visual oddball experiment and showed coherent oscillations in beta 2 frequency band in the prestimulus period, recorded by means of intracranial electrodes in humans. However, these authors did not compare target vs. non-target response or target versus simple sensory stimulation.
Several authors indicated the role of frontal, temporal and parietal areas for the generation of P300 amplitude in healthy subjects (Knight et al. 1989; Knight 1990; Polich 2003; Soltani and Knight 2000; Verleger et al. 1994; Yamaguchi and Knight 1992). Decreased coherence values of pathological subjects for frontal-temporal and/or fronto-parietal has been also reported (Ford et al. 2002; Güntekin et al. 2008; Winterer et al. 2003). Sauseng et al. (2005) calculated the coherence function during a visuospatial working memory task in a group of healthy subjects. Their findings indicated that the involvement of prefrontal areas in executive functions are reflected in a decrease of anterior upper alpha short-range connectivity and a parallel increase of fronto-parietal long distance coherence, mirroring the activation of a fronto-parietal network. Based on the findings of these previous studies, we focused our analysis on long distance intrahemispheric coherence values (F3-P3, F3-T7, F3-O1, F4-P4, F4-T8, F4-O2). Our hypothesis is as follows: we will be able to differentiate more clearly sensory and cognitive processes that are selectively distributed in topologically various areas of the brains.
In order to analyze the role of brain oscillation in brain function a “Brain Dynamics Research Program” was proposed by Başar (1976, 1999). This program introduced the necessity of applying several system theoretical tools and conceptual paradigms in order to enrich the functional interpretation of oscillations in neural systems. This research program also indicates the necessity to introduce a Darwinistic view by measuring oscillatory dynamics in several species including invertebrate ganglia as Aplysia or Helix Pomatia (Başar and Güntekin 2009; Bullock and Başar 1988). Additionally, findings in pathological cases help enormously in understanding the functional correlates of brain oscillations and possible effects of transmitters (Başar and Güntekin 2008). One of the most important features of this program is the necessity to jointly apply the increase of oscillatory responses together with evoked coherences, indicating enhanced links between various brain areas. In the last two decades a number of system theoretical tools and method of thoughts were developed in order to understand brain functions. However, in most of the cases neuroscientist prefer to choose one or two of currently most applied methods. Our research program is suggesting to neuroscientist to look to a broad ensemble of methods that can be useful to understand the problem which will be studied. For example, applications of drugs, application of several paradigms lead to obtain richer amount of data. In the special case of our study we apply different recordings as simple evoked oscillations and event related oscillations by means of oddball paradigm. The comparison of response adaptive filtering results gives a good idea on the auditory signal processing. However, additional to this, the study of event related and evoked coherences establish links between various areas of the brain that are activated differentially upon application of sensory and cognitive paradigms.
Experimental procedure
Subjects
Seventeen subjects (8 females, 9 males), most of them being university students and university members, volunteered for the experiment. Their ages ranged from 19 to 41 years. The mean age of subjects was 27.53 ± 6.08 years. All of the subjects were right handed. All subjects had completed at least 10 years of education. All subjects were interviewed with a questionnaire for their family history, demographic, medical profiles and drinking habits. No subjects reported any current or past neurological or psychiatric illness and all participants had normal or corrected to normal vision. All subjects signed an approved consent form.
Stimuli and paradigms
The subjects’ eyes were open and they were seated in a dimly-lit isolated room.
Two types of stimuli were presented: simple auditory stimuli for analyzing auditory evoked potentials (simple auditory stimulation), and; auditory oddball paradigm for analyzing auditory event related potentials (AERP). The auditory stimuli had 16 ms rising time, 50 ms falling time and a 1,000 ms duration and were presented by two loudspeakers. Eye-movements were controlled by a fixation cross on a screen.
The auditory simple stimuli were tones of 80 dB and 1,500-Hz tones. The inter-stimulus intervals varied randomly between 3 and 7 s. The total number of stimuli was 60.
A classical auditory oddball paradigm was used in the experiments. Two types of stimuli were used: task-relevant target and task- irrelevant non-target (standard). The total number of stimuli was 120 (40 target, 80 non-target). In the oddball paradigm the 80 dB, 1,600-Hz tones (target) and 1,500-Hz tones (non-target) were presented in a random sequence. The interval between tones varied randomly between 3 and 7 s. The subjects were instructed to keep a mental count of the number of 1,600-Hz tones (target). During the elicitation period of event related oscillations, all the subjects had displayed sufficient accuracy in the mental count of the target stimuli.
The evoked coherence responses to the target, non-target and simple auditory stimulation stimuli were analyzed and compared.
Electrophysiological recording
EEG was recorded with 30 Ag-AgCl electrodes mounted in an elastic cap (Easy-cap) according to the international 10–20 system. Additionally, two linked earlobe electrodes (A1 + A2) served as references. The EOG from the medial upper and lateral orbital rim of the right eye was also registered. For the reference electrodes and EOG recordings, Ag-AgCl electrodes were used. All electrode impedances were less than 10 kΩ. The EEG was amplified by means of a BrainAmp 32-channels DC system machine with band limits of 0.01–250 Hz. The EEG was digitized on-line with a sampling rate of 500 Hz.
Artifacts were eliminated by manual off-line selective averaging, taking into consideration the EOG recorded from the right eye. The sweep numbers were equalized randomly between the target, non-target and simple auditory stimulation conditions. In the mean 20 sweeps per modality were used.
Coherence
For the signal analysis, evaluation of oscillatory dynamics and coherence analysis Brainvision Analyzer Software was used. First, the Fast Fourier transform of each epoch with 0–800 ms duration was calculated, and then the coherence analysis was performed with a 1.25 Hz resolution.
The choosing of the time interval 800 ms following the stimulation is based on a rationale, which takes care of the complex biological properties of the EEG. In engineering studies the analyzer usually prefers an analysis period of more than 1,000 ms for the delta band. However, in order to optimize the time period of analysis we first performed a power spectral analysis of EEG response and found a peak around 1.5 Hz in the power spectrum of the EP and ERP responses. Furthermore, we observed that the filtered ERP in the delta frequency range is a dampened aperiodical signal, which is almost completely flattening around 500–600 ms. An analysis of coherence for longer periods would include then unexpected artifacts and/or alpha after discharges. In the theta band the second response window is found around 400 ms (Başar-Eroğlu et al. 1992; Demiralp et al. 1999; Stampfer and Başar 1985; Yordanova et al. 2000). These were the chain of reasoning to choose the period of 0–800 ms as the optimal time period for the coherence analysis. We also extended our analysis to 1,000 ms; here similar results with minimal deviation were found. We also recommend to brain research scientist to develop controls and not to use strict formula presented in some of the engineering textbooks. The method used was the cross-spectrum/autospectrum and the mathematical relations are described in the following:
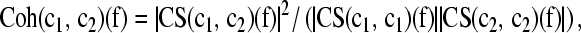 |
in conjunction with
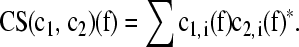 |
Then, Fisher’s Z transformation was used to normalize the distribution of average coherence values.
