Abstract
N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-RGD (Arg-Gly-Asp) conjugates targeting the αvβ3 integrin present on angiogenic blood vessels and some tumor types have shown increased accumulation in solid tumors and possess properties that suggest their use for site-specific drug delivery. Geldanamycin (GDM) is a benzoquinoid ansamycin that binds to heat-shock protein 90 (HSP90), effective for the treatment of multiple cancer types including prostate, but has dose-limiting cytotoxicity. We recently reported the synthesis of HPMA copolymer-aminohexyl-geldanamycin (AH-GDM) conjugates containing RGDfK that demonstrated favorable properties of drug release, in vitro binding to the αvβ3 integrin, cytotoxicity in human prostate cancer cells, and tolerability in nude mice greater than 2-fold equivalent free drug doses. In this study the biodistribution of 125I-radiolabeled HPMA copolymer-AH-GDM conjugates with and without RGDfK in both non-tumor and DU145 prostate tumor xenograft-bearing nude mice was evaluated. At 60 mg/kg drug equivalent polymer doses in non-tumor bearing mice both conjugates showed fast elimination from blood and decreasing accumulation in all other organs. Kidney accumulation predominated and was higher for the conjugate containing RGDfK. In tumor bearing mice, trace quantities of the conjugate containing RGDfK showed increased tumor accumulation as compared to the conjugate without RGDfK. Also evaluated were free drug concentrations in prostate tumor xenografts following treatments of 30 and 60 mg/kg drug-equivalent copolymer-conjugates (with and without RGDfK) compared with 30 mg/kg free AH-GDM. Overall, 60 mg/kg treatment of RGDfK-containing conjugate showed significantly higher (p<0.001) tumor drug concentrations compared with all other treatments. The targetable conjugates can effectively deliver higher amounts of geldanamycin to the tumor compared to non-targetable systems.
Keywords: Geldanamycin, HPMA copolymer, RGDfK, Targeted delivery, Prostate cancer
INTRODUCTION
Recent advances in polymeric drug delivery systems have improved the chemotherapeutic options available to cancer patients1. The use of targeted therapies aids the continued development of highly effective therapies designed to target the tumor2. Copolymers based on N-(2-hydroxypropyl)methacrylamide (HPMA) are one such drug carriers undergoing clinical investigations for chemotherapeutic delivery because of their macromolecular and biologically compatible nature3. Targeted HPMA copolymer-based drug delivery systems have been previously investigated for their ability to deliver radioisotopes to solid prostate and lung tumor xenografts in mice4-7. These conjugates contained cyclic Arg-Gly-Asp (RGD) peptide sequences that target αvβ3 integrins expressed on angiogenic blood vessels and tumor cells8, 9. Increased tumor accumulation of these conjugates compared to non-targeted conjugates and peptide alone was identified and set the stage for the use of these copolymers for the delivery of bioactive agents to solid tumors.
Geldanamycin (GDM) is a benzoquinoid ansamycin that binds to the chaperone protein heat-shock protein 90 (HSP90), inhibiting its ability to fold client proteins into their active conformation10, 11. Many of these proteins (Lattouf, et al.12) are required for cancer cell survival and progression13, 14. Inhibition of HSP90 clients such as the wild-type and mutated androgen receptor, human epidermal growth factor receptor 2 (HER2) and protein kinase B offer particular advantages in GDM therapy for treatment of prostate cancer, among many other cancer types12. Geldanamycin analogs 17-allylamino-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) were developed following the pre-clinical failure of the parent compound due to hepatotoxicity15 and are currently progressing in clinical trials16-20. These analogs were designed to decrease the toxicity of the parent compound while maintaining or increasing potency. While these efforts have been successful to a given extent, clinical response continues to be limited12, 20.
In a previous work21, we reported the synthesis of HPMA copolymers containing aminohexyl-geldanamycin (AH-GDM) and the targeting peptide RGDfK. AH-GDM is attached to copolymer side chains via a Gly-Phe-Leu-Gly (GFLG) peptide spacer that is degraded intracellularly by lysosomal enzymes resulting in drug release3. These systems demonstrated the ability for in vitro drug release, actively bound to the αvβ3 integrin in vitro, and displayed in vitro cytotoxicity comparable to free AH-GDM. Upon single administration of drug-equivalent doses of AH-GDM and copolymer conjugates in vivo the conjugates demonstrated a marked increase in tolerability over 2-fold when compared with free drug.
The purpose of the current study was to further the advancement of these targeted drug delivery systems by testing their in vivo properties for delivering the active agent AH-GDM to prostate tumors. We hypothesized that the copolymer-drug conjugates targeted to tumor vasculature can deliver significantly higher amounts of drug to the tumor environment than can be achieved through delivery of free drug, thereby increasing the therapeutic index with higher local doses and decreased toxicity to background organs. Studies demonstrating the in vivo biodistribution of radiolabeled conjugates and in vivo tumor drug accumulation are reported here in.
EXPERIMENTAL SECTION
Chemicals
Geldanamycin (NSC 122750) was kindly supplied by the National Cancer Institute Developmental Therapeutics Program and was protected from light during all procedures. RGDfK (MW 604.5) was obtained from AnaSpec, Inc. (San Jose, CA). 125I-echistatin (2000 Ci/mmol) was purchased from Perkin Elmer (Waltham, MA). Iodine-125 (100mCi/ml) was obtained as Na125I from Perkin Elmer in 10−5M NaOH. All amino acids used were of L-configuration. All other chemicals were of reagent grade as obtained from Sigma Chemical Co (St. Louis, MO).
