Abstract
The sex pheromone of the navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae), consists of two different types of components, one type including (11Z,13Z)-11,13-hexadecadienal (11Z,13Z-16:Ald) with a terminal functional group containing oxygen, similar to the majority of moth pheromones reported, and another type including the unusual long-chain pentaenes, (3Z,6Z,9Z,12Z,15Z)-3,6,9,12,15-tricosapentaene (3Z,6Z,9Z,12Z,15Z-23:H) and (3Z,6Z,9Z,12Z,15Z)- 3,6,9,12,15-pentacosapentaene (3Z,6Z,9Z,12Z,15Z-25:H). After decapitation of females, the titer of 11Z,13Z-16:Ald in the pheromone gland decreased significantly, whereas the titer of the pentaenes remained unchanged. Injection of a pheromone biosynthesis activating peptide (PBAN) into the abdomens of decapitated females restored the titer of 11Z,13Z-16:Ald and even increased it above that in intact females, whereas the titer of the pentaenes in the pheromone gland was not affected by PBAN injection. In addition to common fatty acids, two likely precursors of 11Z,13Z-16:Ald, i.e., (Z)-11-hexadecenoic and (11Z,13Z)-11,13-hexadecadienoic acid, as well as traces of (Z)-6-hexadecenoic acid, were found in gland extracts. In addition, pheromone gland lipids contained (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoic acid, which also was found in extracts of the rest of the abdomen. Deuterium-labeled fatty acids, (16,16,16-D3)-hexadecanoic acid and (Z)-[13,13,14,14,15,15,16,16,16-D9]-11-hexadecenoic acid, were incorporated into 11Z,13Z-16:Ald after topical application to the sex pheromone gland coupled with abdominal injection of PBAN. Deuterium label was incorporated into the C23 and C25 pentaenes after injection of (9Z,12Z,15Z)- [17,17,18,18,18-D5]-9,12,15-octadecatrienoic acid into 1–2 d old female pupae. These labeling results, in conjunction with the composition of fatty acid intermediates found in pheromone gland extracts, support different pathways leading to the two pheromone components. 11Z,13Z-16:Ald is probably produced in the pheromone gland by Δ11 desaturation of palmitic acid to 11Z-16:Acid followed by a second desaturation to form 11Z,13Z-16:Acid and subsequent reduction and oxidation. The production of 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H may take place outside the pheromone gland, and appears to start from linolenic acid, which is elongated and desaturated to form (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoic acid, followed by two or three further elongation steps and finally reductive decarboxylation.
Key Words: Sex pheromone; Biosynthesis; Amyelois transitella; Linolenic acid; (5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-icosapentaenoic acid; (3Z, 6Z, 9Z, 12Z, 15Z)-3,6,9,12,15-tricosapentaene; (11Z,13Z)-11,13-hexadecadienal; Bifunctional ∆11 desaturase; PBAN; Pyralidae
Introduction
Unsaturated C10–C18 straight chain alcohols, aldehydes, and acetates have been designated as “Type I” sex pheromones of lepidopteran insects, and the majority of the known lepidopteran pheromones fall into this class (Ando et al. 2004). In contrast, a number of species in the families Geometridae, Arctiidae, Lymantriidae, and Noctuidae use polyunsaturated C17–C23 straight chain hydrocarbons and the corresponding mono- and diepoxide derivatives as pheromone components, classed as “Type II” sex pheromones because of their distinct differences from the Type I group (Millar 2000; Ando et al. 2004).
The Type I alcohol, aldehyde, and acetate components are biosynthesized de novo from fatty acid precursors by a series of desaturation, chain elongation, and chain shortening steps that produce pheromone components with specific chain lengths, double bond positions, and double bond geometries. The structures are completed by the adjustment of the terminal functional group by reduction, oxidation, or tranesterification to provide alcohols, aldehydes, or acetates, respectively (Jurenka and Roelofs 1993; reviewed in Tillman et al. 1999). The evidence to date suggests that all of these transformations are carried out in the pheromone gland.
The biosynthesis of Type II pheromones also has been studied, and from reports of pheromone biosynthesis in the Lymantriidae (Kasang et al. 1979), Arctiidae (Rule and Roelofs 1989), and Geometridae (reviewed in Ando et al. 2008), it is clear that there are significant differences between the biosynthetic pathways leading to the two types of pheromones. These include differences in the biosynthetic sites, the substrates, the enzymes involved, and the endocrine regulation of biosynthesis. In distinct contrast to the site of production of Type I pheromones, it is likely that the polyunsaturated hydrocarbons that constitute Type II pheromones are produced in oenocyte cells by chain extension of diet-derived linoleic or linolenic acids, culminating in reductive decarboxylation to remove the terminal functional group (Rule and Roelofs 1989; reviewed in Ando et al. 2008). The final steps are slightly different for odd- vs. even-numbered carbon skeletons. The former probably result from 0–3 cycles of 2-carbon unit chain extension of linoleic or linolenic precursors and subsequent removal of one carbon by reductive decarboxylation to give an odd-numbered chain. In contrast, recent evidence suggests that even-numbered chains probably are produced by the same series of 2-carbon chain extension steps, then α-oxidation and loss of one carbon, followed by loss of a second carbon as CO or CO2 by an oxidative mechanism (Goller et al. 2007). In some cases, further desaturation also occurs somewhere in the pathway to produce compounds such as the tetraene hydrocarbon pheromones of the arctiid moth Utetheisa ornatrix (Linnaeus) (Choi et al. 2007) and the geometrid moth Operophtera brumata (L.) (Zhao and Löfstedt, unpublished data). The newly formed polyene skeletons then are transported through the hemolymph by lipophorin carriers from the oenocytes to the pheromone gland (Wei et al. 2004; Matsuoka et al. 2006). The polyene hydrocarbons are emitted from the pheromone gland unchanged, or are further transformed by one or more epoxidation steps to produce unsaturated epoxide pheromone components for release (Miyamoto et al. 1999; Jurenka et al. 2003; Wei et al. 2003; Fan et al. 2004; Wei et al. 2004; Matsuoka et al. 2006).
The endocrine regulation of Type II pheromone biosynthesis also is markedly different from that of Type I pheromones. According to studies published to date, pheromone biosynthesis activating neuropeptides (PBANs) are not involved in the production of unsaturated hydrocarbon pheromone components in the arctiid moth U. ornatrix (Choi et al. 2007) or the geometrid moth Ascotis selenaria cretacea (Butler) (Wei et al. 2004), but they are involved in the epoxidation of these hydrocarbons in the pheromone gland, for those species that use epoxide pheromones alone or in combination with unsaturated hydrocarbons (e.g., the lymantriid moth Lymantria dispar L. (Jurenka et al. 2003) and the geometrid moth A. selenaria cretacea B. (Ando et al. 1997; Wei et al. 2004)).
For many years, it was presumed that a particular lepidopteran species produced either Type I or Type II pheromones, but not both. However, examples of species whose pheromone blends contain compounds of both types recently have been found in two moth families. For example, the pheromone blend of the crambid moth Neoleucinodes elegantalis consists of (E)-11-hexadecen-1-ol and (3Z,6Z,9Z)-3,6,9-tricosatriene (3Z,6Z,9Z-23:H) (Cabrera et al. 2001), whereas another crambid species, Deanolis sublimbalis, uses a blend consisting of (Z)-11-hexadecenal and 3Z,6Z,9Z-23:H (Gibb et al. 2007). Within the pyralid moth family, several examples are known, including the meal moth, Pyralis farinalis (L.) [ (11Z,13Z)-11,13-hexadecadienal (11Z,13Z-16:Ald) (Landolt and Curtis 1982) + 3Z,6Z,9Z,12Z,15Z-23:H; Leal et al. 2005; Kuenen et al. 2010], the navel orangeworm, Amyelois transitella (Walker) [11Z,13Z-16:Ald (Coffelt et al. 1979) + 3Z,6Z,9Z,12Z,15Z-23:H; Leal et al. 2005; Millar et al. 2005; Kuenen et al. 2010], and the fir coneworm moth, Dioryctria abietivorella (Grote) [(9Z,11E)-9,11-tetradecadienyl acetate (9Z,11E-14:OAc) + 3Z,6Z,9Z,12Z,15Z-25:H; Millar et al. 2005]. Several other Dioryctria species also appear to have analogous pheromone blends containing both Type I and Type II components (Miller et al., 2010a, b; Löfstedt et al. unpub. data).