Coherence was calculated for the target, non-target and simple auditory stimuli for long-distance intrahemispheric pairs for three different frequency bands (delta (1–3.5 Hz); theta (4–7.5 Hz); alpha (8–13 Hz)). The maximum coherence value in each frequency range was included for the purpose of statistical analysis as the coherence value of that range. (If there was more than one peak, the peak with the maximum coherence value was accepted as the coherence value.) The long distance intrahemispheric pairs were F3-P3, F3-T7, F3-O1, F4-P4, F4-T8, F4-O2.
The present study does not include coherence analysis higher than alpha frequency band. In the analysis of gamma frequency band authors mostly consider a wide range between 30 and 70 Hz. This application is erroneous, because most of the investigators do not perform spectral analysis prior choosing the frequency channels to analyze the coherence function. Accordingly, special care must be applied to the higher frequency range in a future publication.
In addition to foregoing methodological description of coherence function we mention the publications by Srinivasan et al. (2007) and Nunez et al. (1999). According to these authors the optimal distance between electrodes must be around 10–20 cm in human EEG-recordings. They assume that Laplacian measure is more suitable since the limitation is around 5 cm (see Fig. 3; Srinivasan et al. 2007). Furthermore, these authors indicate that almost all of volume conduction effects on coherence are removed by the surface Laplacian; however, the Laplacian spatial filter also removes large-scale source activity. In our study the fronto-temporal distance is approximately 8 cm; accordingly the correction of volume conduction has values that can be neglected. On the other hand, in our present work the comparisons of responses between target, non-target and simple auditory stimuli are relevant and not the absolute values. The target responses depict increases of approximately 50% in comparison of single EPs and non-target responses. These are crucial results eliminating the necessity of corrections mentioned about.
Fig. 3.
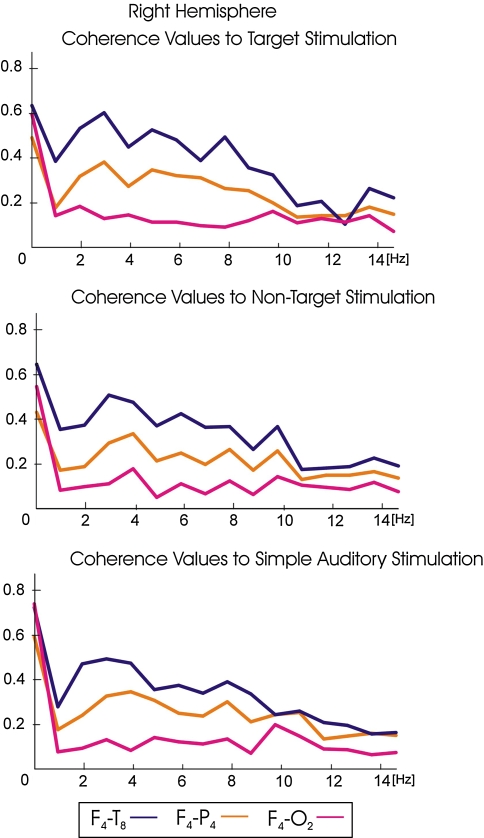
Grand averages of coherences for target, non-target and simple auditory stimulation responses for right hemisphere electrode pairs (right hemisphere: F4-T8, F4-P4, F4-O2)
Moreover, Başar et al. (2009) recently published results on Alzheimer patients showing that the coherence values of responses to simple visual stimuli can be as low as 0.3. This indicates that volume conduction does not have considerable correction necessity when the connectivity between (F3P3, F4P4) is biologically impaired.
Volume conduction is important when two electrode pairs (F3-T7 vs. F3-O1) are compared. But it could be neglected in different conditions: (1) When two different paradigms are compared for the same electrode pair (i.e. Comparing target coherence value to simple auditory coherence value for F3-T7 electrode pair. (2) When two different groups of subjects are compared for same electrode pair (i.e. comparing Alzheimer subjects’ coherence values to healthy subjects’ coherence values for F3-T7 electrode pair). In these two conditions the volume conduction for F3-T7 is same; however the paradigm and group change influences the coherence values (Başar et al. 2009; Güntekin et al. 2008).
Statistics
Fisher’s Z transformation was used to normalize the distribution of coherence values. The Statistical Package for Social Studies (SPSS) program was used for statistical analysis. The differences between stimulus types were assessed by means of a repeated measure ANOVA for each frequency band, and for the intrahemispheric locations. In the analysis of intrahemispheric coherence differences, repeated measures of ANOVA included the between-subjects factor as gender (male, female) (although the number of subjects are not enough for gender comparison, according to our groups’ previous results, including the gender as a factor and controlling for the gender effects seems crucial (Güntekin and Başar 2007a, b)); repeated measure ANOVA included the within-subject factors as stimulus types (target, non-target, simple auditory stimulation); laterality (right, left) and location (F3/4-P3/4 vs. F3/4-T7/8 vs. F3/4-O1/2). Post-hoc comparisons were analyzed with t-tests. Differences between electrode pairs of modalities were also analyzed with t-tests and the significance level was set to P < 0.05 for all post-hoc comparisons. Post-hoc comparisons were corrected using the Bonferroni procedure.
