Abstract
The determination of the dose of inhaled aerosol particles in animal subjects is not a trivial exercise. In its simplest form, the dose is the amount (particle number, mass, or other relevant metric) that deposits in the respiratory tract. The amount deposited will depend on the aerosol particle sizes (e.g. the aerodynamic diameter size distribution), the duration of exposure, the exposure system's delivery efficiency, the subject's ventilation rate, the species and strain, and other factors. Similarly, species differences in the clearance rates of deposited particles will influence the time integrated particle doses. In practice, particle doses are estimated using mathematical models, previous experimental dosimetry data, tracers of the inhaled particles, and biomarkers of exposure. With care, desired aerosol doses can be achieved and documented.
Keywords: animal models, aerosols, inhalation, dosimetry, respiratory tract, bioaerosols
1. Introduction
A fundamental requirement in animal aerosol inhalation studies is that the dose delivered be known. Unlike most other exposure methods, inhalation requires a knowledge of several physical and biological factors in order to deliver a well-defined dose. Without a reliable estimate of the dose deposited, an inhalation study will be difficult to interpret. For simplicity, the inhaled aerosol deposition dose for particles that are all one size can be defined as the product of the aerosol concentration, the exposure time, the subject's ventilation rate, the subject's sampling efficiency (i.e. inhalability), and the deposition efficiency for the particles in the respiratory tract. This dose represents the total initial aerosol deposition: particle clearance and the sites of deposition within the respiratory tract are not included. In practice the dose may be normalized to a biological target, such as lung mass, alveolar surface area, number of alveolar macrophages etc. The purpose of this paper is to provide an overview of several factors that affect aerosol doses.
2. Exposure systems
Aerosol exposure systems can be classified according to the degree of exposure of the subject (Wong, 1999). Whole-body systems are particularly useful for long-term exposures. However, the exposure is not limited to inhalation, as the animals fur and food will be coated with the study material. Eye irritation, and ingestion of particles during grooming can occur. Also, animals in chambers can avoid or limit exposure by burying their noses in their fur or seeking locations that have low concentrations. Head-only and nose-only exposure systems are usually appropriate for brief exposures, but they can be used for repeated exposures (Vick et al., 2007; Jaeger et al., 2006; Wong, 1999). These systems limit the exposure and effectively prevent the ingestion of particles, but they can be stressful to the subjects. With proper design and prior conditioning, a number of species can be exposed comfortably for brief periods (Narciso et al., 2003). From a dosimetry point of view, nose-only exposure systems are particularly useful, especially if they are instrumented in order to record breathing patterns during exposure (Mautz, 1997).
3. Dose Metrics
A dose metric is a measurable property of the exposure agent that is closely related to its biological effect(s). Aerosol particulate mass, or mass per unit volume of air is the most commonly used metric in inhalation studies. However, recent evidence (Kreyling et al, 2006; Moss and Wong, 2006; Oberdörster, 2001) indicates that for some particles, total particle count, surface area, or projected area produce greater effects than total particle mass. Also, ultrafine and nanoparticles (that have one or more dimensions less than 0.1 μm) are not likely to be well-characterized by their mass alone. For bacteria, dose metrics to consider include number of viable-staining organisms and number of colony forming units. For viruses, the number of infectious units and plaque forming units inhaled and deposited may be the best dose metric.
