Abstract
We asked whether sex and adult estrogen exposure influence the detection thresholds for urinary odors used by mice to guide their social behaviors. Gonadectomized (GDX) male and female mice were trained on a two-choice food-motivated task to determine detection thresholds for male urinary odors. There was no significant sex difference in the detection of these odors by GDX subjects without hormone replacement. However, during treatment with estradiol benzoate (EB), GDX females, but not GDX males, showed an enhanced ability to detect these odors. To investigate a possible mechanism for this effect, we measured GDX females’ odor-sampling behavior (sniffing) by monitoring intranasal pressure transients during performance of the urinary odor detection task with and without EB treatment. Under both hormone conditions females decreased their sniffing frequency as the urinary odor concentration decreased, with this decrease being significantly greater while GDX females received EB. Thus, estradiol enhanced detection thresholds for male urine in a sex-specific manner, and this enhanced sensitivity in females was correlated with altered odor-sampling behavior.
Keywords: olfaction, sex hormones, odor detection, sex differences, sniffing
Introduction
Rodents use olfactory cues to assess the sex and reproductive status of conspecifics (Brennan & Kendrick, 2006; Brown & Macdonald, 1985; Johnston, 1983). Both the perception of and behavioral reactions to socially relevant odor cues can vary with animals’ sex and endocrine status. For example, endocrine status modulates odor acuity in female rodents and humans (Dalton et al., 2002; Pietras & Moulton 1984; but see McClintock, 2002). Thus, in female rats there was enhanced detection of several common environmental odors during the estrous phase of the ovarian cycle, with a positive correlation being seen between plasma estrogen levels and females’ odor detection ability (Pietras & Moulton, 1974). Analogous results have been found in human subjects (Dalton et al., 2002; Good et al., 1976; Navarrete-Palacios et al., 2003). By contrast, gonadectomized (GDX) male rats resembled gonadally intact controls in their ability to detect urinary odors (Carr et al., 1962). Sex differences in olfactory detection and/or discrimination abilities also exist (Baum & Keverne, 2002; Dorries et al., 1995; Koelega & Köster, 1974; Pietras & Moulton, 1974; Wesson et al., 2006). With a few exceptions, most previous studies of sex differences in olfactory function have used gonadally intact subjects in which the two sexes potentially experience large differences in the type and quantity of sex hormone exposure across the period of behavioral assessment. In the current study we therefore studied male urinary odor detection thresholds in GDX male and female mice (initially given no replacement hormone) using a two-choice sand digging task adapted from previous studies of olfactory learning (Mihalick, 2003; Schellinck et al., 2001). Progressively lower concentrations of odorants emitted from the urine of gonadally intact male mice served as the discriminative stimulus for food reward in our study. Subsequently, we examined the effects of estradiol benzoate (EB) treatment on urinary odor detection thresholds in groups of GDX males and females. Data collected from GDX mice in the hormone-free condition would provide information on hard-wired sex differences in odor detection capacity that are revealed even in the absence of an adult ‘activational’ sex hormone. By contrast, data collected from GDX mice given adult EB treatment would reveal sex differences in olfactory function that are only seen in the presence of an ‘activational’ sex steroid.
Mammals actively sample odors by sniffing, which is a rhythmic behavior that controls the flow of odors to olfactory receptors in the main olfactory epithelium. It has been previously shown that rats sniff between 2-12Hz when performing odor detection or discrimination tasks (Verhagen et al., 2007; Welker, 1964; Youngentob et al., 1987). Recently, Youngentob (2005) recorded sniffing from mice (via whole-body plesthysmography) while they were passively presented with odors. However, to date, no sniffing data are available from mice during performance of an operant olfactory task. Therefore, after observing that EB treatment enhanced the capacity of GDX female mice to detect male urinary odors, we conducted a second study recording intranasal pressure transients while GDX females performed the odor detection task, first without and then with EB treatment. We hypothesized that estradiol-induced changes in sniffing behavior may have contributed to the ability of females to detect especially low concentrations of male urinary odorants.