Results
Description of coherence function
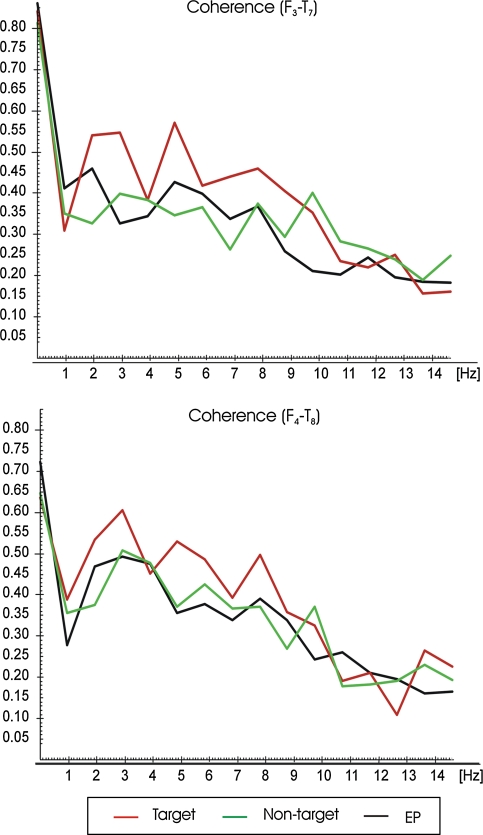
Figure 1 illustrates the grand average of coherence function in the 1–15 Hz frequency range for F3-T7 and F4-T8 electrode pairs. The red line represents the grand average of coherence values for target response; the green line represents the grand average of coherence values for non-target response; the black line represents the grand average of coherence values upon simple auditory stimulation. As seen from Fig. 1 the grand average of coherence values for target responses were higher than the non-target and simple auditory stimulation responses in all frequency bands (2, 5, 8 Hz) and for both electrode pairs (F3-T7, F4-T8). This difference is especially apparent for the left hemisphere (F3-T7). However, in non-target responses alpha coherence is the highest for F3-T7 and also for F4-T8. The grand average of coherence values for target response reached 0.57 for F3-T7 theta response and reached 0.60 for F4-T8 delta response. However, the grand average of coherence values for non-target and simple auditory stimulation responses are between 0.35 and 0.45 for the left hemisphere (F3-T7) and between 0.40 and 0.50 for the right hemisphere (F4-T8).
Fig. 1.
Grand averages of coherences for target, non-target and simple auditory stimulation responses of F3-T7 and F4-T8 electrode sites
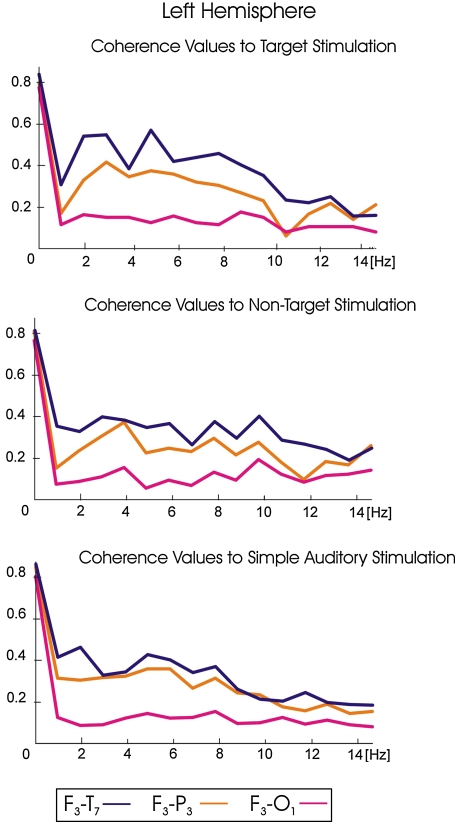
Figure 2 illustrates the grand averages of coherences values for target, non-target and simple auditory stimulation responses for left hemisphere electrode pairs (left hemisphere: F3-T7, F3-P3, F3-O1) in the 1–15 Hz frequency range. Figure 3 illustrates the grand averages of coherences values for target, non-target and simple auditory stimulation responses for right hemisphere electrode pairs (right hemisphere: F4-T8, F4-P4, F4-O2) in the 1–15 Hz frequency range. Blue lines represent the grand average of coherence values for fronto-temporal electrode pairs; orange lines represent the grand average of coherence values for fronto-parietal electrode pairs; pink lines represent the grand average of coherence values for fronto-occipital electrode pairs. The grand averages of coherence values upon target stimulation are illustrated in the upper part of the diagrams; the grand averages of coherence values upon non-target stimulation are illustrated in the middle part of the diagrams; the grand averages of coherence values upon simple auditory stimulation are illustrated in the lower part of the diagrams. As seen in Figs. 2 and 3, fronto-temporal coherences were higher than the fronto-parietal and fronto-occipital coherence values for all modalities (target, non-target, simple auditory stimulation). Furthermore, the fronto-parietal coherence values are higher than fronto-occipital coherence values for all modalities (target, non-target, simple auditory stimulation). The peaks of delta, theta and alpha frequency ranges of grand averages of coherence values for target responses can easily be detected, and they are over 0.55 for fronto-temporal electrode pairs (F3-T7, F4-T8) (Figs. 2 and 3). However, the grand averages of coherence values for non-target and simple auditory stimulation responses do not exceed 0.50 at any location and they are particularly low at the left hemisphere (Fig. 3).
Fig. 2.
Grand averages of coherences for target, non-target and simple auditory stimulation responses for left hemisphere electrode pairs (left hemisphere: F3-T7, F3-P3, F3-O1) right hemisphere: F4-T8, F4-P4, F4-O2)
The descriptions of grand averages presented in section "Description of coherence function" are in accordance with the statistical findings described below.
Statistical evaluation and results of coherence function
Delta (1–3.5 Hz) frequency range
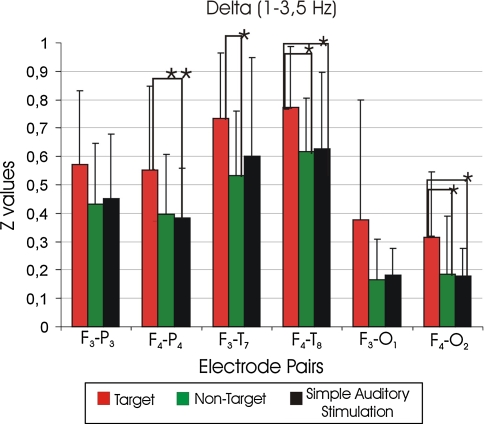
In the analysis of intrahemispheric coherence differences, the ANOVA of the delta coherence revealed a significant effect between stimulus types (F(2,30) = 9.9; P < 0.002). Post-hoc comparisons showed that target delta coherences were significantly higher than the non-target and simple auditory stimulation delta coherences (P < 0.0001; P < 0.0001). The ANOVA of delta coherence revealed a significant effect for location (F(2,30) = 64.2; P < 0.0001). Post-hoc comparisons showed that fronto-temporal delta coherences were significantly higher than the fronto-parietal and fronto-occipital delta coherences (P < 0.0001; P < 0.0001). Furthermore, the fronto-parietal delta coherences were significantly higher than the fronto-occipital delta coherences (P < 0.0001). These results indicate higher connectivity in a cognitive network rather than a sensory network (fronto-parietal hippocampal system versus sensory pathway). This point will be explained in the subsequent Discussion section (see section "The significance of fronto-parietal-hippocampal system in comparison to sensory pathways"). The differences between electrode pairs between target-non-target, target-simple auditory stimulation and non-target-simple auditory stimulation were also analyzed. Figure 4 represents the mean Z values of target, non-target and simple auditory stimulation responses of delta (1–3.5 Hz) frequency range. Table 1 shows the mean Z values of target, non-target and simple auditory stimulation responses for all electrode pairs and for all frequency bands. Post hoc comparisons showed that F4-P4 target delta coherence was higher than the F4-P4 simple auditory stimulation delta coherence (P < 0.01, not significant after correction) (Fig. 4; Table 1).