4. Comparative anatomy and physiology
Both the nasal anatomy and the mode of bronchial branching are species specific. Humans and other primates have relatively simple nasal anatomies in comparison to rodents, lagomorphs and canines (Newton, 1995). Such differences in nasal complexity do not always affect particle deposition per-se because of the uneven distribution of inhaled air in the nose. However, the noses of small mammals are more effective in trapping particles than are the noses of larger species. Humans differ from most other mammals with respect to the mode of bronchial branching. The human has a markedly more-symmetric bronchial branching pattern than the dog, cat, rat, mouse, hamster, guinea pig, pig, rabbit, ferret, and most other mammals (Schlesinger and McFadden, 1981). Another species difference in airway anatomy relates to the presence, or absence, of respiratory bronchioles (RB) (Tyler and Julian, 1991). RB are partially-alvelorized airways situated between the terminal bronchioles and the alveolar ducts. Unlike most mammals, rats and mice appear to lack RB. Because RB are sites of human diseases, including emphysema and fibrosis (Churg and Wright, 2003), rats and mice are limited for these conditions. The presence or absence of RB appears to have implications for particle clearance. Rats and mice have an efficient early alveolar clearance of insoluble particles (Wolff, 1991), probably because their alveolar ducts connect directly to terminal bronchioles (which have an active mucociliary clearance apparatus).
Mammals must produce metabolic heat to compensate for heat loss to the environment. As heat loss depends on the body surface-to-mass ratio, smaller mammals generally consume more oxygen per unit body mass than larger animals. The formulae of Guyton (1947) are useful for estimating the volume of air breathed per unit time (Vm) as a function of body weight (W). Using Guyton's formulae, Vm/W (ml g−1) = 2.18/W¼, one can compare the minute volume per unit body mass of a resting 70 kg adult, a newborn 3.3 kg child, and a 20 g mouse: The air intake/mass ratios for adult/newborn/mouse are 1/2.2/7.9. Small children and small laboratory animals in general can be expected to deposit greater particle doses per unit body mass under similar exposure conditions. However, when aerosol deposition is normalized to other parameters, such as respiratory tract surface area, the comparative dose may be reversed (Jarabek et al., 2005).
5. Aerosol particle deposition
The sampling efficiency (inhalability) of the mammalian nose/mouth for aerosol particles is defined as the ratio of mass concentration of particles that are inhaled to the mass concentration of particles in the air in front of the head. The International Standards Organization (ISO), the Comité Européen de Normalisation (CEN), and the American Conference of Governmental Industrial Hygenists (ACGIH®) agree on the particle size dependent inhalability for mouth-breathing adults at low wind speeds (1-9 m s−1), averaged over all orientations to the wind (Lidén and Harper, 2006; ACGIH®, 2008). Under these conditions, particles with aerodynamic diameters under 2 μm have inhalabilities above 94%, and inhalability decreases to level off at 50% for 100 μm particles. Inhalabilities for calm air and high wind speeds, for children, and for laboratory animals have not been well-studied. Some authors have provided limited inhalability data for rats (Jarabek et al, 2005). The specific exposure system design will influence particle inhalability.
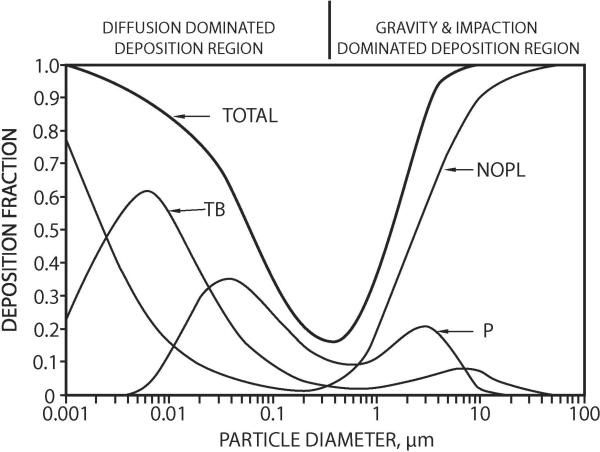
Inhaled particles will deposit in the respiratory tract if they move out of the air streams and touch an airway surface. About one dozen different mechanisms influence particle deposition, but four are generally included in mathematical simulations: inertial impaction; gravitational sedimentation; Brownian diffusion; and interception (e.g. for long fibers). These, and other mechanisms act in the respiratory tract airways to produce complex deposition patterns in the major anatomical regions. The curves depicted in Figure 1 apply to a near-resting “reference” man, and to uncharged, nonhygroscopic, simple-shaped particles. Mathematical models are available for generating such curves for various airway sizes, various levels of exertion, polydisperse particle size distributions, hygroscopic aerosols, and rats (ICRP, 1994; NCRP, 1997; Asgharian et al., 2004; Jarabek et al, 2005; Swift et al., 2007). Nonhuman mammals have aerosol deposition curves that are qualitatively, but not quantitatively, similar to those for humans (Schlesinger, 1985; Newton, 1995). Differences in individuals, species, and even varieties within a species can produce different aerosol deposition efficiencies (Oldham and Phalen, 2002).