Synthesis and characterization of AH-GDM derivative and comonomers
N-(2-hydroxypropyl)methacrylamide (HPMA)22; N-methacryloyl-tyrosinamide (MA-Tyr)23; N-methacryloylglycylglycyl-p-nitrophenyl ester (MA-GG-ONp)24; N-methacryloyl-glycylphenylalanylleucylglycine-p-nitrophenyl ester (MA-GFLG-ONp)25; 17-(6-aminohexylamino)-17-demethoxygeldanamycin (AH-GDM)21, 26-28 and N-methacryloylglycylphenylalanylleucylglycl-17-(6-aminohexylamino)-17-demethoxygeldanamycin (MA-GFLG-AH-GDM)21, 26-28 were synthesized and characterized according to previously described methods. Water-soluble GDM derivative AH-GDM hydrochloride was synthesized according to a previously described method21 and used for animal studies.
Synthesis and characterization of HPMA copolymer conjugates
HPMA copolymer precursor was synthesized via free radical precipitation copolymerization of comonomers in 10% v/v anhydrous dimethyl sulfoxide (DMSO) in acetone using N, N’-azobisisobutyronitrile (AIBN) as the initiator22. The feed composition of comonomers for copolymer precursor was 20 mol% for MA-GG-ONp, 5 mol% for MA-GFLG-AH-GDM, 2 mol% for MA-Tyr and 73 mol% for HPMA. The comonomer mixture was sealed in an ampoule under nitrogen and stirred at 50°C for 24h. Solvent was removed by rotary evaporation, copolymer precursor dissolved in methanol and precipitated and washed in diethyl ether. MA-GG-ONp content in the polymeric precursor was assessed by release of p-nitrophenol (ONp) from the copolymer in 1.0 N sodium hydroxide by UV spectrophotometry (400 nm). Weight average molecular weight (Mw) and polydispersity (Mw/ Mn) were estimated by size exclusion chromatography (SEC) on a Superose 12 column (10 mm × 30 cm) (GE Healthcare, Piscataway, NJ) with fractions of known molecular weight HPMA copolymers using a Fast Protein Liquid Chromatography (FPLC) system (GE Healthcare).
HPMA copolymer-AH-GDM-RGDfK conjugate (P1) (Figure 1) was synthesized via p-nitrophenyl ester aminolysis of polymeric precursors in dry DMF in the presence of pyridine for 48h6. The reaction was terminated with 0.1 N sodium hydroxide and DMF removed under vacuum. Copolymer precipitates were dissolved in deionized water and purified using Amicon Ultra-15 (MWCO 3000, Millipore, Billerica, MA) ultracentrifugal tubes to remove small molecular weight impurities. HPMA copolymer-AH-GDM conjugate (P2) was formed by hydrolyzing the precursor ONp groups with 0.1 N sodium hydroxide followed by purification using the same method as for P1. AH-GDM content was determined by UV spectrophotometric analysis of copolymer product P2 (without RGDfK) using ε340nm = 2.16 × 104 M−1 cm−1 in DMSO. The peptide and MA-Tyr content of copolymer conjugate P1 was determined by amino acid analysis (Commonwealth Biotechnologies, Richmond, VA).
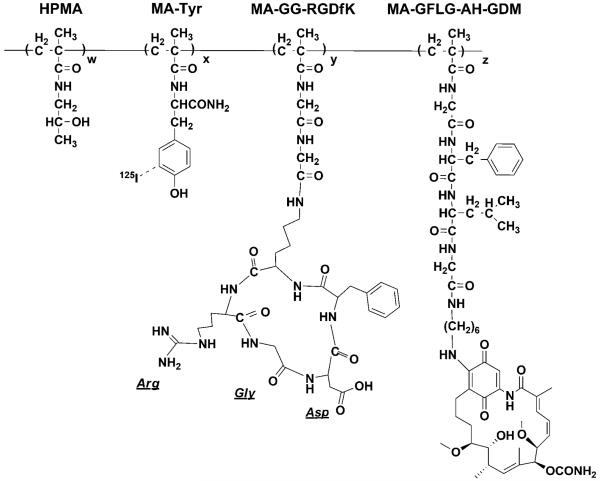
FIGURE 1.
Structure of HPMA copolymer-RGDfK-AH-GDM conjugates. Copolymers P1 and P2 contained the geldanamycin derivative AH-GDM as well as MA-Tyr to label the conjugate with 125I. P1 contained the monocyclized RGDfK peptide targeting moiety to target the αvβ3 integrin.
125I radiolabeling of conjugates
The copolymers were labeled via conjugation of 125I to tyrosinamide groups incorporated using the Iodogen method as previously described29. Briefly, 3 mg of copolymer conjugates were dissolved in 0.5 ml of PBS and added to IODO-GEN tubes (Pierce, Rockford, IL) along with 0.5 mCi of 125I in 0.2 ml saline. The solutions were mixed and incubated for 30 minutes at room temperature with intermittent gentle agitation. Following incubation, the labeled conjugates were isolated in normal saline over a Sephadex G-25 (PD-10) column (GE Healthcare, Piscataway, NJ).
Cell lines
DU145 prostate cancer cells (ATCC, Manassas, VA) were cultured in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 4 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1% 100x antibiotic-antimycotic (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 (v/v). Human umbilical vein endothelial cells30 (HUVECs) were cultured in endothelial cell growth media-2 (EGM-2: Lonza, Walkersville, MD) at 37°C in a humidified atmosphere of 5% CO2 (v/v). For all experimental procedures, sub-confluent cells in 24h culture were harvested with 0.05% trypsin/0.02% EDTA in PBS.
