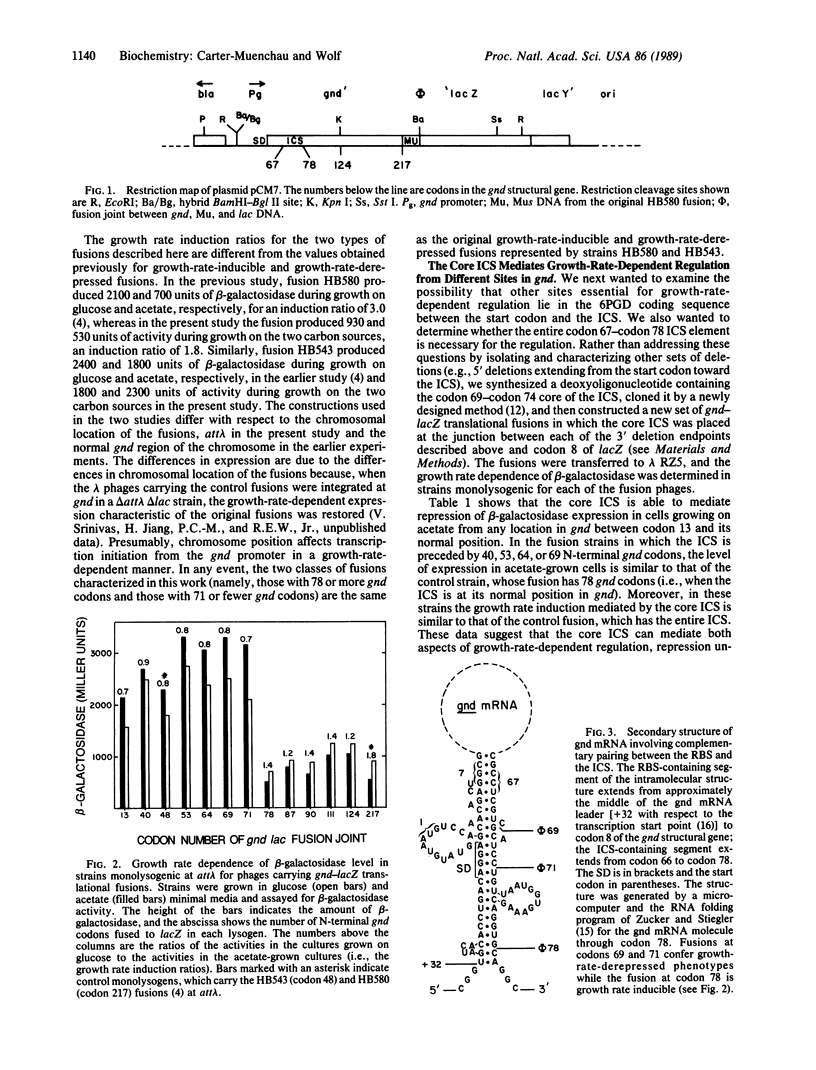
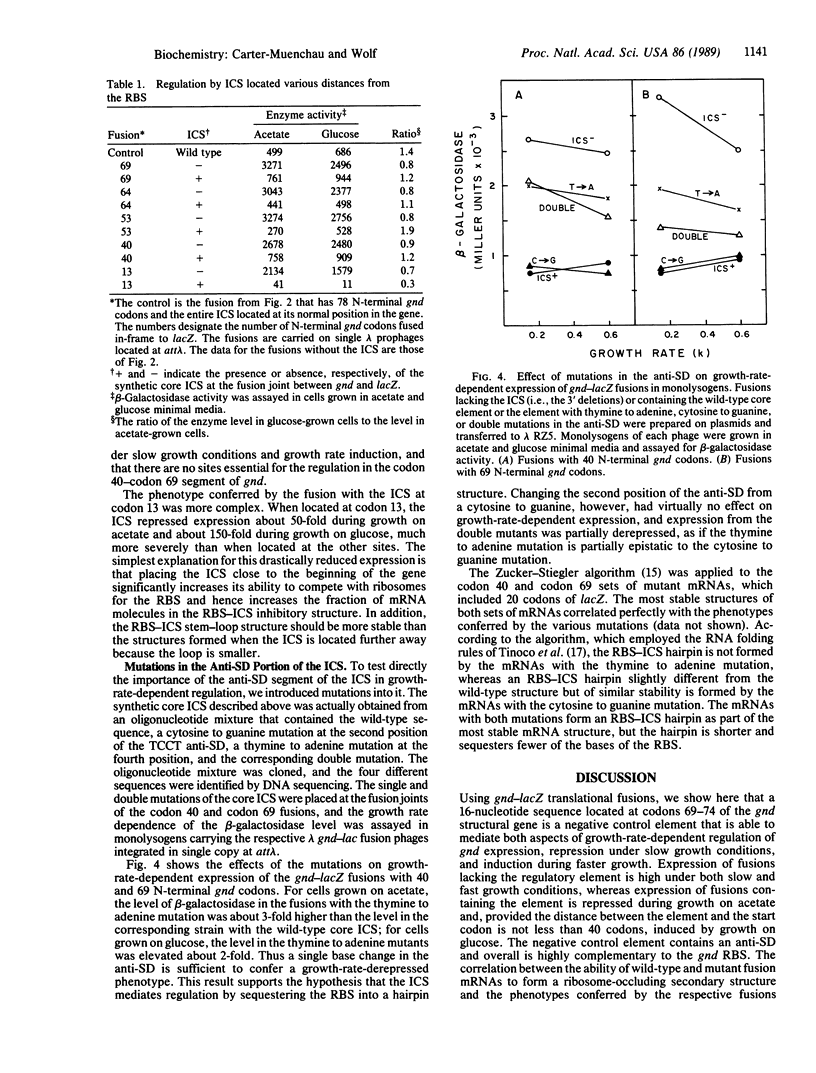
Abstract
Previous work has shown that in Escherichia coli K-12 growth-rate-dependent regulation of expression of 6-phosphogluconate dehydrogenase, encoded by the gnd gene, occurs at the posttranscriptional level and is mediated by a negative control element that lies deep in the coding sequence, somewhere between codons 48 and 118. Deletion analysis of a growth-rate-regulated gnd-lacZ translational fusion showed that the element is the segment of gnd mRNA between codons 67 and 78 that is complementary to an extensive portion of the gnd ribosome-binding site, including its Shine-Dalgarno sequence. The boundaries of the element were further defined by the cloning of a synthetic "internal complementary sequence." The core internal complementary sequence element effected growth-rate-dependent regulation when placed at several sites between codon 40 and codon 69, but it severely reduced gene expression when moved to codon 13. The effect on regulation of single and double mutations introduced into the element by site-directed mutagenesis correlated with the ability of the respective mRNAs to fold into secondary structures that sequester the ribosome-binding site. Thus the gnd gene's internal regulatory element appears to function as a cis-acting antisense RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker H. V., 2nd, Wolf R. E., Jr Essential site for growth rate-dependent regulation within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7669–7673. doi: 10.1073/pnas.81.24.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Growth rate-dependent regulation of 6-phosphogluconate dehydrogenase level in Escherichia coli K-12: beta-galactosidase expression in gnd-lac operon fusion strains. J Bacteriol. 1983 Feb;153(2):771–781. doi: 10.1128/jb.153.2.771-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Comparative nucleotide sequence analysis of growth-rate-regulated gnd alleles from natural isolates of Escherichia coli and from Salmonella typhimurium LT-2. J Bacteriol. 1988 Jan;170(1):372–379. doi: 10.1128/jb.170.1.372-379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr Growth-rate-dependent expression and cloning of gnd alleles from natural isolates of Escherichia coli. J Bacteriol. 1988 Jan;170(1):365–371. doi: 10.1128/jb.170.1.365-371.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Muenchau P., Wolf R. E., Jr A method for cloning mixtures of long, synthetic oligodeoxynucleotides. Gene Anal Tech. 1987 Sep-Oct;4(5):105–110. doi: 10.1016/0735-0651(87)90003-3. [DOI] [PubMed] [Google Scholar]