Methods
Animals
Six-week old, sexually naive male and female Swiss-Webster mice were purchased from Taconic Farms (Hudson, NY) and housed in same-sex groups of 2-4 on a reversed light cycle (12L:12D, lights on at 21:00 h). One week prior to testing, mice were food-deprived to 80-85% of baseline body weight, with water provided ad libitum except during behavioral testing sessions. During food deprivation, body weights in male subjects averaged 34.9 ± 0.64g and in females averaged 29.1 ± 0.25g. All mice were less than 10 months of age by completion of data collection. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Institutional Animal Care and Use Committee.
Surgical Procedures
Two weeks prior to initial behavioral testing, all animals were gonadectomized (GDX) while under 3% isoflurane anesthesia with butorphanol (0.01mg/kg, Henry Schein, Melville, NY) administered immediately following surgery as an analgesic. Additionally, three GDX female mice were implanted with a hollow guide cannula (model #C313G, Plastics One, Roanoke, VA) into the dorsal nasal recess under general anesthesia induced by an IP injection of a mixture of ketamine (75mg/kg, Henry Schein) and medetomidine (1mg/kg, Pfizer Inc., NY, NY) and were also given local injections of Buprivacaine (1%, s.c., Sigma-Aldrich, St. Louis, MO) prior to opening the scalp. Following surgery, mice were also administered Antisedan (4mg/kg, s.c., Pfizer Inc.) to accelerate recovery from anesthetic as well as Rimadyl (5mg/kg, s.c., Webster Veterinary, Sterling, MA) as an analgesic for three days.
Urine Collection and Stimulus Preparation
Urine was collected from eight gonadally intact adult male Swiss-Webster mice as they were held by the nape of the neck. Urine was pooled and stored in 50 μl aliquots at −20° C. Immediately prior to testing, urine was vortexed and diluted in distilled water to final concentrations of 1×10−2, 3×10−3, 1×10−3, 3×10−4, 1×10−4, 3×10−5, 1×10−5, 3×10−6, 1×10−6, or 3×10−7 (all concentrations expressed as volume urine/volume distilled water).
Odor detection threshold testing
Subjects were transported in their home cage during the dark phase of the light/dark cycle to a testing room lit by a dim yellow light. Animals were briefly allowed to acclimate to the testing chamber, consisting of a clean plastic cage (12 × 29 × 15 cm), before training began. Odors were presented by lowering two small cups into the test chamber. The cups were placed on wire mesh platforms separated by a mesh divider. Each cup (1″ diameter, 0.5″ high) contained approximately 10g of clean sand. 25 μl of liquid odor stimulus was dispensed onto the surface of the sand. Mice were initially trained on a 2-choice odor detection task requiring them to dig in the stimulus cup laced with a 1×10−3 concentration of amyl acetate (CS+, Sigma, St. Louis, MO) but not to dig in the cup with mineral oil (CS−). Stimuli were presented on random sides of the wire mesh divider, with no more than three consecutive presentations of the CS+ on the same side. Correct responses (digging in the CS+ cup) resulted in a food reward (¼ of a Cheerio®) being lowered to the mouse by the experimenter using a long forceps. Animals were given 20 trials per day for four days or until a criterion of 85% accuracy (3 or fewer errors in 20 trials) was met. Once animals reached a criterion of 85% accuracy with amyl acetate as the CS+, they were conditioned over 1-2 testing sessions to respond to male urine (1×10−2, diluted with DI water) as the CS+ and distilled water as the CS−. Upon reaching a criterion of 85% accuracy with the urine stimulus, mice were tested on sequentially lower dilutions of male urine on each of nine consecutive days. Animals that did not meet the accuracy criterion for either amyl acetate or male urine were not tested further. Of the 12 GDX males that underwent initial training, only 4 acquired the task to criterion. Of the 10 GDX females that underwent initial training, 7 reached criterion. A repeat presentation of the initial (high) urine concentration (3×10−3 dilution) stimulus condition was performed after animals reached a failing performance level (threshold; ≤ 60% correct responses) to ensure that subjects’ performance failure at low concentrations was due to an inability to detect the stimulus and not to a lack of motivation to perform the task.