Mouse xenograft model of human prostate cancer
DU145 prostate cancer cells were collected, washed, counted and resuspended in RPMI 1640 media. 5×106 cells (50μl) mixed with 50μl Matrigel (Becton Dickinson Biosciences, Bedford, MA) were injected subcutaneously in the flank of each female athymic NCr-nu/nu mice (Animal Production Facility at NCI Frederick, MD) (6-8 weeks old, 20-25 g). After 28 days post-injection, tumor volume reached approximately 400 mm3 and experiements initiated. All studies were conducted under an approved protocol of the University of Maryland Baltimore Institutional Animal Care and Use Committee (IACUC).
Cell receptor binding assay
The binding affinities of free RGDfK peptide and HPMA copolymer-AH-GDM-RGDfK conjugate (P1) were assessed using a competitive binding assay to αvβ3 integrin expressed on HUVECs with 125I-echistatin31-33. HUVECs were harvested, washed with PBS, resuspended in binding buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mmol/L MnCl2, 0.1% BSA) and seeded in 96-well Multiscreen HV filter plates (0.45 μm; Millipore) at 50,000 cells per well. Cells were co-incubated at 4°C for 2h with 125I-echistatin (0.05 nM) and increasing peptide equivalent concentrations of copolymers or free RGDfK (0-500 μM), and final volume adjusted to 200 μL all in binding buffer. Following incubation, the plates were filtered using a Multiscreen vacuum manifold (Millipore) and washed twice with cold binding buffer. Filters were harvested and radioactivity determined by γ-counting (Perkin Elmer Wizard, 1470 Automatic Gamma Counter) to determine percent bound 125I-echistatin. Nonspecific binding was determined by incubating cells with a 200-fold excess of cold echistatin. Nonlinear regression analysis and determination of IC50 values was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Biodistribution studies
Two biodistribution studies with radiolabeled copolymers were conducted. In the first study, non tumor-bearing athymic nude mice were injected via the lateral tail with 200 μl of normal saline containing HPMA copolymer-AH-GDM-RGDfK (P1) or HPMA copolymer-AH-GDM (P2) at 60 mg/kg drug equivalent with 1 μCi 125I-labeled copolymer conjugates as a tracer. Time dependent biodistribution studies were carried out by sacrificing mice at 15 min, 1, 6, 24, 48 and 72h post-injection (p.i.). At the time of euthanasia, blood samples were collected by cardiac puncture. During necropsy, whole organ tissue samples were obtained from the heart, lung, liver, kidneys, spleen, small intestine, large intestine, stomach and skeletal muscle. Blood concentrations of 125I-radiolabeled conjugates were calculated as μCi/ml from radioactivity counts and 2-compartmental pharmacokinetic analyses performed using WinNonlin (Pharsight Corp., Mountain View, CA).
In the second study, tumor-bearing mice were injected via the lateral tail vein with 200 μl of normal saline containing 4 nmol each of 125I labeled HPMA copolymer-AH-GDM-RGDfK (P1) conjugate (4.5-4.7 μCi) or HPMA copolymer-AH-GDM (P2) conjugate (4.2-4.4 μCi) per mouse. Time dependent biodistribution studies were carried out by sacrificing mice at 1, 24, 48 and 72h p.i.. At the time of euthanasia, blood was collected as described above. During necropsy, whole organ tissue samples were obtained from the heart, lung, liver, kidneys, spleen, small intestine, large intestine, stomach and tumor.
In both studies tissue samples were washed with water, counted (Perkin Elmer Wizard, 1470 Automatic Gamma Counter), weighed and the percentage-injected dose per gram tissue (%ID/g) was calculated. Tumor-to-background (T/B) ratios were calculated by dividing %ID/g results for the tumor by indicated background organ for each time point. All biodistribution studies were performed with three mice per group.
Evaluation of tumor drug concentrations
Relative concentrations of drug in tumors following treatment with free drug or copolymer-drug conjugates were evaluated in DU145 prostate tumor bearing mice. Mice were injected intravenously (i.v.) via the tail vein with sterile solutions of 30 and 60 mg/kg drug equivalent P1 and P2, and 30 mg/kg AH-GDM hydrochloride. Cohorts of 3 mice per treatment group were sacrificed at 4, 12, and 24h p.i. Tumors were harvested and stored at −80°C until analysis.
Concentrations of AH-GDM (via enzymatic drug release from copolymers or accumulation of free drug) in tumors was determined with HPLC using modification of previously describe procedures21, 34. Tumor samples were thawed and homogenized using a Powergen 125 homogenizer (Fisher Scientific, Pittsburgh, PA) in 1 part (weight to volume) PBS. 5 μl of 200 μg/ml 17-DMAG (internal standard, IS) in acetonitrile was added to a 250 μl sample of tumor homogenate and mixed. Samples were extracted with 1 ml ethyl acetate (EtOAc) by vortexing, centrifuged at 14,000g for 5 min, and resulting organic layer transferred to 12 × 75 mm glass culture tubes. The samples were extracted with an additional 1 ml EtOAc, vortexed, centrifuged, organic layers combined with the first, and dried under nitrogen. Resulting residue was dissolved in 250 μl of mobile phase and filtered with 0.2 μm polypropylene syringe filter units (Whatman, Florham Park, NJ). The resulting filtrate was placed in Waters Total Recovery glass vials (Waters Corporation, Milford, MA) and 150 μl was injected into the HPLC system. Mobile phase consisted of 0.05 M ammonium acetate buffer pH=4.7 and acetonitrile (70:30, v/v) at a flow rate of 1 ml/min15. HPLC analyses were performed with a Waters 717 autosampler with Waters 2487 dual wavelength detector set at 350 nm using a Waters XBridge column (C18, 4.6 × 150mm, 5 μm). The analytical column was protected with a Waters Xbridge guard column (C18, 4.6 × 20mm, 5 μm).