Fig. 4.
Mean Z values of target, non-target and simple auditory stimulation responses of delta (1–3.5 Hz) frequency range, where ** P < 0.01, * P < 0.05
Table 1.
Mean Z values of target, non-target and simple auditory stimulation responses for all electrode pairs and for all frequency bands
| Delta | Theta | Alpha | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| F3P3 | ||||||
| Target | 0.57 | 0.26 | 0.62 | 0.23 | 0.49 | 0.35 |
| Non-target | 0.43 | 0.21 | 0.42 | 0.24 | 0.45 | 0.24 |
| EP | 0.45 | 0.23 | 0.57 | 0.21 | 0.42 | 0.22 |
| F4P4 | ||||||
| Target | 0.55 | 0.29 | 0.50 | 0.21 | 0.42 | 0.33 |
| Non-target | 0.40 | 0.21 | 0.54 | 0.23 | 0.40 | 0.26 |
| EP | 0.38 | 0.17 | 0.47 | 0.23 | 0.40 | 0.22 |
| F3T7 | ||||||
| Target | 0.73 | 0.23 | 0.83 | 0.51 | 0.60 | 0.49 |
| Non-target | 0.53 | 0.23 | 0.61 | 0.22 | 0.60 | 0.32 |
| EP | 0.60 | 0.35 | 0.67 | 0.25 | 0.47 | 0.23 |
| F4T8 | ||||||
| Target | 0.77 | 0.21 | 0.76 | 0.32 | 0.52 | 0.31 |
| Non-target | 0.62 | 0.19 | 0.65 | 0.18 | 0.51 | 0.35 |
| EP | 0.63 | 0.27 | 0.62 | 0.34 | 0.46 | 0.26 |
| F3O1 | ||||||
| Target | 0.38 | 0.42 | 0.29 | 0.16 | 0.36 | 0.27 |
| Non-target | 0.17 | 0.14 | 0.25 | 0.19 | 0.35 | 0.31 |
| EP | 0.18 | 0.09 | 0.27 | 0.16 | 0.29 | 0.22 |
| F4O2 | ||||||
| Target | 0.31 | 0.23 | 0.24 | 0.11 | 0.35 | 0.22 |
| Non-target | 0.19 | 0.20 | 0.30 | 0.25 | 0.29 | 0.25 |
| EP | 0.18 | 0.10 | 0.25 | 0.16 | 0.31 | 0.25 |
Theta (4–7.5 Hz) frequency range
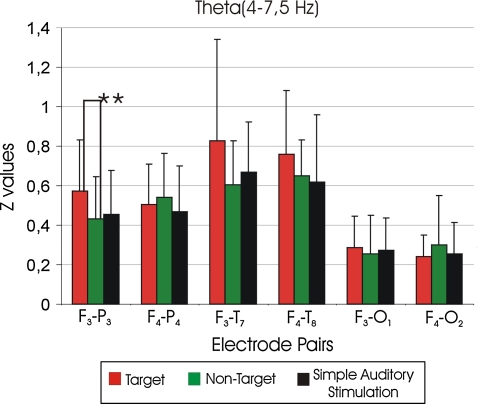
In the analysis of intrahemispheric coherence differences, the ANOVA of the theta coherence revealed a significant effect between stimulus types × laterality (F(2,30) = 4.1; P < 0.03). Post-hoc comparisons showed that left target theta coherences were significantly higher than the left non-target theta coherences (P < 0.005). The ANOVA of theta coherence revealed a significant effect for location (F(2,30) = 64.2; P < 0.0001). Post-hoc comparisons showed that fronto-temporal theta coherences were significantly higher than the fronto-parietal and fronto-occipital theta coherences (P < 0.0001; P < 0.0001). Furthermore, the fronto-parietal theta coherences were significantly higher than the fronto-occipital theta coherences (P < 0.0001). The differences between electrode pairs between target-non-target, target-simple auditory stimulation and non-target-simple auditory stimulation were also analyzed. Figure 5 represents the mean Z values of target, non-target and simple auditory stimulation responses of theta (4–7.5 Hz) frequency range. Post hoc comparisons showed that F3-P3 target response was higher than the F3-P3 non-target response (P < 0.01, not significant after correction) (Fig. 5; Table 1). Although there was a tendency for location × laterality × gender, there were no significant results for gender differences. Further experiments with a larger sample group would be required to analyze gender differences.
Fig. 5.
Mean Z values of target, non-target and simple auditory stimulation responses of theta frequency range (4–7.5 Hz), where ** P < 0.01
Alpha (8–13 Hz) frequency range
In the analysis of intrahemispheric coherence differences, the ANOVA of alpha coherence revealed no significant effect between modalities (F(2,30) = 0.4; P = 0.66). The ANOVA of alpha coherence revealed a significant effect for location (F(2.3) = 9,864; P < 0.002). Post-hoc comparisons showed that fronto-temporal alpha coherences were significantly higher than the fronto-parietal and fronto-occipital alpha coherences (P < 0.0001; P < 0.0001). Furthermore, the fronto-parietal alpha coherences were significantly higher than the fronto-occipital alpha coherences (P < 0.0001). The differences between electrode pairs between target-non-target, target-simple auditory stimulation and non-target-EP were also analyzed and no differences were found (Table 1).
Discussion
The results of the present study clearly indicated that, during the application of an oddball paradigm, the coherence of target responses were, in general, higher than the non-target and simple auditory stimulation responses. This difference was significant for the delta coherence for both hemispheres. The difference was also significant for theta coherences over the left hemisphere. No statistically significant results were observed between target, non-target and simple auditory responses for the alpha frequency range. The highest coherences were registered at fronto-temporal locations for all frequency bands (delta, theta, alpha). Furthermore, fronto-parietal coherences were higher than fronto-occipital coherences for all frequency bands (delta, theta, alpha).