Following completion of the first series of tests, GDX animals of both sexes were given daily subcutaneous injections of 1μg 17β-estradiol benzoate (EB, Sigma, St. Louis, MO; dose from Wesson et al., 2006) dissolved in sesame oil beginning one week prior to retraining sessions with both amyl acetate and male urine and a repeat of the above testing sequence. EB was administrated each day two hours before the testing session began. Again, GDX+EB animals that did not meet the accuracy criterion for both amyl acetate and male urine stimulus conditions were not tested further. Specifically, of the 8 GDX+EB males that underwent training, 7 acquired the task to criterion. This included 2 GDX males tested previously without EB treatment and 5 newly tested GDX males. Furthermore, of the 10 GDX+EB females that underwent training, 8 reached criterion. This included 7 GDX females tested previously without EB treatment and 1 newly tested GDX female. A subset of GDX female mice (n=5) was tested a third time, again in the absence of hormone treatment (EB-withdrawal) to ensure that any differences in performance between the GDX and EB-treated conditions were not due to the effects of extended, repeated testing. Testing of EB-withdrawal GDX female mice began 2 weeks after cessation of EB treatment.
Food motivation test
An assessment of subjects’ food motivation was performed following the final odor detection session in both the GDX and GDX+EB conditions to ensure that all animals had sufficient motivation to perform the task, and to make sure there was no disruptive effect of hormone treatment on food motivation. Approximately 0.55 g of food reward (Cheerio®) was placed into a clean testing cage inside a small cup. This amount matches the maximum reward given in a testing session, and thus the amount of food eaten in ten minutes was recorded as an indication of food motivation. All subjects in this study, regardless of sex or hormone treatment, consumed the entire amount of food provided. This outcome implies that subjects of both sexes and in both endocrine states were hungry and motivated to perform the operant task.
Sniff Data Collection
Three females implanted with sniff cannulae were trained with the same protocols as outlined above, with the exception that training and testing occurred in a modified testing chamber designed to facilitate collection of sniff signals. Odors were presented individually on opposite sides of a divider at one end of the chamber. For sniff signal acquisition, the hollow guide cannula was connected to a pressure transducer (model CPXL04GF, Honeywell Intl, Morristown, NJ), via 22Ga flexible tubing and an air-tight swivel (model 375/22PS; Instech Labs, Plymouth Meeting, PA). Sniff data, acquired at 500Hz, were filtered on-line between 0.1-100Hz in National Instruments LabVIEW (Austin, TX) along with a 5V signal which was manually initiated by the experimenter indicating the times the animal dug in the stimulus cup. Sniff data were collected at high, moderate, and low urine concentrations in both the GDX and GDX + EB conditions. High (1×10−3) and low concentrations (1×10−7) of male urine were chosen to ensure maximal and minimal performance, respectively, and moderate concentrations of urine were chosen as the concentrations at which GDX and GDX + EB-treated females showed approximately 75% accuracy (1×10−5 and 1×10−6 dilutions, respectively). Mice were tested at each urine concentration for 2 sessions (1 session/day), 20 trials/session.
Data Analysis
The number of mice that reached criterion performance was compared to the total number of mice that initiated training with a Kolmogorov-Smirnov test to assess differences in learning the odor detection task. At each urine dilution, between-groups 2-tailed t-tests were used to compare the performance of GDX male vs. GDX female subjects on the odor detection task. Differences in the composition of the groups administered odor detection tests under each endocrine condition led us to use between, as opposed to within-groups t-tests. Thus, at each urine dilution, between-groups 2-tailed t-tests were used to compare the performance of GDX female mice as well as GDX male mice before and during EB treatment. Offline extraction and analyses of sniff data were carried out in LabVIEW and MatLab (The MathWorks, Inc., Natick, MA). Sniff cycles were filtered off-line between 0.5 and 50Hz. After filtering, sniffs were peak detected to the point of maximum inhalation using custom software written in LabVIEW and sorted into 500ms time bins based upon the onset of the dig indicator. The time of each sniff peak was then read into MatLab for analysis. Further, within-groups 2-tailed t-tests were used to compare baseline sniffing frequencies of GDX vs. GDX+EB female mice. To look for an effect of endocrine state on sniffing during ‘odor sampling’, within-groups 2-tailed t-tests were used at each stimulus dilution. Finally, to look for an effect of stimulus dilution on sniffing during ‘odor sampling’, within-groups 2-tailed t-tests were used to compare HI versus LOW dilutions in each endocrine state. Data are expressed as mean ± SEM.