A calibration curve was generated for AH-GDM at concentrations of 0.15, 0.25, 0.5, 1.5, 5, 15, 25 and 50 μg/ml in control tissue homogenate and processed as described above. The curve was constructed by plotting the peak area ratio of AH-GDM to the IS and was linear from 0.15 to 50 μg/ml. Recovery of 17-DMAG internal standard was 91 ± 2.5%. AH-GDM in tissue samples was quantitated using the ratio of found AH-GDM peak area to IS peak area. Drug release was not detected from copolymer standard solutions under these extraction conditions (data not shown).
Statistical Analysis
Differences in organ accumulation of the copolymer conjugates and tumor drug concentration were analyzed using one-way ANOVA. Where differences were detected, Tukey’s test was used to test for pairwise differences between the groups.
RESULTS
Characteristics of HPMA copolymer conjugates
Geldanamycin derivative AH-GDM and corresponding drug comonomer MA-GFLG-AH-GDM were synthesized and characterized as previously reported21, 27. The characteristics of HPMA copolymers P1 and P2 synthesized from the same polymeric precursor are summarized in Table 1. Copolymer precursor had an estimated molecular weight of 28.4 kDa and polydispersity of 1.7 as determined from size exclusion chromatography (SEC). Drug content in the conjugates was determined to be 0.239 mmol/g corresponding to 15.4% (wt/wt), approximately 6.7 units per backbone, as determined spectrophotometrically using P2 (without RGDfK) and SEC. RGDfK content in P1 as determined by amino acid analysis was 0.361 mmol/g, corresponding to approximately 10 peptide units per polymeric backbone. Tyrosine content in the conjugates was 0.027 mmol/g corresponding to one Tyr unit per backbone. The size exclusion profiles indicated the absence of small molecular weight impurities in the polymer conjugates (not shown). Radiolabeling the conjugates P1 and P2 with 125I was achieved using the Iodogen method resulting in 1.16 μCi/nmol (41 μCi/mg) for P1 and 1.07 μCi/nmol (38 μCi/mg) for P2.
TABLE 1.
Physicochemical characteristics of HPMA copolymer conjugates*
| Sample | P1 | P2 | |
|---|---|---|---|
| Structure | P-(GFLG-AH-GDM)- (GG-RGDfK) |
P-(GFLG-AH-GDM)- (GG-OH) |
|
| Estimated Mw (kDa) a | 28.4c | 28.4 | |
| Mw/Mna,b | 1.7 | 1.7 | |
| AH-GDM content d | mmol/g | 0.239 | 0.239 |
| % wt/wt | 15.4 | 15.4 | |
| RGDfK content e | mmol/g | 0.361 | -- |
| peptides/backbone f | 10.1 | -- |
As determined by size exclusion chromatography.
Polydispersity.
Reported as precursor Mw.
Results of UV spectrophotometric analysis.
Results of amino acid analysis.
Average as estimated from molecular weight and amino acid analysis data.
For structure see Figure 1
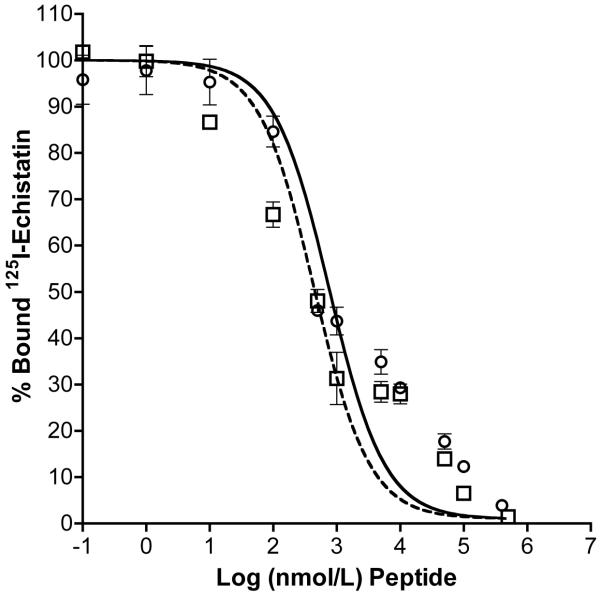
Competitive binding studies with HUVECs showed active binding of RGDfK peptide and copolymer-peptide conjugate (P1) to the αvβ3 integrin (Figure 2). IC50 values (nM peptide) as determined by non-linear regression for binding to HUVECs were 323.8 ± 3.4 and 438.5 ± 3.5 for RGDfK and P1, respectively. At equivalent peptide concentration, free RGDfK showed greater displacement and binding affinity as compared to polymeric conjugates. However it is clear as has been in previous studies21, 33 that targeted conjugates containing RGDfK actively bind to the αvβ3 integrin.
FIGURE 2.
Competitive binding of HPMA copolymer conjugates and free RGDfK peptide. Binding of HPMA copolymer-AH-GDM-RGDfK conjugate P1 was compared to free peptide using HUVEC cells. Open squares and dashed line: RGDfK; open circles and solid line: P1. Results are expressed as means of triplicate ±SD.