Fundamental analysis of the coherence function
According to Başar (2006), the coherence—i.e. the connectivity between brain structures—is selective, depending on cognitive load. This is manifested in the selective distribution of the coherence functions over various brain structures with varied degrees of coherence between 0 and 1. Demonstration of the principle of selective connectivity requires the analysis of oscillations in several neural populations and in several frequency windows. Such analyses and the related findings have been fundamental results for refinement of the concepts of “whole brain” and “cooperation”.
Many studies reported the successful use of EEG coherence to measure functional connectivity (Bendat and Piersol 1967; Lopes Da Silva et al. 1980; Petsche and Etlinger 1998; Rappelsberger et al. 1982). According to these studies, EEG coherence may be considered to be an indispensable large-scale measure of functional relationships between pairs of cortical regions (Nunez 1997).
It is also important to mention the studies of Bullock’s research group: Bullock et al. (1995) clearly showed that the connectivity (coherence) between neural groups is a main factor for the evolution of cognitive processes.
According to Bullock and Başar (1988) and Bullock et al. (1995), no significant coherences were found in the neural networks of invertebrates, in contrast to the higher coherences between distant structures that were recorded in mammalian and human brains. The highest coherences were found in the subdural structures of the human brain (Bullock 2006).
Since coherence is, in essence, a correlation coefficient per frequency band, it is used to describe the coupling or relationship between signals for a certain frequency band. According to Bullock et al. (2003), increased coherence between two structures, namely A and B, can be caused by the following processes: (1) Structures A and B are driven by the same generator; (2) Structures A and B can mutually drive each other; (3) One of the structures, A or B, drives the other.
The implications following the analysis of coherence function by several authors can be summarized as follows:
In simple binding, there is temporal coherence between cells in cortical columns. This has been demonstrated by several authors (Eckhorn et al. 1988; Gray and Singer 1989).
The response susceptibility of the brain activates resonant communications in the brain by facilitating electrical processing between networks (Başar 2004; Başar et al. 1997a, b). This could be also interpreted as a general tuning process between neural populations and feature detectors (Sokolov 2001).
Parallel processing in the brain shows selectivity. The selectivity in parallel processing is produced by variations in the degree of spatial coherences that occur over long distances between brain structures/neural assemblies (Başar 1980, 1983a, b; Başar et al. 1999a, b; Kocsis et al. 2001; Miltner et al. 1999; Schürmann et al. 2000).
Fries (2005) hypothesized that neural communication is mechanistically subserved by neural coherence. Activated neural groups oscillate and thereby undergo rhythmic excitability fluctuations that produce temporal windows for communication. Only coherently activated neural groups can interact effectively.
This hypothesis is in agreement with the general hypothesis on “excitability of neural structures”, “overall coherency increase” and “complex matching” (Başar 2004). In the P300 responses, the excitability is in delta and theta frequency channels and, accordingly, the communication in the brain occurs mostly in those frequency channels. Delta and theta frequency channels show selective coherences upon increased cognitive load (see Başar 2004).
The principle of superposition describes integration over the temporal axis consisting of a relationship between the amplitude and phases of oscillations in various frequency bands. Furthermore, selectively distributed and selectively coherent oscillatory activities in neural populations describe integration over the spatial axis (Başar 1980). Consequently, integrative activity is a function of the coherences among spatial locations of the brain; these coherences vary according to the type of sensory and/or cognitive event and possibly the state of consciousness of the species (Başar 1998, 1999, 2004). The publications of Bressler and Kelso (2001), Varela et al. (2001) and Von Stein and Sarnthein (2000) clearly describe this trend, in which the concerted activity of alpha, theta, and delta and beta oscillations in distributed structures as reticular formation (RF), hippocampus (HI), thalamus and sensory cortices was emphasized.
Fundamental findings in studies of oddball paradigms
As Polich indicates in his review (2007), several authors indicated the role of circuits between frontal, parietal, temporal areas for the generation of P300 amplitude. Moreover, P300 amplitude is affected by temporal–parietal junction integrity, as its absence greatly reduces component size over the parietal area (Knight et al. 1989; Verleger et al. 1994; Yamaguchi and Knight 1992) and P3a and P3b indicate a circuit pathway between frontal and temporal/parietal brain areas (Knight 1990; Polich 2003; Soltani and Knight 2000). These authors performed their studies on subjects who had temporal and/or parietal cortical lesions. The findings of the present study support the mentioned role of “frontal”, “parietal” and “temporal” areas and also the importance of “connectivity” between these areas in the generation of P300 by applying coherence analysis. It should also be mentioned that the paradigm used in the present study was an auditory paradigm. It is likely that analysis of coherence function upon application of a visual oddball paradigm would depict higher fronto-occipital and fronto-parietal coherences in comparison to the present study.
The significance of fronto-parietal-hippocampal system in comparison to sensory pathways
Why are the fronto-parietal delta, theta and alpha response coherences higher in comparison to fronto-occipital coherence? It is well known that cognitive-associative mechanisms are processed in the fronto-parietal and hippocampal system. In contrast, sensory signals are primarily processed in sensory pathways. The paradigm used in the present study involves the cognitive networks and auditory networks; (Başar et al. 2001; Başar-Eroğlu et al. 1992, 2001; Polich 2007). Accordingly, it is to be expected that the fronto-occipital coherence will be weaker than the fronto-parietal coherence. It is also to mention that the distance between pairs of electrodes may play a small deviation of results. Moreover, in the delta frequency range the target coherence is approximately 40% higher than non-target and sensory evoked response coherences (see Fig. 1). Furthermore, the evoked coherences by means of simple auditory stimuli are not highly different from non-target responses.
According to all these results, it can be stated that coherence values are highly increased during cognitive processes in comparison to responses elicited by a simply auditory stimuli. It should also be emphasized that fronto-temporal (F3-T7, F4-T8) coherences are also significantly high, thus indicating that fronto-temporal links are augmented during cognitive processes.
In the alpha frequency range, fronto-parietal and fronto-temporal alpha coherences are also significantly higher than fronto-occipital alpha coherences. The results in all frequency ranges can be interpreted such that the cognitive processes depict more connectivity in fronto-parietal pathways than fronto-occipital connectivity.
Comparison with clinical studies
In our previous study, during a visual oddball paradigm the delta, theta and alpha coherences in the left fronto-parietal electrode pair (F3-P3) of Alzheimer’s disease (AD) patients was significantly decreased, thus indicating reduced connectivity between frontal and parietal sites (Güntekin et al. 2008). Hogan et al. (2003) also reported reduced coherence for AD patients when compared with normal controls during a working memory paradigm. According to Hogan et al. (2003) the AD patients had reduced upper alpha coherence between the central and right temporal cortex. Winterer et al. (2003) investigated schizophrenia patients and their clinically unaffected siblings with an auditory oddball paradigm and reported that schizophrenic patients and their siblings showed a reduction of fronto-temporal coherence. Ford et al. (2002) also reported reduced fronto-temporal coherence for schizophrenia patients when talking.