Results
Odor detection thresholds
Only GDX animals of each sex that reached the 85% correct response criterion using both amyl acetate and a high concentration (1×10−2 dilution) of male urine as stimuli within the first four days of training were tested further under each endocrine condition. Of the 12 GDX males that underwent initial training, only 4 acquired the task to criterion. Of the 10 GDX females that underwent training, 7 reached criterion. Additionally, of the 8 GDX+EB males that underwent training, 7 acquired the task to criterion. Finally, of the 10 GDX+EB females that underwent training, 8 reached criterion. Kolmogorov-Smirnov tests failed to show any significant effect of sex or hormone treatment on the proportion of animals that reached the training criterion.
Performance on the odor detection task decreased dramatically in both GDX male and female subjects over the odor dilution series regardless of whether or not subjects were given EB (Fig. 1; both panels). T-tests carried out on the percentage of correct responses shown at each urine dilution failed to reveal a significant difference in the ability of GDX males and females (when not administered EB) to detect the urinary odor stimulus. By contrast, treatment with EB enhanced the ability of GDX females to detect male urinary odor (Fig. 1; top panel). Thus, significant treatment effects were found at several stimulus concentrations in female mice: 3×10−4 (t(13)=2.28, p=0.04), 1×10−4 (t(13)=2.69, p=0.019), 3×10−6 (t(13)=2.17, p=0.029), and 1×10−6 (t(13)=3.79, p=0.002) dilutions of urine. In a subsequent series of tests given two weeks after the cessation of EB injections (GDX-EB withdrawal), a subset of these same GDX females performed the urinary odor detection task at the same level of accuracy as during the original tests that were initially given to GDX females in the absence of EB treatment (see Supplementary Figure 1).
Figure 1.
Effect of estradiol benzoate treatment (EB) of gonadectomized (GDX) female (top) and male mice (bottom) on odor detection thresholds for different concentrations of gonadally intact male urine. Dashed line at 60% represents the performance criterion below which subjects were deemed as having failed to detect the urinary odor stimulus concentrations presented. *p<0.05; **p<0.01., 2-tailed between-groups t-test comparisons of GDX females’ odor detection performance with and without EB treatment.
In contrast to the response of GDX female subjects, EB treatment failed to enhance the urinary odor detection capacity of GDX male mice at any dilution of urine presented (Fig. 1, bottom panel). All mice successfully completed a recovery session (Fig. 1; both panels) during which they performed at ≥ 80% response accuracy when the highest concentration of male urine again served as the discriminative stimulus for food. This outcome suggests that failing performance scores at low urinary stimulus dilutions were due to subjects’ inability to detect the stimulus as opposed to any deficit in subjects’ motivation to perform the task.