Biodistribution of HPMA copolymer-AH-GDM conjugates
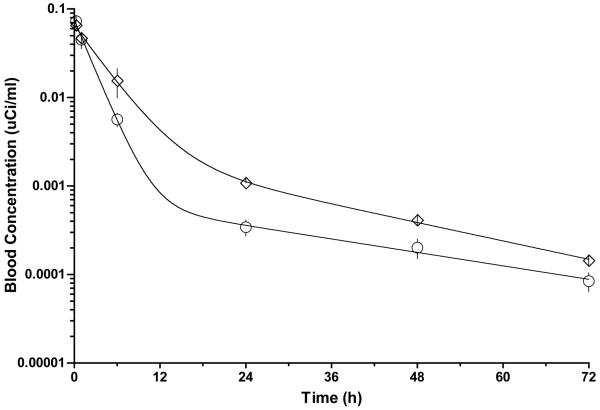
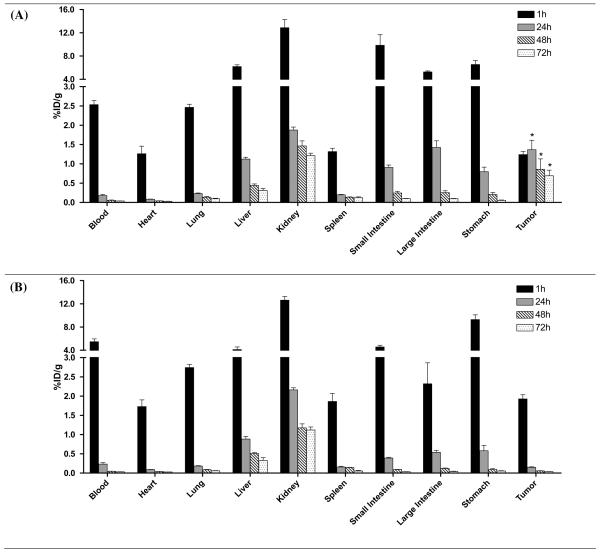
The results of a 3-day biodistribution study of 125I-labeled conjugates containing RGDfK (P1) and non-targeted conjugate (P2) at 60 mg/kg doses in non-tumor bearing mice is shown in Figure 3. Rapid blood clearance was observed for both conjugates with very little activity remaining in blood after 24h. This is also evident in the pharmacokinetic profiles (Figure 4) and pharmacokinetic parameters (Table 2) showing short half-lives of 1.75 and 3.23 hours for P1 and P2, respectively. Accumulation in all other organs appears to decrease with time. Significant differences in accumulation for the targeted conjugate P1 compared with the non-targeted conjugate P2 were analyzed at each time point for a given organ beyond one hour. For both treatments, high accumulation is observed in the kidneys despite a decreasing trend. Significantly higher accumulation is observed in the kidneys for the targeted conjugate containing RGDfK (P1) after one hour and for the remainder of the study. Polymer accumulation in the liver for P1 was significantly higher at later time points but the overall level remained low. Activity in stomach was high out to 6h but rapidly decreased at 24h and beyond with no significant differences between the treatments.
FIGURE 3.
Biodistribution of 125I-labeled HPMA copolymer conjugates P1 (panel A) and P2 (panel B) given at 60 mg/kg drug equivalent dose in non tumor-bearing mice. Activity per organ is expressed as % injected dose per gram of tissue (%ID/g) following necropsy at 15 min, 1, 6, 24, 48 and 72 h post-intravenous injection. Rapid decrease in blood is accompanied by decreasing activity in organs. Significantly higher prolonged activity of conjugate P1 occurs in kidney compared to the non-targeted polymer P2 (B) and is indicated on the graph as * p<0.001. Data is expressed as mean ±SD.
FIGURE 4.
Pharmacokinetic profiles for HPMA copolymer conjugates in blood. Radioactivity measurements in blood expressed as μCi/ml for 125I-labeled conjugates were analyzed by 2-compartment modeling. Circles: HPMA copolymer-AH-GDM-RGDfK conjugate (P1); Diamonds: HPMA copolymer-AH-GDM conjugate (P2). Data is expressed as mean ±SD.
TABLE 2.
Pharmacokinetic parameters of HPMA copolymer conjugates in blood
| Sample | P1 | P2 |
|---|---|---|
| AUC (μCi/ml h) | 0.190 ± 0.01 | 0.304 ± 0.02 |
| T1/2 (h) | 1.748 ± 0.12 | 3.229 ± 0.25 |
| CL (ml/h) | 5.259 ± 0.33 | 3.286 ± 0.18 |
| Vd (ml) | 13.26 ± 1.29 | 15.31 ± 1.15 |
Individually for P1 or P2, significant differences in organ accumulation exist at each time point. Most notably kidney accumulation is significantly higher for both copolymer treatments at each time point compared with all other organs (p<0.001). Liver accumulation of P1 at 24h and beyond was significantly higher than all other organs with the exception of kidney (p<0.01).
