Abstract
The rhythmic activity of neurons in the brain has fascinated neuroscientists ever since electrical potentials were first recorded from the human scalp more than 70 years ago. The rhythms of electrical activity in sensory neurons that encode visual information are known to vary markedly with attention. How does neuronal encoding differ for a visual stimulus that is the center of attention compared with one that is ignored? To answer this question, Fries et al. (1) simultaneously recorded electrical activity from several clusters of neurons in the V4 region of the visual cortex of macaque monkeys that were shown behaviorally relevant and distracter objects (see the figure). On page 1560 of this issue, they report a rapid increase in the synchronization of electrical activity in the gamma frequency range (35 to 90 Hz) in V4 neurons activated by the attended stimulus (that is, the stimulus on which attention is focused) but not in V4 neurons activated by distracter objects (1).
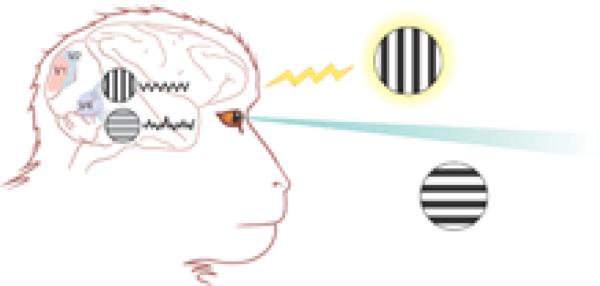
The benefits of paying attention
A halo of attention surrounds one of the two physically similar stimuli (vertical and horizontal stripes) that the monkey can see while his eyes fixate on a point between them. The attended stimulus has a more powerful representation in the V4 area of the visual cortex because the neurons that respond to it tend to fire rhythmically in synchrony with one another, as illustrated by the wiggly trace to the right of the stimulus. V4 neurons that respond to the other (distracter) stimulus fire at similar rates but not in synchrony. Synchronized firing provides the attended stimulus with a more powerful representation, illustrated by the greater clarity of the mental image.
The neurophysiology of attention remains a puzzle. A simple and attractive hypothesis is that an attended stimulus behaves as though it were bigger and brighter than all of the other competing stimuli. To encode this bigger and brighter stimulus, neurons would need to somehow increase their electrical response. Although some experiments have shown the production of a greater (but still equally selective) neuronal electrical discharge in response to attention (2, 3), other studies in the same brain areas have found almost no effect of attention on electrical activity (4), and in still others the effects of attention were disappointingly small. So, it would be attractive indeed to discover a neurophysiological mechanism for attention that is consistent with all of these findings.
About 12 years ago, rhythmic oscillatory activity and neuronal synchronization were proposed as solutions to a different but related problem. In higher mammals, including monkeys and humans, there are many different visual areas in the brain that respond more or less selectively to the different qualities of a visual stimulus: its motion, color, texture, and so on. When more than one object is visible, how are the representations of the different qualities of the individual objects bound to one another so that a person does not associate the color of one object with the movement of another? The Singer (5) and Eckhorn groups (6) suggested that the widespread representations of the different visual qualities of a particular object might be unified by neurons firing together rhythmically on a time scale of 25 milliseconds or so, with representations of different objects encoded in electrical activity of different phases or frequencies. Crick and Koch (7) suggested that the same sort of synchronous oscillation might underlie consciousness or visual awareness. Extreme manipulations of visual awareness--such as presenting a viewer's two eyes with different scenes that alternately appear and disappear (a phenomenon called binocular rivalry--have provided some support for this proposal. However, it has not yet been demonstrated with more natural stimuli that the presence of rhythmic or synchronous activity among a collection of neurons controls whether the features represented by those neurons are bound together either to represent a single object to our perceptual system or to bring an object into conscious awareness. A stringent test of this hypothesis would be to use ambiguous figures (such as the classic face-vase illusion) in which the same physical stimulus can be perceived in different ways, to determine whether the way the object is perceived depends on which clusters of neurons are firing together (8).