- Farrish E. E., Baker H. V., 2nd, Wolf R. E., Jr Different control circuits for growth rate-dependent regulation of 6-phosphogluconate dehydrogenase and protein components of the translational machinery in Escherichia coli. J Bacteriol. 1982 Nov;152(2):584–594. doi: 10.1128/jb.152.2.584-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke L., Wulff D. L. OOP RNA, produced from multicopy plasmids, inhibits lambda cII gene expression through an RNase III-dependent mechanism. Genes Dev. 1987 Nov;1(9):1005–1013. doi: 10.1101/gad.1.9.1005. [DOI] [PubMed] [Google Scholar]
- Lee N., Zhang S. Q., Cozzitorto J., Yang J. S., Testa D. Modification of mRNA secondary structure and alteration of the expression of human interferon alpha 1 in Escherichia coli. Gene. 1987;58(1):77–86. doi: 10.1016/0378-1119(87)90031-x. [DOI] [PubMed] [Google Scholar]
- McPheeters D. S., Christensen A., Young E. T., Stormo G., Gold L. Translational regulation of expression of the bacteriophage T4 lysozyme gene. Nucleic Acids Res. 1986 Jul 25;14(14):5813–5826. doi: 10.1093/nar/14.14.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melefors O., von Gabain A. Site-specific endonucleolytic cleavages and the regulation of stability of E. coli ompA mRNA. Cell. 1988 Mar 25;52(6):893–901. doi: 10.1016/0092-8674(88)90431-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoff M. S., Baker H. V., 2nd, Wolf R. E., Jr DNA sequence of the Escherichia coli gene, gnd, for 6-phosphogluconate dehydrogenase. Gene. 1984 Mar;27(3):253–264. doi: 10.1016/0378-1119(84)90070-2. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Schoner B. E., Hsiung H. M., Belagaje R. M., Mayne N. G., Schoner R. G. Role of mRNA translational efficiency in bovine growth hormone expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5403–5407. doi: 10.1073/pnas.81.17.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Prather D. M., Shea F. M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J Bacteriol. 1979 Sep;139(3):1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]