Sniffing results
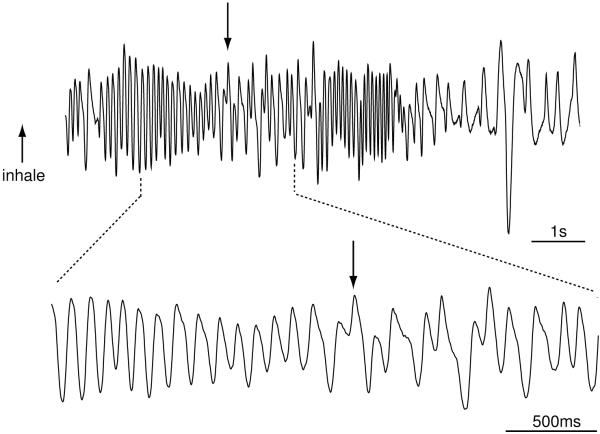
To determine a mechanism by which estradiol may modulate subjects’ odor detection thresholds, and thus potentially explain why EB treatment enhanced females’ odor detection performance, we measured sniffing in 3 GDX female mice as they performed the urinary odor detection task. Mouse sniffing appeared as rapid pressure transients, reflecting inhalation and exhalation cycles (Fig. 2). Furthermore, these pressure transients changed in frequency throughout the odor detection task (Fig. 2). To determine if EB treatment had a general effect on sniff frequency (independent of ‘odor sampling’ per se), we performed T-tests of post-dig (i.e., after the odor-detection decision) sniffing frequencies throughout the dilution sequence. This analysis failed to show an overall baseline respiratory difference due to EB treatment, with GDX female mice sniffing at 10.5 ± 0.12 Hz in the absence of EB treatment and at 10.1 ± 0.15 Hz during EB treatment. For a measure of ‘odor sampling’, we choose to look at the 3 seconds prior to the time when animals first dug in the sand cup. This seemed like a reasonable interval during which to assume that the animal was engaged in ‘odor sampling.’ While there was no significant difference in sniffing frequencies during odor sampling between the high and low stimulus concentrations in the GDX condition, this comparison was significant in the GDX+EB condition (t(4)=3.99, p=0.016; Fig. 3). There was also a significant effect of endocrine status on sniff frequencies at the lowest stimulus concentration (t(4)=2.91, p=0.043), but not at the high or middle concentrations.
Figure 2.
Intranasal pressure transients (sniffing) recorded from a female mouse performing the odor detection task when a male urinary odor was presented as the discriminative stimulus for food reward. Sniff frequency was recorded using a hollow cannula implanted into the dorsal nasal recess and connected to a pressure transducer. Inhalation is indexed by an upward trace whereas exhalation is indexed by a downward trace. The downward arrow marks the time when the mouse began digging in the sand. This trace was recorded from a gonadectomized female mouse given no EB while making a correct response to dig to the CS+, which was a 1×10−5 (middle) dilution of male urine.
Figure 3.
Sniff frequencies (mean ± SEM) monitored in three gonadectomized (GDX) female mice before and after estradiol benzoate (EB) treatment while they performed the odor detection task. The high (HI), middle (MID) and low concentrations (CONC) of male urine presented were 1×10−3, 1×10−5, and 1×10−7 dilutions, respectively. Data points indicate individual subject’s performance. *p<0.05., 2-tailed within-groups t-tests.
Discussion
In the present study GDX male and female mice, when given no sex hormone replacement, showed an equivalent capacity to detect decreasing concentrations of male urinary odors in a food-motivated task. This outcome differs from results of two earlier studies (Baum & Keverne, 2002; Pierman et al., 2006) in which GDX female mice consistently investigated lower concentrations of male as well as female urinary odorants more reliably than GDX males in home-cage habituation/dishabituation tests. It seems likely that the latter sex difference reflected a difference in subjects’ motivation to approach and investigate the urinary odors presented, as opposed to a sex difference in the capacity of subjects’ olfactory nervous systems to detect these odors. In the present study subjects were food deprived and motivated to perform the odor detection task by hunger and the expectation of receiving a food reward. When hunger, as opposed to subjects’ intrinsic interest in conspecifics’ odors, served as the motivation for the behavioral responses being observed, olfactory detection capacity in gonadectomized subjects given no sex hormone priming showed no evidence of being sexually dimorphic.