The biosynthesis of the blends of Type I and Type II pheromone components in such species has not yet been studied. On the basis of previous pheromone biosynthesis studies, the aldehyde and pentaene pheromone components found in A. transitella pheromone glands probably arise from two independent biosynthetic pathways. The conjugated diene aldehyde, 11Z,13Z-16:Ald, may be produced from palmitic acid in the pheromone gland, by pathways analogous to those for other conjugated diene Type I pheromones. For example, production of 11Z,13Z-16:Acid, a precursor to the conjugated pheromone in the pine processionary moth, Thaumetopoea pityocampa (Denis and Schiffermüller), involves a bifunctional ∆11 desaturase (Quero et al. 1997; Abad et al. 2007). By analogy to other systems, it seems likely that the two long-chain pentaenes, 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H, are synthesized in oenocyte cells via chain elongation, desaturation, and decarboxylation of diet-derived linolenic acid or other unsaturated fatty acid precursors, and transported to the gland through the hemolymph (Schal et al. 1998a, b; Jurenka and Subchev 2000; Matsuoka et al. 2006). It also is noteworthy that the above polyenes differ in structure by only one two-carbon unit, suggesting that the polyene motif that is common to both components may be synthesized first, followed by chain length adjustment in the later steps of the biosynthesis.
Thus, the objectives of the work reported here were to study the biosynthesis of the polyene hydrocarbon components and the diene aldehyde pheromone component present in the navel orangeworm pheromone gland, and to gain a better understanding of the mechanisms by which the biosynthesis of these two distinct types of pheromone components are regulated in A. transitella, including examining the possible role of PBAN.
Methods and Materials
Insects
The A. transitella moths used in this study were obtained from the Department of Entomology, University of California, Riverside, CA, USA. The larvae were fed on a wheat germ diet as previously described (Coffelt et al. 1978; Girling and Cardé 2006), and kept in 4 liter glass jars in controlled environment chambers with 17:7 L:D, 25 ± 1°C and 65% relative humidity. Newly emerged adult females were collected daily and held in single-sex cohorts fed with a 10% honey solution. One- to 2-d old female pupae and adults were used throughout this study. Pupae were sexed by the morphological differences in their external genitalia.
Chemicals
PBAN (Helicoverpa zea pheromone biosynthesis-activating neuropeptide) was purchased from Bachem (Weil am Rhein, Germany). Methyl (9Z,12Z,15Z,18Z,21Z)-9,12,15,18,21-tetracosapentaenoate and (16,16,16-D3)-hexadecanoic acid (D3-16:Acid) were purchased from Larodan Fine Chemicals, Malmö, Sweden. The synthesis of (Z)-[13,13,14,14,15,15,16,16,16-D9]-11-hexadecenoic acid (D9-11Z-16:Acid) was described in Löfstedt et al. (1994), and (9Z,12Z,15Z)-[9,10,12,13,15,16-D6]-9,12,15-octadecatrienoic acid (D6-linolenic acid), (9Z,12Z,15Z)-[17,17,18,18,18-D5]-9,12,15-octadecatrienoic acid (D5-linolenic acid), and (11Z,14Z,17Z)-[8,8,9,9-D4]-icosatrienoic acid (D4-icosatrienoic acid) were obtained from R. Adlof as gifts. 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H were synthesized as described by Millar et al. (2005), and 11Z,13Z-16:Ald and 11Z,13Z-16:OH were synthesized as described in Kuenen et al. (2010).
(Z)- and (E)-6-Hexadecenol (6Z-16:OH and 6E-16:OH) from our laboratory collection of pheromone components were used to prepare the reference standards needed to identify the double bond configuration of methyl 6-hexadecenoate in the insect extracts. Thus, 2 mg of 6Z-16:OH or 6E-16:OH were dissolved in 1 ml of dimethylformamide (DMF) in a 4 ml screw-cap vial, 100 mg of pyridinium dichromate were added, and the mixture was stirred overnight at room temperature. Then 1 ml of diethyl ether was added, and the mixture was vortexed briefly to precipitate the chromium salts. Distilled water was added, and the ether layer was then collected, washed twice with distilled water to remove traces of DMF, dried over anhydrous sodium sulfate, and evaporated to dryness. Methyl esters were produced by acid-catalyzed esterification (MeOH/HCl at 80°C for 1 h).
A reference standard of methyl (11Z,13Z)-11,13-hexadecadienoate (11Z,13Z-16:Me) also was prepared from 11Z,13Z-16:Acid by acid-catalyzed esterification.
Extraction of Insect Tissues
The pheromone glands of female A. transitella, a broad, chevron-shaped structure located on the ventrolateral surface of the intersegmental membrane between abdominal scleromata VIII and IX (Srinivasan et al. 1986), were dissected from cohorts of individual 1- to 2-d-old intact virgin female moths in both the photophase (1 h before lights off) and scotophase (6 h after lights off). Each gland was extracted for 30 min in 10 μl of hexane containing 1 ng of (3Z,6Z,9Z)-3,6,9-henicosatriene (3Z,6Z,9Z-21:H) as internal standard. After removing the pheromone glands, the hemolymph, the abdominal cuticle, and the whole abdomen (i.e., cuticle, hemolymph, and abdominal tissues) were extracted separately. For extraction of the hemolymph, ca. 10 μl of modified Weever’s saline (21 mM KCl, 12 mM NaCl, 3 mM CaCl2, 18 mM MgCl2, 170 mM glucose, 5 mM PIPES, 9 mM KOH, adjusted to pH 6.6; Carrow et al. 1981) were injected first into the abdomen, and then the mixture of hemolymph and saline was transferred into a glass vial insert by syringe. Methanol (ca. 30–40 μl per 3–4 female equivalents of hemolymph) was added, and the mixture was extracted × 3 with 10 μl hexane containing 0.1 ng/μl of internal standard (3Z,6Z,9Z-21:H).
The abdominal cuticle was extracted as previously described (Jurenka et al. 2003), first removing the majority of the scales on the abdomen by application of a gentle vacuum and then removing the abdomen at the junction of the thorax. The abdomen was cut laterally and pinned open. The ovaries were removed along with the majority of the remaining fat body and organs, leaving mostly epidermal tissue attached to the cuticle. The cuticle was rinsed first with 30 μl methanol and then extracted by vortexing 3× with 100 μl hexane containing 0.1 ng/μl 3Z,6Z,9Z-21:H internal standard, 10 min each time. After extraction, the hexane layers were combined into a new glass tube. The external abdominal cuticle also was extracted in this study by successively dipping five intact female abdomens into 100 μl hexane, with 5 sec dipping per individual. The hexane extract then was concentrated to approximately 10 μl for GC-MS analysis.
To extract the hydrocarbons from the whole abdomen with only the pheromone gland removed, the abdominal tissues were immersed in 100 μl methanol in a 2 ml vial and disrupted with a probe sonicator for a few seconds. The resulting homogenate was extracted by sequential vortexing 3× with 100 μl hexane containing 0.1 ng/μl 3Z,6Z,9Z-21:H internal standard. The hemolymph, cuticle, and abdomen tissue homogenate extracts were dried with anhydrous sodium sulfate and purified by passage through a 7 × 0.2 cm column of 100-200 mesh Florisil. Approximately 30-40 μl of a crude hexane extract were added to the column, and hydrocarbons were eluted with 1 ml hexane.