The biodistribution of 125I-radiolabeled copolymers P1 and P2 at a lower dose of total polymer (4 nmol) was evaluated in prostate tumor bearing mice to demonstrate the targeting capability of the targeted conjugate P1 containing RGDfK to the αvβ3 integrin in vivo (Figure 5). As is seen in the biodistribution study with high polymer doses (Figure 3), both conjugates undergo rapid blood clearance. In this study, kidney accumulation is lower for both and the conjugates displayed similar renal elimination profiles in contrast to what was observed for higher doses. Additionally, polymer accumulation in all background organs decreased with time with no significant differences existing in organ accumulation between the two conjugates with the exception of tumor. Tumor accumulation was significantly higher for the targeted conjugate P1 after 1h (p<0.05) and demonstrated prolonged accumulation for the duration of the study. At 24h, tumor accumulation of P1 was significantly higher than blood, heart, lung, spleen and stomach (p<0.05). Additionally, at 72h tumor accumulation was significantly higher than all organs except kidneys (p<0.01). Very little tumor accumulation for the non-targeted conjugate P2 was observed beyond 1h. Kidney accumulation predominated for P2 with significantly higher accumulation than all other organs for the duration of the study after 1h (p<0.001).
FIGURE 5.
Biodistribution of 125I-labeled HPMA copolymer conjugates P1 (panel A) and P2 (panel B) in prostate tumor-bearing mice. Activity per organ is expressed as % injected dose per gram of tissue (%ID/g) following necropsy at 1, 24, 48 and 72 h post-intravenous injection. Rapidly decreasing blood concentrations are observed with no significant accumulation differences in normal organs. Significantly higher localization of the targeted conjugate P1 occurs in the tumor after 1h compared with the non-targeted conjugate P2 as indicated on the graph as *p < 0.05. Data is expressed as mean ±SD.
Table 3 shows the tumor-to-background ratios for conjugates P1 and P2. An increasing trend for P1 is observed for most organs indicating prolonged tumor residence with clearance from background organs. In contrast, T/B ratios for P2 show generally consistent or decreasing numbers for which the majority are below one, indicating low tumor accumulation.
TABLE 3.
Tumor-to-background ratios for HPMA copolymer-AH-GDM conjugates with and without RGDfK
| 1 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| HPMA-AH-GDM-RGDfK (P1) | ||||
|
| ||||
| Blood | 0.49 | 7.66 | 15.43 | 18.04 |
| Heart | 0.98 | 16.50 | 21.94 | 23.04 |
| Lung | 0.50 | 5.97 | 6.32 | 7.25 |
| Liver | 0.20 | 1.21 | 1.95 | 2.24 |
| Kidney | 0.10 | 0.73 | 0.59 | 0.57 |
| Spleen | 0.94 | 6.94 | 6.26 | 5.61 |
| Small Intestine | 0.13 | 1.51 | 3.51 | 6.97 |
| Large Intestine | 0.24 | 0.96 | 3.41 | 7.25 |
| Stomach | 0.19 | 1.72 | 4.13 | 12.53 |
|
| ||||
| HPMA-AH-GDM (P2) | ||||
|
| ||||
| Blood | 0.35 | 0.64 | 1.21 | 1.18 |
| Heart | 1.12 | 1.66 | 1.43 | 1.29 |
| Lung | 0.66 | 0.82 | 0.65 | 0.65 |
| Liver | 0.47 | 0.17 | 0.11 | 0.12 |
| Kidney | 0.15 | 0.07 | 0.05 | 0.03 |
| Spleen | 1.04 | 0.94 | 0.40 | 0.71 |
| Small Intestine | 0.42 | 0.38 | 0.62 | 1.13 |
| Large Intestine | 0.83 | 0.28 | 0.47 | 0.89 |
| Stomach | 0.21 | 0.26 | 0.62 | 0.71 |
Tumor concentrations of free drug
To study the relative concentrations of drug delivered to the tumor environment through targeted and non-targeted polymers as well as free drug, DU145 prostate tumor mice were injected with 30 and 60 mg/kg drug equivalent doses of targeted conjugate P1 and non-targeted conjugate P2. These doses were compared with the maximum tolerated dose of 30 mg/kg AH-GDM as previously determined21. The mice tolerated all doses administered as previously observed21. However, doses of 30 mg/kg AH-GDM and 60 mg/kg of drug equivalent copolymers caused a decrease in general movement and activity of the mice for approximately 5 minutes following injection.
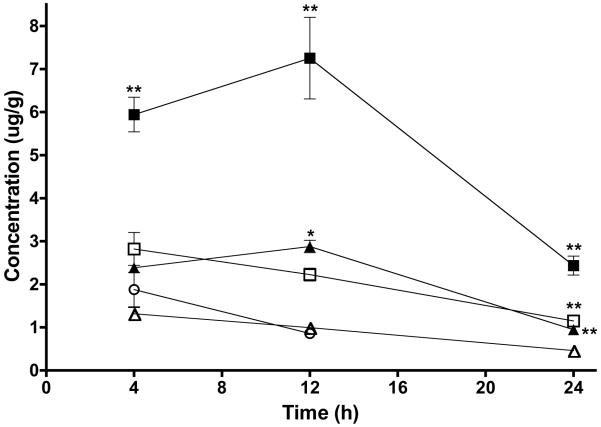
Drug concentrations in prostate tumors at 4, 12 and 24h p.i. are displayed in Figure 6. Free AH-GDM following in vivo polymer release was detected in tumors for all polymer treatments at all three time points sampled out to 24h. AH-GDM in tumor following administration of AH-GDM hydrochloride at 30 mg/kg was detected at 4 and 12h with no detectable concentrations in the tumor at 24h. Significant differences existed in tumor drug levels for each time point. Most notably, targeted conjugate P1 at 60 mg/kg resulted in significantly higher tumor drug concentrations compared to all other treatments at all time points (p<0.001). Following 12h p.i., P1 30 mg/kg dose had significantly higher tumor drug levels than compared with 30 mg/kg drug equivalent doses of non-targeted conjugate P2 and AH-GDM (p<0.05). Also observed in Figure 6 are increasing drug concentrations for the targeted conjugate (P1) at both dose levels between 4 and 12h. This effect is not seen for the non-targeted conjugates and can be attributed to the prolonged accumulation of the targeted copolymers as shown in the biodistribution analysis (Figure 5).