In the present study administration of EB significantly enhanced the capacity of GDX female mice to detect progressively lower concentrations of male urinary odor whereas this treatment had no such effect in GDX males. In some ways this observation parallels results of a previous study (Dorries et al., 1995) in which ovary-intact female pigs detected a low concentration of the male pheromone, androstenone, more reliably than testis-intact males in an operant task motivated by sucrose reward. The fact that these two groups of pigs were gonadally intact at the time of testing raises the possibility that differences in their endocrine state at the time of testing (e.g., presence of estradiol in females) may have contributed to the observed sex difference in odor detection. Interestingly, in the study by Dorries et al. (1995) there was no difference in the capacity of gonadally intact and castrated boars to detect progressively lower concentrations of androstenone. This outcome also parallels the present observation that adult administration of EB to GDX male mice failed to influence their ability to detect progressively lower concentrations of male urine in an operant task. The absence of any activational effect of EB treatment on odor detection capacity of adult GDX male mice also parallels an early report (Carr et al., 1962) that castration of adult male rats failed to affect their capacity to detect progressively lower concentrations of estrous female urinary odors in a water-motivated operant task.
To investigate the possibility that EB may enhance the ability of female mice to detect low concentrations of male urinary odors by altering their sampling of these odors, we systematically examined the sniffing behavior of GDX female mice with and without EB replacement while they performed the odor-detection task. At each of three concentrations of male urine presented, GDX females showed lower sniff frequencies (during odor sampling) when they received EB as opposed to no hormone, although the effect of EB only reached statistical significance at the lowest stimulus concentration. The effect of EB on sniffing frequency in GDX females was correlated with the ability of this treatment to enhance odor detection capacity. One possibility is that EB treatment facilitated the access of low concentrations of male urinary odor to olfactory receptors in the main olfactory epithelium. There is some independent evidence supporting such a view. For example, nasal patency and airflow resistance was enhanced in post-menopausal women by estradiol/progesterone replacement therapy (Caruso et al., 2004), perhaps due to structural changes in the olfactory turbinates (Arimondi et al., 1993), and this effect correlated with enhanced detection of several odorants following hormone treatment (Caruso et al., 2004). Also, the viscosity of the olfactory mucosa is hormone dependent (Mair et al., 1978), which might consequently affect the sorption of odor molecules and their binding to olfactory receptors. In another study (Massaro et al., 2006) ovariectomy of female rats led to decreased surface area in the alveoli of the lungs, a change that was prevented by estrogen replacement. Thus, the presence of estrogen may control gas exchange efficiency, which is a potential explanation for the overall lower respiratory rate exhibited by GDX+EB females.
Another possibility, which is not mutually exclusive from an effect of EB on odor access to the main olfactory epithelium, is that estradiol directly affects the processing of odor information in the female’s central nervous system. Both estradiol receptor (ER)-α and ER-β expression has been reported in several olfactory regions, including the olfactory bulb, anterior olfactory nucleus, pyriform cortex, entorhinal cortex, and the bed nucleus of the stria terminalis (BNST) (Shughrue et al. 1997). In female rats, variations in ER-β distribution occurred in the amygdala and BNST across the estrous cycle (Isgor et al., 2002). Estradiol receptors are also expressed in the locus coeruleus (Heritage et al., 1980; Shughrue et al., 1997), a midbrain nucleus that provides centrifugal noradrenergic input to the granule cell layer of the main olfactory bulb. In addition, estrogen’s role in protecting against cell death and facilitating synaptic plasticity is widely acknowledged (for a review, see Brann et al., 2007). The rostral migratory stream is a well-established site of adult neurogenesis (Lledo et al., 2006) that provides a steady influx of new granule as well as periglomerular cells into the main olfactory bulb. Research in ovariectomized female voles (Smith et al., 2001) showed increased neurogenesis occurring in the rostral migratory stream in response to estradiol treatment. The resultant increase in the influx of main olfactory bulb granule cells may have enhanced olfactory function. Further convincing evidence of a direct modulatory role of estrogen on olfactory neurons comes from an early study by Schmidt & Schmidt (1980). Odor-evoked potentials were monitored in the olfactory bulb of naturally cycling female rats. Neuronal activation thresholds to odor stimuli were lowest in female rats during the proestrous phase of the estrous cycle, pointing to an enhancing effect of estrogen on olfactory sensitivity.