Base Methanolysis and Methylthiolation
After the first extraction with hexane, the remaining glandular and abdominal tissues were extracted further for fatty acyl moieties. Thus, the glandular tissue was extracted with 20 μl chloroform:methanol (2:1 v:v) in a glass vial insert for 24 h at room temperature, then the tissue was removed and the extract was concentrated under a stream of nitrogen. The residue was subjected to base methanolysis to convert fatty acyl moieties to the corresponding methyl esters as described by Bjostad and Roelofs (1984). First, 20 μl of 0.5 M KOH in methanol were added, and the vial insert was placed inside a 4 ml screw-cap vial that was heated for 1 h at 40°C. After cooling, 20 μl of 0.5 M HCl in methanol were added to neutralize the alkali, and the mixture was extracted 3× with 20 μl hexane. The combined hexane layers were washed twice with 30 μl distilled water, then dried over anhydrous sodium sulfate.
The residue from extraction of the abdominal tissue with hexane, in 100 μl methanol, was re-extracted with 200 μl chloroform for 24 h at room temperature. The chloroform/methanol extract was transferred to a new 2 ml vial and concentrated under a stream of nitrogen, then subjected to methanolysis by addition of 200 μl of 0.5 M KOH in methanol and warming to 40°C for 1 h. After cooling, 200 µl of 0.5 M HCl in methanol were added, and the mixture was extracted 3× with 200 μl hexane, vortexing for 10 min each time. The hexane layers were combined and washed 2× with 200 μl distilled water, and dried over anhydrous sodium sulphate. The resulting solutions of fatty acid methyl esters were analyzed by GC-MS as described below. The double bond positions of monounsaturated methyl esters were determined by GC-MS analysis of their dimethyldisulfide (DMDS) adducts (Buser et al. 1983; Dunkelblum et al. 1985). DMDS derivatizations were carried out by adding 50 µl DMDS and 10 µl of 5% (by weight) I2 in diethyl ether to 20 µl of the solution of methylated fatty acids extracted from the pheromone glands, or 50 µl of the methylated fatty acids extracted from the abdomen tissues. Each reaction was stirred in a 2 ml screw-cap vial overnight at 40°C, then 200 µl of hexane were added, and the mixture was washed with 50 µl 5% aqueous sodium thiosulfate. The organic layer then was dried over anhydrous sodium sulfate and evaporated to dryness. The product was taken up in hexane for analysis.
In addition, an extract of artificial diet (1 g diet in 1 ml chloroform/methanol for 24 h) was used to analyze fatty acids from the diet.
Effect of PBAN on Pheromone Production
Unmated female moths (0–24-h-old) were decapitated at the end of the photophase and kept individually in small plastic containers. A piece of wet filter paper was placed inside each container to maintain high humidity so that the decapitated animals would not desiccate. After 24 h, 10 pmol PBAN in 4 μl of Weever’s saline were injected into the abdomen of each decapitated female. Equal volumes of Weever’s saline were injected into decapitated animals as controls. The pheromone glands of treated and control insects were dissected 2 h later, and each gland was extracted with 10 μl hexane containing 0.1 ng (Z)-12-tetradecenyl acetate and 0.2 ng (1,3Z,6Z,9Z)-3,6,9-nonadecatetraene as internal standards.
Labeling Experiment
The proposed pheromone precursors, D3-16:Acid, D9-11Z-16:Acid, D6-linolenic acid, D5-linolenic acid, and D4-11Z,14Z,17Z-20:Acid were used to probe the aldehyde and pentaenes biosynthetic pathways. D3-16:Acid, D9-11Z-16:Acid, and D6-linolenic acid were dissolved in dimethylsulfoxide (DMSO) at a concentration of 12.5 μg/μl and topically applied to the extruded pheromone gland (0.2 μl of DMSO solution; 2.5 μg/gland) of PBAN-injected decapitated female adults. D5-linolenic acid and D4-11Z,14Z,17Z-20:Acid dissolved in DMSO also were topically applied to pheromone glands of the control group of intact female adults that had not been injected with PBAN. After a 2 h incubation, pheromone glands were dissected and extracted as described above.
For injection of the possible pheromone precursors, D5-linolenic acid and D4-11Z,14Z,17Z-20:Acid were dissolved in vegetable oil (75% rapeseed and 25% sunflower oil, ICA, Sweden.) and injected into the abdomens of 1- to 2-d-old female pupae (1 µl of 100 μg/μl solution per female). After adult emergence, at the 6th h of the scotophase, the pheromone gland was removed from 2-d-old females and extracted with 10 μl hexane containing 0.1 ng/μl 3Z,6Z,9Z-21:H as internal standard. After removal of the pheromone gland, the remaining abdominal tissue including the scales was extracted 3× with 100 μl hexane containing 0.1 ng/μl 3Z,6Z,9Z-21:H as internal standard as described above. D4-11Z,14Z,17Z-20:Acid dissolved in vegetable oil also was injected into 2-d-old female adults at the 2nd h in the scotophase, and the pheromone gland and remaining abdominal tissue were subsequently extracted at the 6th h in the scotophase, followed by the same protocol.
Coupled Gas Chromatography-Mass Spectrometry
The aldehyde and pentaene components in pheromone gland extracts, the corresponding deuterium labeled compounds and precursors, and the fatty acid methyl esters derived from methanolysis of tissue and diet extracts were analyzed with a Hewlett-Packard (Palo Alto CA, USA) 5972 mass selective detector coupled to an HP 5890 series II GC equipped with a capillary column (HP-1MS or InnoWax (SGE, Austin, TX, USA), both 30 m × 0.25 mm i.d.). The HP-1MS column was programmed from 80°C at 10°C/min to 220°C, hold for 3 min, then to 250°C at 4°C/min, hold for 5 min, and finally to 280°C at 20°C/min, hold for 5 min. The InnoWax column was programmed from 80°C at 10°C/min to 200°C, then to 230°C at 1°C/min, with a final hold for 20 min at 230°C.
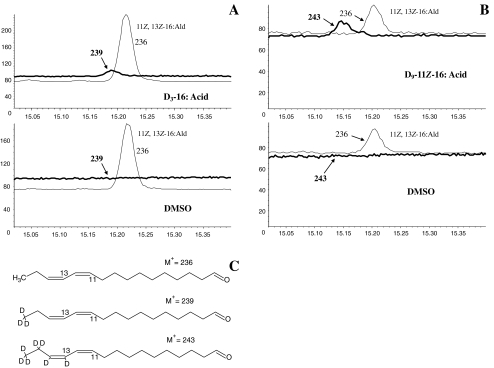
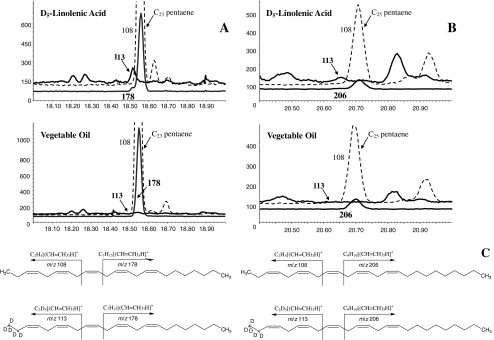
Selected ion monitoring (SIM) was used to monitor the pheromone compounds and the incorporation of labeled precursors. The following characteristic ions were chosen for the aldehyde and the hydrocarbons: when each of D3-16:Acid and D9-11Z-16:Acid was applied, the molecular ion at m/z 236 was chosen for 11Z,13Z-16:Ald, with m/z 239 and 243 being used to monitor the corresponding D3-11Z,13Z-16:Ald and D7-11Z,13Z-16:Ald, respectively. When D6-linolenic acid was applied to probe the pentaenes biosynthetic pathway, the diagnostic ions at m/z 108 and 178 were used to monitor unlabeled 3Z,6Z,9Z,12Z,15Z-23:H, and m/z 108 and 206 were used to monitor unlabeled 3Z,6Z,9Z,12Z,15Z-25:H, whereas m/z 112 (108 + 4) and m/z 178 or 206 were used to monitor labeled 3Z,6Z,9Z,12Z,15Z-23:H or 3Z,6Z,9Z,12Z,15Z-25:H, respectively. Specifically, the ions at m/z 178 and 206 remain unchanged in the labeled pentaenes because they arise from cleavages from the nondeuterated end of the chains (Millar et al. 2005). When D5-linolenic acid was applied, m/z 113 (108 + 5) and m/z 178 or 206 were used to monitor labeled 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H, respectively; and when D4-Z11,Z14,Z17-20:Acid was used, m/z 108 and m/z 180 (178+2) were chosen for monitoring labeled 3Z,6Z,9Z,12Z,15Z-23:H.