Figure 1.
Particle deposition curves for an adult male at rest according to the NCRP (1997) model. Not corrected for inhalability. NOPL = naso-oro-pharyngo-laryngeal; TB= tracheobronchial; and P = pulmonary
The actual inhaled particle deposition dose in an aerosol exposure should be measured, or at least carefully estimated: otherwise, the delivered dose will be unknown. If a reliable biomarker of dose (such as a radioactive label, a direct assay, or a unique metabolite) is available, it should be measured in a sufficient number of animals in each study group to determine the delivered particle dose. Use of a co-present inert tracer with the same aerodynamic size distribution as the study aerosol can also be useful. If such direct measurements of delivered particle dose are not practical, the inhalation exposure system can be “calibrated” using tracer aerosols prior to the study.
Mathematical aerosol deposition modeling, or, if available, published aerosol deposition data (e.g. Raabe et al., 1988, for mice, rats, hamsters, guinea pigs, and rabbits) can also be used to estimate the deposited particle dose in a study if the specific strains and similar exposure systems are used. In order to use these methods, an accurate aerosol particle size distribution should be obtained; and the actual respiratory frequencies and tidal volumes of the animals in the study should be measured (e.g. Mautz, 1997), taken from the literature, or at least calculated from Guyton's (1947) or similar suitable equations.
6. Particle Clearance
The clearance of deposited inhaled particles depends on the: particle size; particle dissolution rate in the respiratory tract; the site(s) of deposition; the animal species, size, and state of health; and other factors. The deposition pattern will determine the particle clearance mechanisms that act to remove or redistribute the study particles. In the nasal, oral and pharyngeal airways, clearance is primarily produced by particle dissolution, and extrinsic mechanisms (e.g. cough, expectoration, swallowing, and grooming). Particles in the nm size range may be partially cleared by movement along the olfactory nerve to the brain (Dorman et al., 2002; Oberdörster et al., 2004). The tracheobronchial region (TB) is primarily cleared of insoluble particles by cilia-propelled mucus to the glottis, where the particles are swallowed. In the larger airways, mucous movement is faster than in the smaller bronchi and bronchioles, but most particles will be cleared by 24 hours post deposition in healthy animals. Infections, and some other respiratory tract diseases, can greatly impair mucociliary clearance for several days or weeks in humans, and presumably in other animals (Pavia, 1987). There is evidence for a slowly-cleared component of particles deposited in the TB region (ICRP, 1994; Kreyling and Scheuch, 2000). Particle clearance from the alveolar regions of the respiratory tract is strongly dependent on particle properties including their sizes, shapes, dissolution rates, and toxicity. Some particles may not be effectively removed by resident alveolar macrophages (mobile phagocytic cells) and, if they are not readily solubilized, they may remain in the alveolar region for long periods (up to several years). Unphagocytyzed particles may cross the alveolar epithelium and enter interstitial spaces, lymphatics, and blood vessels. Long insoluble fibers, such as some asbestos fibers, resist removal by macrophages, and can kill these cells. Similarly, other toxic insoluble particles such as some forms of quartz, can resist clearance and produce deep-lung dust diseases (pneumoconioses). Another consideration in alveolar clearance of slowly-dissolving particles is the phenomenon of “particle overload” or clearance stasis (Morrow, 1988; Mauderly and McCunney, 1996). This stasis results from overloading macrophages when heavy particle deposits are present. Such nonphysiological clearance stasis, which may not occur at smaller, more realistic doses, can invalidate an inhalation study. Species differences in alveolar clearance are known (Wolff, 1991; chapter 6 in NCRP, 1997): Rats and mice have much more rapid long term particle clearance (when overload does not occur) than do hamsters, dogs and humans. Infectious bioaerosols are cleared from the respiratory tract by both physical clearance and inactivation by immune and non-immune host factors present in the mucus layer. Also, bronchial associated lymphoid tissue located at branching points in the airways and resident phagocytic cells can capture and initiate immune responses to eliminate the infection (Reynolds, 1997).