In a sense, the Fries et al. study brings us full circle. Their experiments show that it is the rhythmic coordination of a subpopulation of neurons, and not just the amount of nerve cell activity per se, that is associated with finding what we are looking for and missing the unexpected. Their work suggests that the rhythmic synchrony of electrical signals may not be the hallmark of perceptual unity or of conscious awareness. Instead it may be a consequence of a decision to focus attention on a relevant stimulus. A synchronous neural response makes the representation of the stimulus more prominent and thereby more likely to enter the consciousness of the viewer.
The basic biophysical properties of neurons and synapses allow rhythmic synchronization to enhance the effect of a fixed amount of neuronal activity both in sensory neurons in the periphery and in the brain's central processing stations, which receive inputs from these neurons. The enhancing effect of synchronous activity would cause larger responses to the attended stimulus in neurons at the next stage of signal processing. At each stage of processing, responses to the attended stimulus would become stronger, whereas those to the distracter stimuli would remain weak or would fade away entirely.
Despite the attractiveness of this proposal, it is not at all clear how attention causes responses to become more oscillatory and better synchronized. Modifying the strength of particular cortical interneuronal circuits could, in principle, favor certain frequencies of electrical discharge, but it is not known whether or how such circuits receive the inputs that turn them on.
Perhaps it makes sense to regard rhythmic synchronization as only one of a number of processes that enhance responses to attended stimuli. Other processes might include increases in background or “spontaneous” discharges (9) or changes in neuronal “gain.” At least for these two hypotheses, there are clear pharmacological demonstrations that different classes of synaptic receptors can have additive or multiplicative effects on neuronal activity (10).
It is also important to note that rhythmic synchronization may be important in other activities besides attention to a stimulus. For example, synchronization may be used to signal the persistence of stimuli even when the neurons responding to those stimuli with increased rates of discharge do so only transiently (11). Finally, one must remember that synchronization has its costs as well as its benefits. Pooling the outputs from many neurons adds information only if the activity of those neurons is not coordinated. Zohary et al. (12) have shown that the information provided by many thousands of neurons in a higher cortical visual area is only marginally greater than that provided by a few neurons in that area if the electrical discharges of the many are simultaneous. Thus, even minimal synchronization can drastically limit the ability of the cortex to take advantage of its vast numbers of neurons.
References
- 1.Fries P, Reynolds JH, Rorie AE, Desimone R. Science. 2001;291:1560. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds JH, et al. Neuron. 2000;26:703. doi: 10.1016/s0896-6273(00)81206-4. Medline. [DOI] [PubMed] [Google Scholar]
- 3.Treue S, Maunsell JH. J. Neurosci. 1999;19:7591. doi: 10.1523/JNEUROSCI.19-17-07591.1999. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidemann E, Newsome WT. J. Neurophysiol. 1999;81:1783. doi: 10.1152/jn.1999.81.4.1783. Medline. [DOI] [PubMed] [Google Scholar]
- 5.Gray CM, et al. Nature. 1989;338:334. doi: 10.1038/338334a0. Medline. [DOI] [PubMed] [Google Scholar]
- 6.Eckhorn R, et al. Biol. Cybern. 1988;60:121. doi: 10.1007/BF00202899. Medline. [DOI] [PubMed] [Google Scholar]
- 7.Crick F, Koch C. Cold Spring Harbor Symp. Quant. Biol. 1990;55:953. doi: 10.1101/sqb.1990.055.01.089. Medline. [DOI] [PubMed] [Google Scholar]
- 8.Stryker MP. Nature. 1989;338:297. doi: 10.1038/338297a0. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez G, et al. Hippocampus. 1998;9:35. Medline. [Google Scholar]
- 10.Fox K, Sato H, Daw N. J. Neurophysiol. 1990;64:1413. doi: 10.1152/jn.1990.64.5.1413. Medline. [DOI] [PubMed] [Google Scholar]
- 11.deCharms RC, Merzenich MM. Nature. 1996;381:610. doi: 10.1038/381610a0. Medline. [DOI] [PubMed] [Google Scholar]
- 12.Zohary E, et al. Nature. 1994;370:140. doi: 10.1038/370140a0. Medline. [DOI] [PubMed] [Google Scholar]