Results
Regulation of Pheromone Production
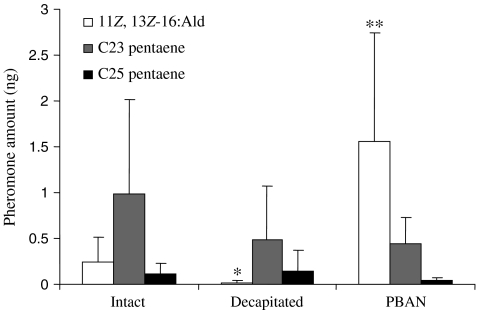
The amount of 11Z,13Z-16:Ald in pheromone gland extracts from decapitated females 24 h after decapitation and 2 h after injection of saline was barely detectable (0.02 ± 0.02 ng) and was significantly lower than the titer in intact control females (0.25 ± 0.27 ng, P = 0.016, N = 10) (Fig. 1). However, 2 h after injection of PBAN into the abdomens of decapitated females, the pheromone gland titer of 11Z,13Z-16:Ald increased to 1.6 ± 1.2 ng (N = 9), significantly higher than the titer in either decapitated, saline-injected control females (P < 0.001) or intact females (P = 0.001). By contrast, the titers of 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H were not affected by PBAN; the levels of each compound found in glands from normal, decapitated PBAN-injected, or decapitated saline-injected control females were not significantly different (Fig. 1).
Fig. 1.
Titers of 11Z,13Z-16:Ald, 3Z,6Z,9Z,12Z,15Z-23:H, and3Z,6Z,9Z,12Z,15Z-25:H in pheromone gland extracts from Amyelois transitella intact females, decapitated females injected with saline, and decapitated females injected with 10 pmol PBAN in saline (PBAN). For each column color, significant differences between means were determined by Student’s t-tests (“*”: P < 0.05; “**”: P < 0.01)
The two long-chain pentaenes were found in hexane extracts from various tissues including the pheromone gland, the abdominal cuticle, the abdominal tissue, and the hemolymph of 1- to 2-d-old virgin females (Table 1). The titers of each of the two pentaenes in the pheromone gland and abdominal tissue extracts did not differ significantly between the photophase and scotophase (Table 1). There was no evidence for either of the pentaenes on external cuticular surfaces; extracts prepared by quickly dipping intact adult insects in solvent contained no detectable amounts of the pentaenes.
Table 1.
Titer (ng) of C23 and C25 pentaenes in various tissues of 1–2 day old virgin female navel orangeworm mothsa
| Compound | Pheromone Gland | Abdomenb | Abdominal Cuticlec | Hemolymphd | ||
|---|---|---|---|---|---|---|
| (N = 5) | (N = 5) | (N = 7) | (N = 2) | |||
| photophase | scotophase | photophase | scotophase | scotophase | scotophase | |
| 3Z,6Z,9Z,12Z,15Z-23:H | 0.40 ± 0.47 | 0.45 ± 0.47 | 135 ± 70 | 111 ± 39 | 17 ± 8 | 2.9 ± 0.2 |
| 3Z,6Z,9Z,12Z,15Z-25:H | 0.03 ± 0.01 | 0.07 ± 0.05 | 10 ± 6 | 18 ± 11 | 2.0 ± 1.7 | 0.71 ± 0.12 |
aIndividual 1- to 2-d-old intact virgin female moths (or in the case of hemolymph, samples of four females) were extracted during the last hour of photophase or the 6th hour of scotophase, respectively. The titer of each polyene (mean ± SD) was determined by GC-MS in selected ion monitoring mode using the base peak (m/z 79) for quantification versus 3Z,6Z,9Z-21:H as internal standard. Student’s t-tests showed no significant differences at the 5% level between photophase and scotophase extracted polyenes in the pheromone gland or whole abdominal tissue
bAbdomen = the whole abdomen with the pheromone gland removed, homogenized and extracted
cAbdominal cuticle = the abdominal cuticle with the pheromone gland, hemolymph, fat body, and other tissues removed
dHemolymph = the mixture of hemolymph and saline extracted from the abdomen, after injection of 10 µl Weever’s saline per female
Fatty Acid Composition in Abdominal Tissue and Pheromone Gland
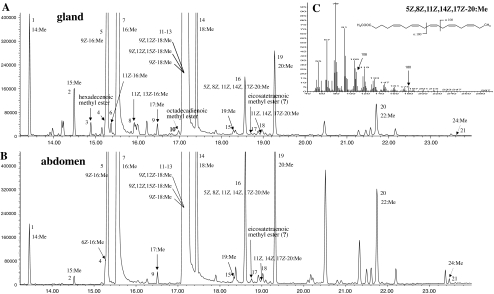
In addition to saturated and unsaturated fatty acid methyl esters such as methyl tetradecanoate (14:Me, related abbreviations are used hereafter for analogous fatty acid methyl esters), 15:Me, 16:Me, 9Z-16:Me, 17:Me, 18:Me, 9Z-18:Me, 9Z,12Z-18:Me, and 9Z,12Z,15Z-18:Me, the methanolized abdominal tissue and pheromone gland extracts contained the methyl esters of some longer chain saturated and unsaturated fatty acids, including 20:Me, 22:Me, 24:Me, 5Z,8Z,11Z,14Z,17Z-20:Me, as well as traces of two compounds tentatively identified as 11Z,14Z,17Z-20:Me and a C20:4 methyl ester (Fig. 2, Table 2). In particular, the mass spectrum of 5Z,8Z,11Z,14Z,17Z-20:Me contained diagnostic ions at m/z 108 and m/z 180 from the characteristic cleavages and rearrangements of a fatty acid methyl ester with a 5,8,11,14,17 pentaene system (Fellenberg et al. 1987), as well as m/z 74 from McLafferty rearrangement of the methyl ester. The molecular ion (m/z 316) was not detected in full scan, but it was found in the more sensitive selected ion monitoring mode. Furthermore, this acid must be produced by the insect because it was not found in extracts of the diet used to rear the insects. 11Z,14Z,17Z-20:Acid also was tentatively identified on the basis of its matching retention time with that of an authentic standard, the characteristic base peak at m/z 79, and the diagnostic fragment at m/z 108 from the same cleavage and rearrangement as shown in Fig. 2c for 5Z,8Z,11Z,14Z,17Z-20:Acid.
Fig. 2.
Total ion chromatograms of the fatty acid compositions (as the methyl esters) of representative pheromone gland (a) and abdominal tissue (b) extracts (HP-1MS capillary column). Fatty acid methyl esters are coded with a number indicating chain length, followed by a number indicating the number of double bonds, with the positions and geometries given if known. Peak numbers correspond to those listed in Table 2. The omega ion at m/z 108, diagnostic for a 3,6,9-triene motif, and the alpha ion at m/z 180, diagnostic for a 5,8,11-triene motif counting from the other end of the chain are indicated in the mass spectrum of the C20:5 acid methyl ester (c). The weak molecular ion at m/z 316 was not detected in full scan mode, but it was detected in the more sensitive selected ion monitoring mode, at a same retention time as the two diagnostic ions at m/z 79 and 108
Table 2.