We cannot rule out the possibility that EB treatment enhanced attention non-specifically in GDX female mice so that these subjects performed better than non-hormone primed GDX females in our olfactory detection task, especially when low concentrations of male urine were presented. Indeed, reductions in estradiol following ovariectomy or aging of female rats impaired performance on a complex serial reaction time task, and this effect was reversed by administration of estradiol (Barnes et al., 2006).
In summary, we have shown that EB treatment enhanced olfactory detection thresholds in GDX female mice, allowing subjects reliably to detect low concentrations of male urine, thereby providing a potential reproductive advantage in mate location. We also showed that during treatment with estrogen, GDX female mice exhibited a greater modulation of sniff frequency over 3 dilutions of male urine. We propose that this action of estradiol in conjunction with direct effects of the hormone on the influx and/or activity of neurons in the olfactory bulb may underlie the observed enhancement of females’ odor detection capacity.
Supplementary Material
Acknowledgments
This research was supported by NIH grants HD21094 and HD044897 (MJB), and by a grant to KGS from the Boston University Undergraduate Research Opportunity Program.
References
- Arimondi C, Vannelli GB, Mathe F, Mrowinski D. Importance of olfaction in the sexual life: Morphofuctional and psychological studies in man. Biomed. Res. (India) 1983;4:43–52. [Google Scholar]
- Barnes P, Staal V, Muir J, Good MA. 17-Beta estradiol administration attenuates deficits in sustained and divided attention in young ovariectomized rats and aged acyclic female rats. Behavioral Neuroscience. 2006;120:1225–1234. doi: 10.1037/0735-7044.120.6.1225. [DOI] [PubMed] [Google Scholar]
- Barni T, Maggi M, Fantoni G, Granchi S, Mancina R, Gulisano M, Marra F, Macorsini E, Luconi M, Rotella C, Serio M, Balboni GC, Vannelli GB. Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. Journal of Clinical Endocrinology and Metabolism. 1999;84:4266–4273. doi: 10.1210/jcem.84.11.6150. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Hormones and Behavior. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philosophical Transactions of the Royal Society B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Macdonald D. Social Odours in Mammals. Clarendon Press; Oxford: 1985. [Google Scholar]
- Carr WJ, Loeb LS, Wylie NR. Response to feminine odors in normal and castrated male rats. Journal of Comparative and Physiological Psychology. 1966;62:336–338. doi: 10.1037/h0023693. [DOI] [PubMed] [Google Scholar]
- Caruso S, Grillo C, Agnello C, Di Mari L, Farina M, Serra A. Olfactometric and rhinomametric outcomes in post-menopausal women treated with hormone therapy: a prospective study. Human Reproduction. 2004;19:2959–2964. doi: 10.1093/humrep/deh465. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Breslin PAS. Gender-specific induction of enhanced sensitivity to odors. Nature Neuroscience. 2002;5:199–200. doi: 10.1038/nn803. [DOI] [PubMed] [Google Scholar]
- Davies VJ, Bellamy D. The olfactory response of mice to urine and effects of gonadectomy. Journal of Endocrinology. 1972;55:11–20. doi: 10.1677/joe.0.0550011. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Olfactory sensitivity to the pheromone, androstenone, is sexually dimorphic in the pig. Physiology and Behavior. 1995;57:255–259. doi: 10.1016/0031-9384(94)00225-t. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Olfactory control of the sexual behavior of male and female mice. Physiology and Behavior. 1973;11:867–872. doi: 10.1016/0031-9384(73)90282-5. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Griffis KT, Tardivel C. Olfactory bulb removal: effects on sexual behavior and partner-preference in male rats. Physiology and Behavior. 1990;48:447–450. doi: 10.1016/0031-9384(90)90342-2. [DOI] [PubMed] [Google Scholar]
- Good PR, Geary N, Engen T. The effect of estrogen on odor detection. Chemical Senses and Flavor. 1976;2:45–50. [Google Scholar]