In the present study, left hemisphere theta response was higher for the target response when compared with non-target response. Our earlier reports analyzing the oscillatory dynamics of AD patients emphasized the left laterality of abnormal phase locking of theta (Yener et al. 2007); amplitude reduction in delta oscillatory activity over the left frontocentral (Yener et al. 2008) and also lower values of evoked coherence in “delta”, “theta” and “alpha” bands in the left fronto-parietal electrode pairs in the untreated AD group when compared to healthy subjects upon appliance of a visual oddball paradigm (Güntekin et al. 2008). Schack et al. (1999) reported higher left hemisphere beta coherence within the left frontal and left parietal areas for an incongruent situation compared to a congruent situation upon appliance of the Stroop task. According to these results, the dominance of the left hemisphere during a cognitive task seems to be crucial.
No differences between coherence values of target, non-target and simple auditory stimulation responses were found for the alpha coherence. In contrast, our previous study comparing the coherence values of elderly healthy subjects and Alzheimer patients showed that the alpha coherence of elderly healthy subjects was higher than the alpha coherence of non-treated Alzheimer patients at F3-P3 electrode pairs. The treated AD patients had also higher alpha coherence than the non-treated AD patients at F3-P3 electrode pairs. The subjects of the present study were healthy young adults, which could affect the coherence values between frontal electrode and other brain structures, compared with other clinical or demographic groups. Yordanova et al. (1996) showed that elderly subjects had higher frontal alpha response then young adults. Future research in this field could examine the target, non-target and simple auditory stimulation coherence results of elderly healthy subjects in order to establish the role of alpha long distance networks upon application of an oddball paradigm. Frontal alpha increases with age, so it is possible that the coherence between frontal and other areas could also increase.
Concluding remarks
It was shown that the connectivity between distant brain structures upon application of an auditory oddball paradigm was mainly controlled by both hemispheric delta and left hemispheric theta oscillations.
The results from healthy subjects upon application of cognitive paradigms may be very important to understand brains of those with cognitive impairments. The results of the present report may provide a basis for coherence analysis and, in parallel, for oddball paradigm analysis.
The increase of response coherences and their functional significance, and the difference between the right and left hemisphere were not discussed in this study. The presented results are only discussed globally, in order to show important differences among target, non-target and simple auditory stimuli. Distances between electrodes higher than 6 cm are enough to eliminate the errors due to volume conduction according to the empirical criteria of Srinivasan et al. (2007).
The most important conclusion of the present study is the following: According to Luria (1966) there are no anatomical centers for the psychological functions of the mind. Mental functions, too, are the products of complex systems, the component parts of which may be distributed throughout the structures of the brain. The task of neuroscience is therefore not to localize the “centers”, but, rather, to identify the components of the various complex systems that interact to generate the mental functions. Luria called this task “dynamic localization”.
Mental functions, in short, are not localized in any of the component structures, but rather between them. Like the mental apparatus as a whole, they are virtual entities (Solms and Turnbull 2002).
According to the present results the understanding of whole brain function also requires the analysis of functional coherences, i.e. the increased connectivity between structures upon cognitive load, together with enhanced temporal oscillatory responses. Furthermore, in addition to Luria’s view, it seems that Brodmann’s (1909) areas should be extended to a more dynamic presentation, in which, sensory and cognitive areas should be described as superposition of multiple primary and secondary functions.
As a consequence of the above concluding remarks (1–6), and especially the views of Luria and the concept of dynamically linked Brodmann’s areas, we propose that all sensory-cognitive paradigms (as is here the case in P300-oddball) must be jointly analyzed in terms of oscillatory responses and related coherences. Only in this way, it is possible to open new avenues for description of whole cortex organization.
Acknowledgments
Authors are thankful to Elif Tülay and Bilge Turp for technical assistance and for their help in data processing.
References
- Adey WR. Cell membranes, electromagnetic fields and intercellular communication. In: Başar E, Bullock TH, editors. Brain dynamics: progress and perspectives. Berlin: Springer; 1989. pp. 26–42. [Google Scholar]
- Adey WR, Dunlop CW, Hendrix CE. Hippocampal slow waves: distribution and phase relationships in the course of approach learning. Arch Neurol. 1960;3:74–90. doi: 10.1001/archneur.1960.00450010074007. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Baal GCM, Beijsterveldt CEM. Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behav Genet. 2001;31(6):545–554. doi: 10.1023/A:1013341310865. [DOI] [PubMed] [Google Scholar]
- Başar E. Biophysical and physiological systems analysis. Reading: Addison-Wesley; 1976. [Google Scholar]
- Başar E. EEG-Brain dynamics. Relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. [Google Scholar]
- Başar E. Toward a physical approach to integrative physiology I. Brain dynamics and physical causality. Am J Physiol. 1983;14:510–533. doi: 10.1152/ajpregu.1983.245.4.R510. [DOI] [PubMed] [Google Scholar]
- Başar E. Synergetics of neuronal populations: a survey of experiments. In: Başar E, Flohr H, Haken H, Mandell A, editors. Synergetics of the brain. Berlin: Springer; 1983. [Google Scholar]
- Başar E. Brain functions and oscillations: I. Brain oscillations principles and approaches. Berlin: Springer; 1998. [Google Scholar]
- Başar E. Brain function and oscillations: II. İntegrative brain function. Neurophysiology and cognitive processes. Heidelberg: Springer; 1999. [Google Scholar]
- Başar E. Memory and brain dynamics: oscillations integrating attention, perception, learning and memory. Florida: CRC Press; 2004. [Google Scholar]
- Başar E. The theory of the whole-brain-work. Int J Psychophysiol. 2006;60:133–138. doi: 10.1016/j.ijpsycho.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;15:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B. Darwin’s evolution theory, brain oscillations, and complex brain function in a new “Cartesian view”. Int J Psychophysiol. 2009;71:2–8. doi: 10.1016/j.ijpsycho.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Başar E, Stampfer HG. Important associations among EEG-dynamics, event-relate potentials, short-term-memory, and learning. Int J Neurosci. 1985;26:161–180. doi: 10.3109/00207458508985615. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Başar E. A compound P300–40 Hz response of the cat hippocampus. Int J Neurosci. 1991;60(3–4):227–237. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Başar E, Gönder A, Özesmi C, Ungan P. Dynamics of brain rhythmic and evoked potentials. I. Some computational methods for the analysis of electrical signals from the brain. Biol Cybern. 1975;20:137–143. doi: 10.1007/BF00342634. [DOI] [PubMed] [Google Scholar]