Fatty acid methyl esters detected in the pheromone gland and abdomen of female navel orangeworm
| Compound | Peak No.a | Pheromone gland | Abdomenc | Criteria used in identifications | ||
|---|---|---|---|---|---|---|
| Relative amountb | Relative amount | MSd | Retention timee | DMDSf derivative | ||
| (N = 7) | (N = 6) | |||||
| 14:Me | 1 | 12.8 ± 8.8 | 2.0 ± 0.9 | √ | √ | |
| 15:Me | 2 | 8.2 ± 5.7 | 0.20 ± 0.04 | √ | √ | |
| Hexadecenoic methyl esterg | 3 | 2.2 ± 1.4 | – | |||
| 6Z-16:Meh | 4 | – | 0.4 ± 0.2 | √ | √ | √ |
| 9Z-16:Me | 5 | 31.8 ± 12.0 | 20.1 ± 7.3 | √ | √ | √ |
| 11Z-16:Me | 6 | 1.4 ± 0.8 | – | √ | √ | √ |
| 16:Me | 7 | 100 | 100 | √ | √ | |
| 11Z,13Z-16:Me | 8 | 1.9 ± 1.0 | – | √ | √ | |
| 17:Me | 9 | 1.9 ± 1.1 | 0.26 ± 0.03 | √ | √ | |
| Octadecadienoic methyl esterg | 10 | 1.3 ± 0.8 | – | |||
| 9Z,12Z-18:Me | 11 | 33.3 ± 4.5 | 38.3 ± 6.2 | √ | √ | |
| 9Z,12Z,15Z-18:Me | 12 | 18.5 ± 4.7 | 10.7 ± 2.8 | √ | √ | |
| 9Z-18:Me | 13 | 90.7 ± 8.4 | 76.3 ± 10.2 | √ | √ | √ |
| 18:Me | 14 | 21.2 ± 5.2 | 10.1 ± 1.4 | √ | √ | |
| 19:Me | 15 | 0.8 ± 0.5 | 0.14 ± 0.07 | √ | √ | |
| 5Z,8Z,11Z,14Z,17Z-20:Me | 16 | 2.5 ± 0.8 | 2.0 ± 0.4 | √ | √ | |
| eicosatetraenoic methyl ester(?)g, i | 17 | <0.1 | 0.10 ± 0.04 | |||
| 11Z,14Z,17Z-20:Mei | 18 | <0.1 | <0.1 | √ | √ | |
| 20:Me | 19 | 3.6 ± 1.1 | 2.2 ± 0.4 | √ | √ | |
| 22:Me | 20 | 3.1 ± 2.2 | 1.6 ± 0.3 | √ | √ | |
| 24:Me | 21 | 0.8 ± 0.5 | 0.17 ± 0.14 | √ | √ | |
aNumbers refer to gas chromatographic peaks in Fig. 2
bAmounts are reported relative to that of methyl palmitate, the most abundant component
cAbdomen = the whole abdomen with the pheromone gland removed, homogenized and extracted
dFull scan mass spectrum
eRetention time match on both the HP1-MS and InnoWax columns
fDMDS derivative confirmation of double bond location and configuration
gThe double bond position was not determined
h6Z-16:Me in pheromone gland extract was well separated from 9Z-16:Me on the InnoWax column, but the two compounds were not resolved on the HP-1MS column. However, the same compounds in the abdomen extracts were separated on both columns. The double bond position of 6Z-16:Me was confirmed by DMDS analysis and the configuration was confirmed by comparing the mass spectrum and retention time of both the methyl ester and the corresponding DMDS adduct with those of the reference 6Z-16 and 6E-16 isomers
iPresent in trace amount
In extracts of the pheromone gland, the aldehyde pheromone intermediates 11Z-16:Me and 11Z,13Z-16:Me were found, along with traces of a nonconjugated doubly unsaturated C18 fatty acid that was not completely identified (peak 6, peak 8, peak10, Fig. 2a). These three compounds were not found in the abdominal tissue extracts.
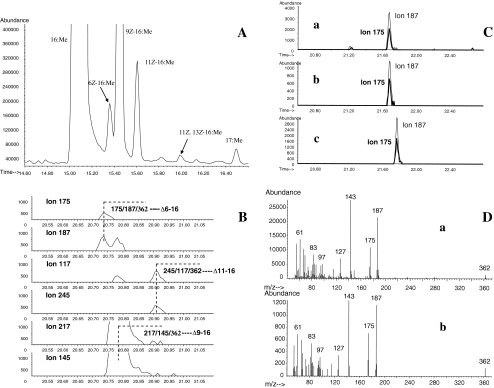
Another unusual monoenoic C16 fatty acid methyl ester was found in the gland and abdomen extracts after DMDS derivatization of the methyl esters fraction. Specifically, a C16 DMDS adduct was found with a molecular ion at m/z 362 (6%) and diagnostic fragment ions at m/z 175 (42%) and 187 (81%), corresponding to the DMDS derivative of Δ6-16:Me. By comparing the mass spectrum and gas chromatographic retention times of the methyl ester and its DMDS adduct with those of authentic 6Z-16:Me and 6E-16:Me and their DMDS adducts, the natural Δ6-16 component was shown to be the 6Z-16:Me isomer (Fig. 3).
Fig. 3.
Representative sections of the total ion chromatograms (TIC) of fatty acid methyl ester DMDS adducts, analyzed on an InnoWax capillary column. a Section of TIC from pheromone gland fatty acid methyl esters. b Selected ion monitoring GC-MS analyses of monounsaturated C16 fatty acid methyl ester DMDS adducts. Double bond positions were identified from the diagnostic ions from cleavage of the C-C bond between the two carbons bearing methylthiol groups: Δ6-16, 175/187/362; Δ9-16, 217/145/362; Δ11-16, 245/117/362. c Confirmation of the “cis” configuration in the insect-produced Δ6-16 monounsaturated fatty acid methyl ester by comparison of the chromatographic retention times of the DMDS adducts, using the diagnostic ions at m/z 175 and 187. a: Δ6-16:Me DMDS adduct in insect abdomen extract; b: authentic 6Z-16:Me DMDS adduct; c: authentic 6E-16:Me DMDS adduct. d Full scan mass spectrum of the Δ6-16 monounsaturated fatty acid methyl ester DMDS adduct from insect abdomen extract (a) and authentic 6Z-16:Me DMDS adduct (b)
Labeling Experiment Results
Topical application of D3-16:Acid and D9-11Z-16:Acid to the pheromone gland of PBAN-injected decapitated females resulted in incorporation of the labeled precursors into 11Z,13Z-16:Ald, with a labeled to unlabelled compounds ratio of 2.7 ± 2.0 % (N = 4) and 44 ± 23% (N = 6), respectively (Fig. 4a and b). These results demonstrated that both of these acids likely are biosynthetic precursors to 11Z,13Z-16:Ald, and that 11Z,13Z-16:Ald synthesis occurs in the pheromone gland.
Fig. 4.
Selected ion monitoring chromatograms showing incorporation of labeled fatty acid precursors into 11Z,13Z-16:Ald, using ions 239 and 243 to monitor D3-11Z,13Z-16:Ald and D7-11Z,13Z-16:Ald, respectively. a Deuterium label incorporation into D3-11Z,13Z-16:Ald after topical application of D3-16:Acid in DMSO onto the pheromone gland; b Deuterium label incorporation into D7-11Z,13Z-16:Ald after topical application of D9-11Z-16:Acid in DMSO onto the pheromone gland; c diagnostic ions for unlabeled 11Z,13Z-16:Ald, D3-labeled 11Z,13Z-16:Ald and D7-labeled 11Z,13Z-16:Ald
When D5-linolenic acid was injected into 1-to 2-d-old female pupae, the labeled precursor was incorporated into both the C23 and C25 pentaenes (Fig. 5), with a labeled to unlabeled compounds ratio of 4.9 ± 0.6% for C23 pentaene in the pheromone gland extracts, a 4.0 ± 1.2% ratio in C23 pentaene extracted from the abdominal tissues (N = 3), and a 3.6 ± 0.2% ratio (N = 3) in C25 pentaene extracted from abdominal tissues. Because of the small amount of native C25 pentaene in the pheromone gland, among the labeled samples only one provided clear evidence for incorporation of label (6.0%). These results demonstrated that linolenic acid is a precursor for both of the pentaene components, but they do not reveal where the biosynthesis of the pentaenes occurs. However, topical application of D6-linolenic acid to the pheromone gland coupled with PBAN injection, or topical application of D5-linolenic acid onto the pheromone gland without PBAN injection resulted in no detectable incorporation of label into either of the pentaenes. This suggests that the pentaenes are not synthesized in the pheromone gland, and consequently, that they must be synthesized elsewhere in the abdomen and transported to the pheromone gland for release.