7. Conclusion
Inhaled aerosol dose determinations are far more complex than those associated with other administration techniques. Much is known about aerosol deposition and clearance phenomena in healthy adults and a few strains of commonly-used species. For compromised animal models, and less-commonly used species, the basis for determining inhaled aerosol doses is less well known. In such cases, current literature searches, and including tracers and other dosimetric tools in a study will be necessary.
Acknowledgements
The authors thank Ms. Leslie Owens for word-processing and administrative support, Dr. Loyda B. Mendez is supported by NIH Award #AI065359. Additional support was provided by the Charles S. Stocking Family Fund, by way of an endowment to Dr. Robert F. Phalen.
References
- ACGIH® . 2008 TLVs® and BEIs®. American Conference of Governmental Industrial Hygienists; Cincinnati: 2008. [Google Scholar]
- Asgharian B, Menache MG, Miller FJ. Modeling age-related particle deposition in humans. Journal of Aerosol Medicine. 2004;17:213–224. doi: 10.1089/jam.2004.17.213. [DOI] [PubMed] [Google Scholar]
- Churg A, Wright JL. Bronchiolitis caused by occupational and ambient atmospheric particles. Seminars in Respiratory and Critical Care Medicine. 2003;24:577–584. doi: 10.1055/s-2004-815605. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. Journal of Toxicology and Environmental Health Part A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Analysis of respiratory patterns in laboratory animals. American Journal of Physiology. 1947;150:78–83. doi: 10.1152/ajplegacy.1947.150.1.78. 1947. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiatiological Protection, Task Group of Committee 2) Human Respiratory Tract Model for Radiological Protection. Pergamon Press; New York: 1994. Publication 66. [Google Scholar]
- Jaeger RJ, Shami SG, Tsenova L. Directed-flow aerosol inhalation exposure systems: Application to pathogens and highly toxic agents. In: Salem H, Katz SA, editors. Inhalation Toxicology. 2nd Ed. CRC Press; Boca Raton: 2006. pp. 73–90. chap. 4. [Google Scholar]
- Jarabek AM, Asgharian B, Miller FJ. Dosimetric adjustments for interspecies extrapolation of inhaled poorly soluble particles (PSP). Inhalation Toxicology. 2005;17:317–334. doi: 10.1080/08958370590929394. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Scheuch G. Clearance of particles deposited in the lungs. In: Gehr P, Heyder J, editors. Particle-Lung Interactions. Marcel Dekker; New York: 2000. pp. 323–376. chap. 7. [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Möller W. Ultrafine particle-lung interactions: Does size matter? Journal of Aerosol Medicine. 2006;19:74–83, 2006. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- Lidén G, Harper M. The need for an international sampling convention for inhalable dust in calm air. Journal of Occupational and Environmental Hygiene. 2006;3:D94–D101. doi: 10.1080/15459620600920580. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, McCunney RJ, editors. Particle Overload in the Rat Lung and Lung Cancer: Implications for Human Risk Assessment. Taylor & Francis; Washington: 1996. [Google Scholar]
- Mautz WJ. Animal monitoring. In: Phalen RF, editor. Methods in Inhalation Toxicology. CRC Press; Boca Raton: 1997. pp. 85–99. chap. 6. [Google Scholar]
- Moss OR, Wong VA. When nanoparticles get in the way: Impact of projected area on in vivo and in vitro macrophage function. Inhalation Toxicology. 2006;18:711–716. doi: 10.1080/08958370600747770. [DOI] [PubMed] [Google Scholar]
- Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Fundamental and Applied Toxicology. 1988;10:369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Narcisco SP, Nadziejko E, Chen LC, Gordon T, Nadziejko C. Adaptation to stress induced by restraining rats and mice in nose-only inhalation holders. Inhalation Toxicology. 2003;15:1133–1143. doi: 10.1080/08958370390228592. [DOI] [PubMed] [Google Scholar]
- NCRP (National Council on Radiation Protection and Measurements) Deposition Retention and Dosimetry of Inhaled Radioactive Substances. National Council on Radiation Protection and Measurements; Bethesda: 1997. NCRP SC 57-2 Report. [Google Scholar]
- Newton PE. Inhalation toxicology. In: Derelanko MJ, Hollinger MA, editors. CRC Handbook of Toxicology. CRC Press; Boca Raton: 1995. pp. 217–276. chap. 5. [Google Scholar]
- Oberdörster G. Pulmonary effects of inhaled ultrafine particles. International Archives of Occupational and Environmental Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling K, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicology. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Phalen RF. Dosimetry implications of upper tracheobronchial airway anatomy in two mouse varities. Anatomical Record. 2002;268:59–65. doi: 10.1002/ar.10134. [DOI] [PubMed] [Google Scholar]
- Pavia D. Acute respiratory infections and mucociliary clearance. European Journal of Respiratory Diseases. 1987;71:219–226. [PubMed] [Google Scholar]
- Raabe OG, Al-Bagati MA, Teague SV, Rasolt A. Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Annals of Occupational Hygiene. 1988;32(Suppl. 1):53–63. [Google Scholar]
- Reynolds HY. Integrated host defense against infections. In: Crystal RG, West JB, Barnes PJ, Weibel ER, editors. The Lung: Scientific Foundations. 2nd Ed. Lippincot-Raven Publishers; Philadelphia: 1997. pp. 2353–2365. chap 181. [Google Scholar]
- Schlesinger RB, McFadden LA. Comparative morphometry of the upper bronchial tree in 6 mammalian species. Anatomical Record. 1981;199:99–108. doi: 10.1002/ar.1091990110. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: A review. Toxicology and Environmental Health. 1985;15:197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Swift D, Asgharian B, Kimbell JS. Use of mathematical aerosol deposition models in predicting the distribution of inhaled therapeutic aerosols. In: Hickey AJ, editor. Inhalation Aerosols: Physical and Biological Basis for Therapy. 2nd Ed. Informa; New York: 2007. pp. 55–82. Chap. 3. [Google Scholar]
- Tyler WS, Julian MD. Gross and subgross anatomy of lungs, pleura, connective tissue septa, distal airways and structural units. In: Parent RA, editor. Comparative Biology of the Normal Lung. CRC Press; Boca Raton: 1991. pp. 37–48. chap. 4. [Google Scholar]
- Vick A, Wolff R, Koester A, Reams R, Deaver D, Heidel S. A 6-month inhalation study to characterize the toxicity, pharmacokinetics and pharmacodynamics of human insulin inhalation powder (HIIP) in Beagle dogs. Journal of Aerosol Medicine. 2007;20:112–126. doi: 10.1089/jam.2007.0586. [DOI] [PubMed] [Google Scholar]
- Wolff RK. Mucociliary function. In: Parent RA, editor. Comparative Biology of the Normal Lung. CRC Press; Boca Raton: 1991. pp. 659–680. chap. 35. [Google Scholar]
- Wong BA. Inhalation exposure systems design, methods and operation. In: Gardner DE, Crapo JD, McClellan RO, editors. Toxicology of the Lung. 3rd Ed. Taylor & Francis; Philadelphia: 1999. pp. 1–53. chap. 1. [Google Scholar]