- Başar E, Gönder A, Özesmi C, Ungan P. Dynamics of brain rhythmic and evoked potentials. II. Studies in the auditory pathway, reticular formation, and hippocampus during the waking stage. Biol Cybern. 1975;20:145–160. doi: 10.1007/BF00342635. [DOI] [PubMed] [Google Scholar]
- Başar E, Gönder A, Özesmi C, Ungan P. Dynamics of brain rhythmic and evoked potentials. III. Studies in the auditory pathway, reticular formation, and hippocampus during sleep. Biol Cybern. 1975;20:161–169. doi: 10.1007/BF00342636. [DOI] [PubMed] [Google Scholar]
- Başar E, Demir N, Gönder A, Ungan P. Combined dynamics of EEG and evoked potentials. I. Studies of simultaneously recorded EEG-EPograms in the auditory pathway, reticular formation, and hippocampus of the cat brain during the waking stage. Biol Cybern. 1979;34(1):1–19. doi: 10.1007/BF00336852. [DOI] [PubMed] [Google Scholar]
- Başar E, Gönder A, Ungan P. Comparative frequency-analysis of single EEG-evoked potential records. J Biomed Eng. 1980;2:9–14. doi: 10.1016/0141-5425(80)90086-2. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Rosen B, Schütt A. A new approach to endogenous event-related potentials in man: relation between EEG and P300-wave. Int J Neurosci. 1984;24:1–21. doi: 10.3109/00207458409079530. [DOI] [PubMed] [Google Scholar]
- Başar E, Hari R, Lopes da Silva FH, Schürmann M (eds) (1997a) Brain alpha activity—new aspects and functional correlates. Int J Psychophysiol 26:1–482 [DOI] [PubMed]
- Başar E, Schürmann M, Başar-Eroğlu C, Karakaş S (1997b) Alpha oscillations in brain functioning: an integrative theory. In: Başar E, Hari R, Lopes da Silva FH, Schürmann M (eds) Brain alpha activity—new aspects and functional correlates, Int J Psychophysiol 26:5–29 [DOI] [PubMed]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;15:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng Med Biol. 1999;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]
- Başar E, Guntekin B, Yener G. Evoked coherence in Alzheimer patients upon application of basic visual paradigm. Biol Psychiatry. 2009;65(8):53S–53S. [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels: a review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-G. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Demiralp T, Schürmann M, Başar E. Topological distribution of oddball ‘P300’ responses. Int J Psychophysiol. 2001;39:213–220. doi: 10.1016/S0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Measurement and analysis of random data. New York: Wiley; 1967. [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. Int J Psychophysiol. 2007;64(1):62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;1:26–36. doi: 10.1016/S1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende lokalisationslehre der grosshirnrinde in ihren prinzipien dargestellt auf grund de zellenbaues. Leipzig: JA Barth; 1909. [Google Scholar]
- Bullock TH. How do brains evolve complexity? An essay. Int J Psychophysiol. 2006;60:106–109. doi: 10.1016/j.ijpsycho.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Başar E. Comparison of ongoing compound field potentials in the brains of invertebrates and vertebrates. Brain Res Rev. 1988;13:57–75. doi: 10.1016/0165-0173(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Bullock TH, McClune MC, Achimowicz JZ, Iragui-Madoz VJ, Duckrow RB, Spencer SS. EEG coherence has structure in the millimeter domain: subdural and hippocampal recordings from epileptic patients. Electroencephalogr Clin Neurophysiol. 1995;95:161–177. doi: 10.1016/0013-4694(95)93347-A. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Mcclune MC, Enright JT. Are the electroencephalograms mainly rhythmic? Assessment of periodicity in wide-band time series. Neuroscience. 2003;121:233–252. doi: 10.1016/S0306-4522(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A. Decomposition of event-related brain potentials into multiple functional components using wavelet transform. Clin Electroencephalogr. 2001;32:122–138. doi: 10.1177/155005940103200307. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Yordanova J, Kolev V, Ademoglu A, Devrim M, Samar VJ. Time–frequency analysis of single-sweep event-related potentials by means of fast wavelet transform. Brain Lang. 1999;66:129–145. doi: 10.1006/brln.1998.2028. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan R, Brosch W, Kruse M, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Fell J, Hinrichs H, Röschke J. Time course of human 40 Hz EEG activity accompanying P3 responses in an auditory oddball paradigm. Neurosci Lett. 1997;235(3):121–124. doi: 10.1016/S0304-3940(97)00730-1. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/S0006-3223(01)01335-X. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fründ I, Schadow J, Busch NA, Naue N, Körner U, Herrmann CS. Anticipation of natural stimuli modulates EEG dynamics: physiology and simulation. Cogn Neurodyn. 2008;2(2):89–100. doi: 10.1007/s11571-008-9043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Brain oscillations are highly influenced by gender differences. Int J Psychophys. 2007;65:294–299. doi: 10.1016/j.ijpsycho.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Gender differences influence brain’s beta oscillatory responses in recognition of facial expressions. Neurosci Lett. 2007;424(2):94–99. doi: 10.1016/j.neulet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Saatçi E, Yener G. Decrease of evoked delta, theta and alpha coherences in Alzheimer patients during a visual oddball paradigm. Brain Res. 2008;15:109–116. doi: 10.1016/j.brainres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Iriarte J, Artieda J. Gamma band activity in an auditory oddball paradigm studied with the wavelet transform. Clin Neurophysiol. 2001;112(7):1219–1228. doi: 10.1016/S1388-2457(01)00557-0. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Artieda J. Gamma band responses to target and non-target auditory stimuli in humans. Neurosci Lett. 2004;367(1):6–9. doi: 10.1016/j.neulet.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Haig AR, Pascalis V, Gordon E. Peak gamma latency correlated with reaction time in a conventional oddball paradigm. Clin Neurophysiol. 1999;110(1):158–165. doi: 10.1016/S0013-4694(98)00112-6. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Swanwick GRJ, Kaiser J, Rowan M, Lawlor B. Memory-related EEG power and coherence reductions in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49:147–163. doi: 10.1016/S0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Ishii R, Canuet L, Herdman A, Gunji A, Iwase M, Takahashi H, Nakahachi T, Hirata M, Robinson SE, Pantev C, Takeda M. Cortical oscillatory power changes during auditory oddball task revealed by spatially filtered magnetoencephalography. Clin Neurophysiol. 2009;120(3):497–504. doi: 10.1016/j.clinph.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Kang K, Williams LM, Hermens D, Gordon E. Neurophysiological markers of contextual processing: the relationship between P3b and Gamma synchrony and their modulation by arousal, performance and individual differences. Brain Res. 2005;25(2):472–483. doi: 10.1016/j.cogbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Erzengin OU, Başar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000;111:1719–1732. doi: 10.1016/S1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Kawamata M, Kirino E, Inoue R, Arai H. Event-related desynchronization of frontal-midline theta rhythm during preconscious auditory oddball processing. Clin EEG Neurosci. 2007;38(4):193–202. doi: 10.1177/155005940703800403. [DOI] [PubMed] [Google Scholar]