Fig. 5.
Selected ion monitoring chromatograms showing incorporation of labeled fatty acid precursors into 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H after abdominal injection of D5-linolenic acid into 1–2 d old pupae. a Deuterium label incorporation into D5-3Z,6Z,9Z,12Z,15Z-23:H; b Deuterium label incorporation into D5-3Z,6Z,9Z,12Z,15Z-25:H; c Ions 108 and 178, and ions 108 and 206 were used to monitor unlabeled 3Z,6Z,9Z,12Z,15Z-23:H and 3Z,6Z,9Z,12Z,15Z-25:H, respectively. Ions 113 and ion 178 were used to monitor D5-3Z,6Z,9Z,12Z,15Z-23:H, and ions 113 and 206 were used to monitor D5-3Z,6Z,9Z,12Z,15Z-25:H. Because of the small amounts of both native and labeled 3Z,6Z,9Z,12Z,15Z-25:H, the 206 ion was not detected in labeled D5-3Z,6Z,9Z,12Z,15Z-25:H
Topical application of D4-11Z,14Z,17Z-20:Acid onto the pheromone gland of intact females without PBAN injection or injection of D4-11Z,14Z,17Z-20:Acid into the abdomens of either pupae or adults also did not result in detectable label incorporation into the pentaenes, indicating that this acid probably is not a precursor to the pheromone.
Discussion
This is the first example of a lepidopteran insect in which both of the biosynthetic pathways leading to Type I and Type II moth pheromone components, respectively, have been demonstrated. The general steps of the pathways are parallel to those found in moths which use only one or the other pathway, with our results suggesting that navel orangeworm females synthesize 11Z,13Z-16:Ald from palmitic acid in the pheromone gland. In contrast, the two pentaenes apparently originate from linolenic acid. The fact that the two pentaenes were found in extracts of the pheromone gland, the abdominal tissue, the abdominal cuticle, and the hemolymph of female navel orangeworm moths (Table 1) are consistent with the hypothesis that the pentaenes are synthesized outside the pheromone gland, possibly in oenocyte cells, and then transferred to the gland through the hemolymph by carrier proteins, as has been shown in other lepidopteran species that produce Type II pheromone components (Jurenka et al. 2003; Wei et al. 2003; Matsuoka et al. 2006; Choi et al. 2007).
As expected, the production of 11Z,13Z-16:Ald in pheromone glands of decapitated females was increased significantly by PBAN injection, whereas the titer of the pentaenes in the gland extracts was not affected by PBAN injection. In addition, the titers of both pentaenes in the gland extracts were the same regardless of whether the glands were extracted during the photophase or the scotophase (Table 1). Thus, PBAN does not appear to be involved in the production of the pentaenes, similar to what was found with the production of polyenes in the geometrid moth A. selenaria cretacea (Wei et al. 2004) and the arctiid moth U. ornatrix (Choi et al. 2007), and the titer of the pentaenes does not fluctuate with the light-dark cycle.
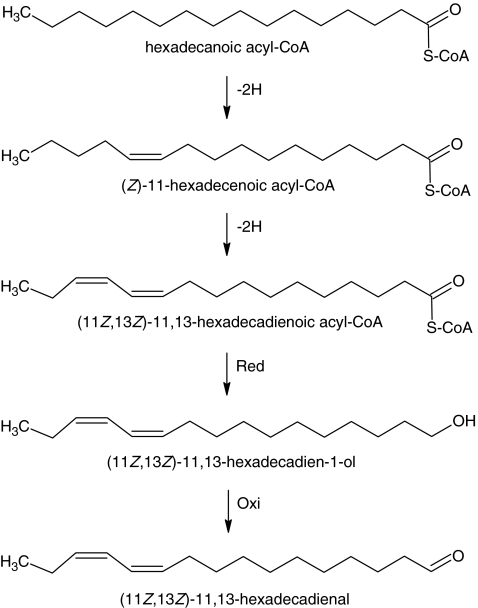
The fatty acid composition of extracts of pheromone glands and abdominal tissues, coupled with the results showing incorporation of labeled precursors, suggest two distinctly different biosynthetic pathways for the aldehyde and the pentaenes (Figs. 6 and 7). In the pheromone gland, the fatty acid compositions found in the extracts suggested that saturated C16 palmitic acid may be a key substrate, being first desaturated at the 11 position to form 11Z-16:Acid. A second desaturation, possibly mediated by a bifunctional desaturase such as the one known from T. pityocampa (Quero et al. 1997; Abad et al. 2007) then may form 11Z,13Z-16:Acid, which is subsequently reduced to the corresponding alcohol and finally oxidized to the aldehyde (Fig. 6). The incorporation of deuterium label from saturated D3-16:Acid and monoenoic D9-11Z-16:Acid precursors into 11Z,13Z-16:Ald is in agreement with this proposed biosynthetic pathway.
Fig. 6.
Proposed biosynthetic pathway for the Type I 11Z,13Z-16:Ald sex pheromone component produced by female Amyelois transitella. “–2H” represents desaturation to form a double bond, “Red” indicates functional group reduction and “Oxi” indicates terminal oxidation
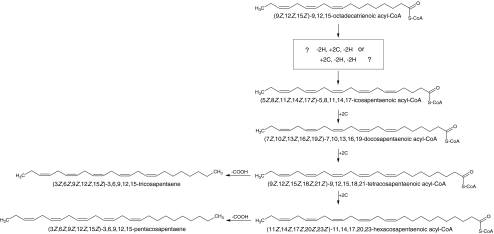
Fig. 7.
Proposed biosynthetic pathway for the Type II pentaene sex pheromone components produced by female Amyelois transitella. “–2H” represents desaturation to form a double bond, “+2C” represents one cycle of chain elongation by two carbons, “–COOH” means decarboxylation
In contrast, our results suggest that the biosynthesis of the two long-chain pentaenes must take place in a markedly different fashion, beginning with different precursors, and likely occurring at a site outside the pheromone gland. The incorporation of deuterium label from labeled linolenic acid into the two pentaenes confirms that linolenic acid can serve as a precursor to these compounds. Furthermore, (5Z,8Z,11Z,14Z,17Z)-icosapentaenoic acid, a likely later-stage intermediate in the biosyntheses of the pentaene hydrocarbons, was found in both abdominal tissue and pheromone gland extracts. Two or three 2-carbon chain extensions of this icosapentaenoic acid would provide the homologous tetracosapentaenoic and hexacosapentaenoic acids, respectively, which would yield the C23 and C25 pentaene hydrocarbons after decarboxylation. These data suggest that the icosapentaenoic acid with all the double bonds in place is a key intermediate in the biosynthesis of the pentaene hydrocarbons. The fact that we could detect no trace of the corresponding C22, C24, or C26 homologs suggests that this intermediate then undergoes a series of chain extensions and the final decarboxylation with all of the intermediates being continuously bound to the relevant enzymes, so that none of them are available as free entities that would have shown up in the analysis (Fig. 7).
The presence of (5Z,8Z,11Z,14Z,17Z)-icosapentaenoic acid in the pheromone gland tissue was unexpected, given that most of the total pentaene titer was found in the abdominal tissues (Table 1). However, this acid actually may have been present in the gland extracts only as the result of contamination of the pheromone gland preparations with abdominal tissues, i.e., it is difficult to dissect out pheromone glands that are completely free of surrounding tissues.