FIGURE 6.
Tumor accumulation of free AH-GDM at 4, 12 and 24 h post intravenous administration of P1 and P2 copolymer conjugates at 30 and 60 mg/kg drug equivalent and 30 mg/kg AH-GDM hydrochloride. Data shows increasing tumor concentrations of drug for copolymer P1 treatments compared with non-targeted copolymer P2. Tumor concentrations following treatment with AH-GDM 30 mg/kg fall rapidly and are not detectable at 24h. Closed triangles: P1 30 mg/kg; closed squares P1 60 mg/kg; open triangles: P2 30 mg/kg; open squares: P2 60 mg/kg; open circle: AH-GDM 30 mg/kg. Data expressed as mean ±SD. Significant differences for polymer treatment groups compared with drug alone at each time point indicated as: *p<0.05, ** p<0.001.
Following 12h p.i., tumor drug levels appear to reach a maximum for the targeted conjugates. At this time point, the amount of drug in tumor for P1 at 60 mg/kg dose was measured at 7.25 ± 1.64 μg AH-GDM/g tumor tissue. This is approximately 3 times higher than P1 at 30 mg/kg and 7.3 and 3.3 times higher than P2 at 30 and 60 mg/kg drug equivalent doses, respectively. Most importantly, it is 8.4 times higher than drug concentrations for 30 mg/kg AH-GDM. Finally, direct comparison of 30 mg/kg doses at 12h shows that P1 resulted in 2.8 times higher tumor drug concentrations than AH-GDM.
DISCUSSION
In this study the in vivo biodistribution and tumor drug delivery of a previously described HPMA copolymer containing the geldanamycin (GDM) derivative aminohexyl-GDM (AH-GDM) and the αvβ3 integrin targeting peptide RGDfK were described21. The goal is to improve geldanamycin chemotherapy by reducing dose-limiting toxicities and improving tumor response through higher localized concentrations of drug15, 17. Our laboratory has previously characterized HPMA copolymers bearing RGD peptides as having enhanced accumulation in solid tumors compared to non-targeted conjugates and peptide alone4-6. The αvβ3 integrin is expressed on the surface of endothelial cells of angiogenic blood vessels and is absent on the luminal surface of cells in established vessels8. Such angiogenesis-targeted copolymers are directed to the αvβ3 integrin expressed on angiogenic blood vessels through conjugation of cyclic RGD peptides (namely RGDfK) to the copolymer backbone that have increased affinity and stability as compared to linear RGD sequences35, 36. Expression of the αvβ3 integrin on tumor cells can also contribute to the enhanced tumor accumulation of these copolymers in vivo9, 21.
The enhanced tumor accumulation of these HPMA copolymer-RGD conjugates were exploited for the delivery of chemotherapeutic drugs and radionuclides7, 21. The anti-tumoral effect of targeted conjugates containing a therapeutic radionuclide was previously demonstrated in mice7. HPMA copolymer-RGDfK conjugates bearing the chemotherapeutic agent AH-GDM were also evaluated and showed higher toxicity towards DU145 prostate cancer cells in vitro and increased tolerability of greater than 2-fold in non tumor bearing mice compared with free AH-GDM21.
In this study, HPMA copolymers were synthesized containing the geldanamycin derivative AH-GDM with and without the targeting moiety RGDfK. The geldanamycin derivative AH-GDM was necessary for covalent attachment to comonomers and subsequent copolymerization, and has shown favorable stability and in vitro activity compared with other amino-alkyl derivatives27. The estimated molecular weight was also similar and below the renal threshold for HPMA (approximately 45 kDa37), allowing for the elimination of the copolymer conjugates predominantly by the kidneys. Active binding of the copolymer conjugate P1 containing RGDfK was demonstrated in vitro with HUVECs. Binding affinities of RGDfK and P1 are similar to those previously reported21, 33. It was also shown that the presence of AH-GDM on the copolymer backbone does not influence binding because copolymers not containing RGDfK showed no radioligand displacement from the αvβ3 integrin on HUVECs21.
The biodistribution of copolymers P1 and P2 was evaluated using therapeutically relevant doses of polymer (60 mg/kg drug equivalent) in non-tumor bearing mice to assess normal tissue distribution and evaluate the clearance of the conjugates from the blood and sensitive organs. Sequestration of the polymer was not observed in any organs with all showing clearance of the conjugate with time (Figure 3). Evaluating the distribution to and clearance from normal organs is essential to establishing the utility of the conjugates for future therapeutic applications. Renal accumulation of the RGDfK-bearing conjugate is higher than the conjugate without peptide. It has been demonstrated that RGD peptides show high kidney accumulation when administered as mono- and multimeric units alone or as part of a macromolecule38-41. Our previous studies demonstrated that compared to peptide alone renal uptake of HPMA copolymer-RGD conjugates was decreased through the conjugation of the peptide to the copolymer4, 5. In the current study we demonstrated higher kidney uptake for the 60 mg/kg dose of RGDfK-bearing conjugate P1. This differential renal uptake was not observed when copolymer conjugates were administered at the lower dose (4 nmol, Figure 5). It is possible that RGDfK has mediated increased renal activity of the P1 conjugate only at higher doses. This phenomenon needs further investigation.