- Knight R. Neural mechanisms of event-related potentials from human lesion studies. In: Rohbraugh J, Parasuraman R, Johnson R, editors. Event-related brain potentials: basic issues and applications. New York: Oxford University Press; 1990. pp. 3–18. [Google Scholar]
- Knight RT, Scabini D, Woods D, Clayworth C. Contributions of temporal parietal junction to the human auditory P3. Brain Res. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Viana Di Prisco G, Vertes RP. Theta synchronization in the limbic system: The role of Gudden’s tegumental nuclei. Eur J Neurosci. 2001;13:381–388. doi: 10.1046/j.0953-816X.2000.01392.x. [DOI] [PubMed] [Google Scholar]
- Kolev V, Demiralp T, Yordanova J, Ademoglu A, Isoglu-Alkaç Ü. Time–frequency analysis reveals multiple functional components during oddball P300. Neuroreport. 1997;8:2061–2065. doi: 10.1097/00001756-199705260-00050. [DOI] [PubMed] [Google Scholar]
- Kukleta M, Brázdil M, Roman R, Bob P, Rektor I. Cognitive network interactions and beta 2 coherence in processing non-target stimuli in visual oddball task. Physiol Res. 2009;58:139–148. doi: 10.33549/physiolres.931404. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, Vos JE, Mooibroek J, Rotterdam A. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher cortical functions in man. New York: Basic Books; 1966. [Google Scholar]
- Mazaheri A, Picton TW. EEG spectral dynamics during discrimination of auditory and visual targets. Brain Res Cogn Brain Res. 2005;24(1):81–96. doi: 10.1016/j.cogbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Miltner W, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Nunez PL. EEG coherence measures in medical and cognitive science: a general overview of experimental methods, computer algorithms, and accuracy. In: Eselt M, Zwiener U, Witte H, editors. Quantitative and topological EEG and MEG analysis. Jena: Universitätsverlag Druckhaus Mayer; 1997. [Google Scholar]
- Nunez PL, Silberstein RB, Shi Z, Carpenter MR, Srinivasan R, Tucker DM, Doran SM, Cadusch PJ, Wijesinghe RS. EEG coherency II: experimental comparisons of multiple measures. Clin Neurophysiol Mar. 1999;110(3):469–486. doi: 10.1016/S1388-2457(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Öniz A, Başar E. Prolongation of alpha oscillations in auditory oddball paradigm. Int J Psychophysiol. 2009;71:235–241. doi: 10.1016/j.ijpsycho.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Petsche H, Etlinger SC. EEG and thinking: power and coherence analysis of cognitive processes. Wien: Verlag Der Österreichischen Akademie Der Wissenscaften; 1998. [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston: Kluwer; 2003. pp. 83–98. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappelsberger P, Pockberger H, Petsche H. The contribution of the cortical layers to the generation of the EEG: field potential and current source density analyses in the rabbit’s visual cortex. Electroencephalogr Clin Neurophysiol. 1982;53:254–269. doi: 10.1016/0013-4694(82)90083-9. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Schack B, Chen AC, Mescha S, Witte H. Instantaneous EEG coherence analysis during the Stroop task. Clin Neurophysiol. 1999;110:1410–1426. doi: 10.1016/S1388-2457(99)00111-X. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Demiralp T, Başar E, Başar-Eroğlu C. Electroencephalogram alpha (8–15 Hz), responses to visual stimuli in cat cortex, thalamus, and hippocampus: a distributed alpha network? Neurosci Lett. 2000;292:175–178. doi: 10.1016/S0304-3940(00)01456-7. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Başar-Eroğlu C, Kolev V, Başar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;39:229–239. doi: 10.1016/S0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Toward new theories of brain function and brain dynamics. Int J Psychophysiol. 2001;39:87–89. doi: 10.1016/S0167-8760(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Solms M, Turnbull O. Emotion and motivation. In: Solms M, Turnbull O, editors. The brain and the inner world. New York: Other Press; 2002. pp. 105–137. [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Spencer KM, Polich J. Poststimulus EEG spectral analysis and P300: attention, task, and probability. Psychophysiology. 1999;36:220–232. doi: 10.1017/S0048577299971615. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer H, Başar E. Does frequency-analysis lead to better understanding of endogenous evoked-potentials. Int J Neurosci. 1985;29:189. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Tomberg C, Desmedt JE. Human perceptual processing: inhibition of transient prefrontal-parietal 40 Hz binding at P300 onset documented in non-averaged cognitive brain potentials. Neurosci Lett. 1998;255(3):163–166. doi: 10.1016/S0304-3940(98)00740-X. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase syncronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–232. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Verleger R, Heide W, Butt C, Kömpf D. Reduction of P3b in patients with temporo-parietal lesions. Cogn Brain Res. 1994;2:103–116. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long distance alpha-theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/S0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biol Psychiatry. 2003;54:1181–1192. doi: 10.1016/S0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Başar E. Evoked brain rhythms are altered markedly in middle-aged subjects: single-sweep analysis. Int J Neurosci. 1996;85:155–163. doi: 10.3109/00207459608986360. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Effects of temporal-parietal lesions on the somatosensory P3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol. 1992;84:139–148. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]
- Yener GG, Güntekin B, Öniz A, Başar E. Increased frontal phase-locking of event-related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int J Psychophysiol. 2007;64:46–52. doi: 10.1016/j.ijpsycho.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Yener G, Güntekin B, Başar E. Event related delta oscillatory responses of Alzheimer patients. Eur J Neurol. 2008;15:540–547. doi: 10.1111/j.1468-1331.2008.02100.x. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. A single-sweep analysis of the theta frequency band during an auditory oddball task. Psychophysiology. 1998;35:116–126. doi: 10.1017/S0048577298000663. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]