Prior studies in other organisms from a broad array of taxa, such as plants, algae, and mammals, suggested a conserved “Δ6 pathway”, i.e., the Δ6 desaturation of linolenic acid followed by elongation to produce 8Z,11Z,14Z,17Z-icosatetraenoic acid, which is further desaturated at the Δ5 position to produce the icosapentaenoic acid (Napier 2007). In the zebrafish, a bifunctional Δ6/Δ5 desaturase has been identified (Hastings et al. 2001), providing a precedent for this proposed pathway. In the present study, small amounts of the unusual (Z)-6-hexadecenoic acid were found in the pheromone gland and the abdomen extracts, which implies that the navel orangeworm may have a corresponding Δ6 desaturase. The fact that labeled 11Z,14Z,17Z-icosatrienoic acid was not incorporated into the pentaenes also suggests that at least the fourth double bond is introduced before elongation of the 18-carbon chain.
The shift from Type I to Type II pheromone components represents a major evolutionary transition that involves a new site of production, a mechanism for transport and selective uptake of polyunsaturated hydrocarbons into the pheromone gland before release, as well as for the evolution of corresponding receptors in male moths, with matching specificity for the detection of the novel pheromone components. It is difficult to envisage all these changes taking place simultaneously, and it is surprising that there are not more reports of species with mixed/intermediate pheromone systems in, for instance, Geometroidea and Noctuoidea, because these two moth superfamilies have many species that possess either Type I or Type II pheromones. The majority of the species for which mixed pheromones have been reported instead belong to the Pyraloidea, from which no cases of pure Type II (hydrocarbon-type) pheromones have been described (Witzgall et al. 2004; El-Sayed 2008). This suggests that the detailed mechanisms and sites of hydrocarbon biosynthesis may differ between the Geometroidea and Noctuoidea on one hand and the Pyraloidea on the other.
Once the hydrocarbon compounds started being exported to and taken up by the pheromone gland, this opened up the possibility for the modification of these compounds by the gland before release. The production of the epoxide derivatives of polyunsaturated hydrocarbons used by some species in the Geometroidea and Noctuoidea should, thus, represent a more advanced character state. Continued radiation then resulted in epoxidation at different positions, yielding one or the other, or in some cases both of the two possible enantiomers of each epoxide, thus allowing a variety of different semiochemicals to be made from a common precursor.
In vitro molecular cloning and functional analysis of the genes for the specific desaturase, elongase, and decarboxylase enzymes postulated in the biosynthesis of the hydrocarbon pheromone components would be helpful in explaining the entire pathway. Likewise, studies of tissue specific expression and in situ hybridization could clarify where the different reactions actually take place.
Acknowledgments
We thank Robbie D. Girling at University of California, Riverside for advice on insect rearing, Erling Jirle at Lund University for laboratory assistance, R. Adlof with USDA-ARS Peoria for providing labeled compounds, and Stefan Schulz of Technische Universität Braunschweig and two anonymous reviewers for helpful suggestions. This work was financially supported by the Swedish Research Council (VR).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- ABAD JL, CAMPS F, FABRIAS G. Substrate-dependent stereochemical course of the (Z)-13-desaturation catalyzed by the processionary moth multifunctional desaturase. J. Am. Chem. Soc. 2007;129:15007–15012. doi: 10.1021/ja0751936. [DOI] [PubMed] [Google Scholar]
- ANDO T, OHTANI K, YAMAMOTO M, MIYAMOTO T, QIN XR. Sex pheromone of Japanese giant looper, Ascotis selenaria cretacea: identification and field tests. J. Chem. Ecol. 1997;23:2413–2423. doi: 10.1023/B:JOEC.0000006683.58028.1e. [DOI] [Google Scholar]
- ANDO T, Inomata SI, Yamamoto M. Lepidopteran sex pheromones. In: Schulz S, editor. The Chemistry of Pheromones and Other Semiochemicals I: Topics in Current Chemistry. Berlin, Heidelberg, New York: Springer; 2004. pp. 51–96. [DOI] [PubMed] [Google Scholar]
- Ando T, Kawai T, Matsuoka K. Epoxyalkenyl sex pheromones produced by female moths in highly evolved groups: biosynthesis and its endocrine regulation. J. Pestic. Sci. 2008;33:17–20. doi: 10.1584/jpestics.R07-06. [DOI] [Google Scholar]
- Bjostad LB, Roelofs WL. Sex pheromone biosynthetic precursors in Bombyx mori. Insect Biochem. 1984;14:275–278. doi: 10.1016/0020-1790(84)90060-X. [DOI] [Google Scholar]
- Buser HR, Arn H, Guerin P, Rauscher S. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 1983;55:818–822. doi: 10.1021/ac00257a003. [DOI] [Google Scholar]
- Cabrera A, Eiras AE, Gries G, Gries R, Urdaneta N, Miras B, Badji C, Jaffe K. Sex pheromone of tomato fruit borer, Neolucinodes elegantalis. J. Chem. Ecol. 2001;27:2097–2107. doi: 10.1023/A:1012299021967. [DOI] [PubMed] [Google Scholar]
- Carrow GM, Calabrese RL, Williams CM. Spontaneous and evoked release of prothoracicotropin from multiple neurohemal organs of the tobacco hornworm. Proc. Natl. Acad. Sci. USA. 1981;78:5866–5870. doi: 10.1073/pnas.78.9.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M-Y, Lim H, Park KC, Adlof R, Wang S, Zhang A, Jurenka R. Identification and biosynthetic studies of the hydrocarbon sex pheromone in Utetheisa ornatrix. J. Chem. Ecol. 2007;33:1336–1345. doi: 10.1007/s10886-007-9306-1. [DOI] [PubMed] [Google Scholar]
- Coffelt JA, Sower LL, Vick KW. Quantitative analysis of identified compounds in pheromone gland rinses of Plodia interpunctella and Ephestia cautella at different times of day. Environ. Entomol. 1978;7:502–505. [Google Scholar]
- Coffelt JA, Vick KW, Sonnet PE, Doolittle RE. Isolation, identification, and synthesis of a female sex pheromone of the navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae) J. Chem. Ecol. 1979;5:955–966. doi: 10.1007/BF00990218. [DOI] [Google Scholar]
- Dunkelblum E, Tan SH, Silk PJ. Double bond location in monounsaturated fatty acids by dimethyl disulfide derivatisation and mass spectrometry: application to analysis of fatty acids in pheromone glands of four Lepidoptera. J. Chem. Ecol. 1985;11:265–277. doi: 10.1007/BF01411414. [DOI] [PubMed] [Google Scholar]
- El-Sayed, A. M. 2008. The Pherobase: Database of Insect Pheromones and Semiochemicals. <http://www.pherobase.com>.