- Gréco B, Lubbers LS, Blaustein JD. Estrogen receptor beta messenger ribonucleic acid expression in the forebrain of proestrous, pregnant and lactating female rats. Endocrinology. 2003;144:1869–1875. doi: 10.1210/en.2002-220807. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–9. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Hoyk Z, Varga C, Párducz A. Estrogen-induced region specific decrease in the density of 5-bromo2-deoxyuridine-labeled cells in the olfactory bulb of adult female rats. Neuroscience. 2006;141:1919–1924. doi: 10.1016/j.neuroscience.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Isgor C, Huang GC, Akil H, Watson J. Correlation of estrogen beta-receptor messenger RNA with endogenous levels of plasma estradiol and progesterone in the female rat hypothalamus, the bed nucleus of the stria terminalis and the medial amygdala. Brain Research: Molecular Brain Research. 2002;106:30–41. doi: 10.1016/s0169-328x(02)00407-2. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical signals and reproductive behavior. In: Vandenbergh JG, editor. Pheromones and Reproduction in Mammals. Academic Press; New York: 1983. pp. 3–37. [Google Scholar]
- Koelega HS, Köster EP. Some experiments on sex differences in odor perception. Annals of the New York Academy of Sciences. 1974;237:234–46. doi: 10.1111/j.1749-6632.1974.tb49859.x. [DOI] [PubMed] [Google Scholar]
- Mair RG, Bouffard JA, Engen T, Morton TH. Olfactory sensitivity during the menstrual cycle. Sensory Processes. 1978;2:90–98. [PubMed] [Google Scholar]
- Massaro GD, Mortola JP, Massaro D. Estrogen modulates the dimensions of the lung’s gas-exchange surface area and alveoli in female rats. American Journal of Physiology. 1996;270:110–4. doi: 10.1152/ajplung.1996.270.1.L110. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Pheromones, odors, and vasanas: the neuroendocrinology of social chemosignals in humans and animals. In: Pfaff DW, editor. Hormones, Brain, & Behavior. Academic Press; New York: 2002. pp. 797–845. [Google Scholar]
- Mihalick SM. Perinatal exposure to diethylstilbestrol improves olfactory discrimination learning in male and female Swiss-Webster mice. Neurobiology of Learning and Memory. 2003;80:55–62. doi: 10.1016/s1074-7427(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nature Reviews: Neuroscience. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Navarrete-Palacios E, Hudson R, Reyes-Guerrero G, Guevara-Guzman R. Lower olfactory threshold during the ovulatory phase of the menstrual cycle. Biological Psychology. 2003;63:269–79. doi: 10.1016/s0301-0511(03)00076-0. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Moulton DG. Hormonal influences on odor detection in rats: changes associated with the estrous cycle, pseudopregnancy, ovariectomy, and administration of testosterone propionate. Physiology and Behavior. 1973;12:475–491. doi: 10.1016/0031-9384(74)90125-5. [DOI] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Balthazart J, Baum MJ, Bakker J. Attraction thresholds and sex discrimination of urinary odorants in male and female aromatase knockout (ArKO) mice. Hormones and Behavior. 2006;49:96–104. doi: 10.1016/j.yhbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Research. 2006;1123:89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Forestell CA, LoLordo VM. A simple and reliable test of olfactory learning and memory in mice. Chemical Senses. 2001;26:663–672. doi: 10.1093/chemse/26.6.663. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schmidt U. Changes of olfactory sensitivity during the estrus cycle in female laboratory mice. Chemical Senses. 1980;5:359–365. [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and –beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Hormones and Behavior. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nature Neuroscience. 2007;10:631–9. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing in the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Hormones and Behavior. 2006;49:580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. Journal of Neuroscience. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Hormones and Behavior. 2003;44:110–118. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiology & Behavior. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Youngentob SL. A method for rapid automated assessment of olfactory function. Chemical Senses. 2005;30:219–229. doi: 10.1093/chemse/bji017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.