- Fan Y, Schal C, Vargo EL, Bagnères A-G. Characterization of termite lipophorin and its involvement in hydrocarbon transport. J. Insect Physiol. 2004;50:609–620. doi: 10.1016/j.jinsphys.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Fellenberg AJ, Johnson DW, Poulos A, Sharp P. Simple mass spectrometric differentiation of the n-3, n-6 and n-9 series of methylene interrupted polyenoic acids. Biomed. Environ. Mass Spectrom. 1987;14:127–130. doi: 10.1002/bms.1200140306. [DOI] [Google Scholar]
- GIBB AR, PINESE B, TENAKANIA D, KAWI AP, BUNN B, RAMANKUTTY P, SUCKLING DM. (Z)-11-Hexadecenal and (3Z, 6Z, 9Z)-tricosatriene: sex pheromone components of the red banded mango caterpillar Deanolis sublimbalis. J. Chem. Ecol. 2007;33:579–589. doi: 10.1007/s10886-006-9221-x. [DOI] [PubMed] [Google Scholar]
- Girling RD, Cardé RT. Analysis of the courtship behavior of the navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae), with a commentary on methods for the analysis of sequences of behavioral transitions. J. Insect Behav. 2006;19:498–520. doi: 10.1007/s10905-006-9041-4. [DOI] [Google Scholar]
- Goller S, Szöcs G, Francke W, Schulz S. Biosynthesis of (3Z, 6Z, 9Z)-3, 6, 9-Octadecatriene: the main component of the pheromone blend of Erannis bajaria. J. Chem. Ecol. 2007;33:1505–1509. doi: 10.1007/s10886-007-9324-z. [DOI] [PubMed] [Google Scholar]
- Hastings N, Agaba M, Tocher DR, Leaver MJ, Dick JR, Sargent JR, Teale AJ. A vertebrate fatty acid desaturase with Δ5 andΔ6 activities. Proc. Natl. Acad. Sci. USA. 2001;98:14304–14309. doi: 10.1073/pnas.251516598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka RA, Roelofs WL. Biosynthesis and endocrine regulation of fatty acid derived sex pheromone in moths. In: Stanley-Samuelson DW, Nelson DR, editors. Insect Lipids: Chemistry, Biochemistry, and Biology. Lincoln, NB/London: Univ. Nebr. Press; 1993. pp. 353–88. [Google Scholar]
- Jurenka RA, Subchev M. Identification of cuticular hydrocarbons and the alkene precursor to the pheromone in hemolymph of the female gypsy moth. Lymantria dispar. Arch. Insect Biochem. Physiol. 2000;43:108–115. doi: 10.1002/(SICI)1520-6327(200003)43:3<108::AID-ARCH2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Jurenka RA, Subchev M, Abad J-L, Choi M-Y, Fabriàs G. Sex pheromone biosynthetic pathway for disparlure in the gypsy moth. Lymantria dispar. Proc. Natl. Acad. Sci. USA. 2003;100:809–914. doi: 10.1073/pnas.0236060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasang G, Schneider D, Beroza M. Biosynthesis of the sex pheromone disparlure by olefin-epoxide conversion. Naturwissenschaften. 1979;61:130–131. doi: 10.1007/BF00606291. [DOI] [Google Scholar]
- Kuenen, L. P. S., McElfresh, J. S., and Millar, J. G. 2010. Identification of critical secondary components of the sex pheromone of the navel orangeworm, Amyelois transitella. J. Econ. Entomol. 103: In press. [DOI] [PubMed]
- Landolt PJ, Curtis CE. Interspecific sexual attraction between Pyralis farinalis L. and Amyelois transitella (Walker) (Lepidoptera: Pyralidae). J. Kansas Entomol. Soc. 1982;55:248–252. [Google Scholar]
- Leal WS, Parra-Pedrazzoli AL, Kaissling K-E, Morgan TI, Zalom FG, Pesak DJ, Dundulis EA, Burks CS, Higbee BS. Unusual pheromone chemistry in the navel orangeworm: novel sex attractants and a behavioral antagonist. Naturwissenschaften. 2005;92:139–146. doi: 10.1007/s00114-004-0598-5. [DOI] [PubMed] [Google Scholar]
- Löfstedt C, Hansson BS, Tóth M, Szöcs G, Buda V, Bengtsson M, Ryrholm N, Svensson M, Priesner E. Pheromone differences between sibling taxa Diachrysia chrysitis (Linnaeus, 1758) and D. tutti (Kostrowicki, 1961)(Lepidoptera: Noctuidae) J. Chem. Ecol. 1994;20:91–109. doi: 10.1007/BF02065993. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Tabunoki H, Kawai T, Ishikawa S, Yamamoto M, Sato R, Ando T. Transport of a hydrophobic biosynthetic precursor by lipophorin in the hemolymph of a geometrid female moth which secretes an epoxyalkenyl sex pheromone. Insect Biochem. Mol. Biol. 2006;36:576–583. doi: 10.1016/j.ibmb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Millar JG. Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu. Rev. Entomol. 2000;45:575–604. doi: 10.1146/annurev.ento.45.1.575. [DOI] [PubMed] [Google Scholar]
- Millar JG, Grant GG, McElfresh JS, Strong W, Rudolph C, Stein JD, Moreira JA. (3Z, 6Z, 9Z, 12Z, 15Z)-Pentacosapentaene, a key pheromone component of the fir coneworm moth, Dioryctria abietivorella. J. Chem. Ecol. 2005;31:1229–1234. doi: 10.1007/s10886-005-5813-0. [DOI] [PubMed] [Google Scholar]
- Miller, D. R., Millar, J. G., Mangani, A., Crowe, C. M., and Grant, G. G. 2010a. (3Z,6Z,9Z,12Z,15Z)-Pentacosapentaene and (Z)-11-hexadecenyl acetate: sex attractant blend for Dioryctria amatella (Lepidoptera: Pyralidae). J. Econ. Entomol. In press. [DOI] [PubMed]
- Miller, D. R., Millar, J. G., Grant, G. G., McDonald, L., and Debarr, G. L. 2010b. (3Z,6Z,9Z,12Z,15Z)-Pentacosapentaene and (9Z,11E)-tetradecadienyl acetate: attractant lure blend for Dioryctria ebeli (Lepidoptera: Pyralidae) in a slash pine seed orchard. J. Entomol. Sci. 45(1):54–57.
- Miyamoto T, Yamamoto M, Ono A, Ohtani K, Ando T. Substrate specificity of the epoxidation reaction in sex pheromone biosynthesis of the Japanese giant looper (Lepidoptera: Geometridae) Insect Biochem. Mol. Biol. 1999;29:63–69. doi: 10.1016/S0965-1748(98)00105-2. [DOI] [Google Scholar]
- Napier JA. The production of unusual fatty acids in transgenic plants. Annu. Rev. Plant Biol. 2007;58:295–319. doi: 10.1146/annurev.arplant.58.032806.103811. [DOI] [PubMed] [Google Scholar]
- Quero C, Malo EA, Fabrias G, Camps F, Lucas P, Renou M, Guerrero A. Reinvestigation of female sex pheromone of processionary moth (Thaumetopoea pityocampa): no evidence for minor components. J. Chem. Ecol. 1997;23:713–726. doi: 10.1023/B:JOEC.0000006406.30444.69. [DOI] [Google Scholar]
- Rule GS, Roelofs WL. Biosynthesis of sex pheromone components from linolenic acid in arctiid moths. Arch. Insect Biochem. Physiol. 1989;12:89–97. doi: 10.1002/arch.940120203. [DOI] [Google Scholar]
- Schal C, Sevala V, Cardé RT. Novel and highly specific transport of a volatile sex pheromone by hemolymph lipophorin in moths. Naturwissenschaften. 1998;85:339–342. doi: 10.1007/s001140050511. [DOI] [Google Scholar]
- Schal C, Sevala VL, Young HP, Bachmann JAS. Sites of synthesis and transport pathways of insect hydrocarbons: cuticle and ovary as target tissues. Amer. Zool. 1998;38:382–393. [Google Scholar]
- Srinivasan, A., Coffelt, J. A., Norman, P., and Williams, B. 1986. Sex pheromone gland of the navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae) location, bioassay and in vitro maintenance. Florida Entomol. 69:169-174.
- Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 1999;29:481–514. doi: 10.1016/S0965-1748(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Wei W, Miyamoto T, Endo M, Murakawa T, Pu G-Q, Ando T. Polyunsaturated hydrocarbons in the hemolymph: biosynthetic precursors of epoxy pheromones of geometrid and arctiid moths. Insect Biochem. Mol. Biol. 2003;33:397–405. doi: 10.1016/S0965-1748(03)00002-X. [DOI] [PubMed] [Google Scholar]
- Wei W, Yamamoto M, Asato T, Fujii T, Pu G-Q, Ando T. Selectivity and neuroendocrine regulation of precursor uptake by pheromone glands from hemolymph in geometrid female moths which secrete epoxyalkenyl sex pheromones. Insect Biochem. Mol. Biol. 2004;34:1215–1224. doi: 10.1016/j.ibmb.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Witzgall, P., Lindblom, T., Bengtsson, M., and Toth, M. 2004. The Pherolist. (http://www-pherolist.slu.se/pherolist.php).