Slightly higher concentrations for P2 in blood at 6 hours and beyond translate into a blood half-life that is approximately 1.5 hours longer than P1 (Table 2). Accordingly, clearance is higher for P1. Aside from these differences, both conjugates have a rapid half-life and high clearance when compared with other HPMA copolymers42. Greater exposure of P2 in blood as reflected by the AUC values could presumably result in higher tumor concentrations of polymer or drug, however as shown in Figures 5 and 6 this is not the case.
The biodistribution of the copolymers were carried out in DU145 prostate tumor bearing mice. This cell line has demonstrated higher αvβ3 expression in vitro21 with effective accumulation of HPMA copolymer-RGD conjugates in vivo4, 6. The DU145 mouse tumor model is also potentially useful for evaluating the efficacy of these conjugates because the cell line demonstrated greater susceptibility to HPMA copolymer-AH-GDM-RGDfK conjugates in vitro compared with AH-GDM alone21. The targeted conjugate P1 demonstrated effective tumor targeting when compared with the non-targeted conjugate P2 at time points beyond 1h (Figure 5). The data show that the dose of polymer received by the tumor at 1h was retained with modest increase between 1 and 24h. However, the overall dose received by the tumor in the current study is lower than what our laboratory has previously demonstrated5. A study of HPMA copolymer-RGDfK biodistribution showed that accumulation in tumor reached a maximum of approximately 5% ID/g at 48h with sustained activity of approximately 4% ID/g after 192 hours. The dose of the polymer in the tumor at 1h (approximately 1% ID/g) increased over 4 times after 48h. The polymeric architecture of these conjugates was substantially different whereby they contained no drug comonomers and higher estimated molecular weight. Increased tumor accumulation of these conjugates may be attributed to extended polymer activity in the blood pool still above 1% ID/g at 48h, allowing for further trafficking to the tumor and accumulation mediated by RGDfK and passively through the enhanced permeability and retention (EPR) effect43. One possible strategy to improve the overall extent of tumor localization of copolymers and maximize the tumor concentration of drug is to increase the molecular weight of the conjugates to allow for longer blood circulation time and improved EPR effect44. However, synthesizing these conjugates with higher molecular weight closer to the renal threshold is challenging because of the high percentage of the chain transfer agent MA-GG-ONp in the comonomer feed limiting polymer chain extension. The high feed ratio of this comonomer is intended to allow for sufficient conjugation sites and increased content of RGDfK peptide per polymer backbone. However, it may be necessary to sacrifice some peptide on the backbone to synthesize larger copolymers that do not undergo such rapid clearance.
To measure the tumor concentrations of AH-GDM achievable through copolymer localization of these conjugates, injections of 30 and 60 mg/kg drug equivalent polymer were administered and compared with 30 mg/kg free AH-GDM in DU145 prostate tumor-bearing mice. Free AH-GDM hydrochloride was administered at the maximum tolerated dose (MTD) as previously determined21. Doses of the copolymers were chosen for comparison with the MTD for free drug and double the MTD of the free drug (60 mg/kg drug equivalent). The high drug content in the copolymers aided formulation of the doses in saline preventing the solutions from becoming too viscous at high polymer concentration (approximately 40 mg/ml).
Higher tolerated doses of drug-equivalent targeted copolymer P1 successfully increased tumor drug concentrations compared with doses of free drug. The 60 mg/kg dose of targeted copolymer-drug conjugate P1 resulted in over eight times higher drug concentrations in the tumor at 12h. The goal of polymer therapeutics is to increase the therapeutic index of a given drug through the ability to administer higher tolerated doses1. In the context of effective therapy this comparison is valid because we are assessing two equally tolerated doses of drug or drug-equivalent polymer. However, to make a direct comparison 30 mg/kg drug equivalent P1 still resulted in nearly 3 times higher tumor drug concentrations than free AH-GDM at the same dose at 12h. Doses of P1 at 30 and 60 mg/kg also had 2.4 and 3.3 times higher tumor drug concentrations compared to P2 at respective doses. Both P1 targeted conjugate doses increased drug concentration between 4 and 12h compared to decreasing drug concentrations at each time point for P2 conjugates and AH-GDM alone. This is most likely a result of the sustained accumulation of P1 in the tumor and the time required for tumor localization, cellular uptake and intracellular drug release. HPMA copolymers of higher molecular weight containing 5-flurouracil (5-FU) have also shown the ability for tumor drug delivery45. These copolymers showed higher amounts of drug in tumor at earlier time points and concentrations below 1 μg/g at 24h. This is unlike what we see for our targeted conjugates where the highest tumor drug concentrations occur after 12h. Similarly observed between the previously mentioned and current study is the rapid decrease of tumor drug concentrations following free drug administration (Figure 6). Drug accumulation in tumors can be increased with larger molecular weight polymers especially at earlier time points.
The increase in tumor concentrations of free AH-GDM through polymer delivery is further impacted by considerations of polymer behavior and analytical detection. AH-GDM was attached to the copolymer backbone via the enzymatically degradable peptide spacer GFLG and conjugates were previously shown to release the drug in vitro21. Some studies evaluating tumor drug concentrations following HPMA copolymer-drug treatments utilizing the same drug release mechanism have employed analytical hydrolysis techniques to measure the total drug (free plus polymer-bound) in tumors45-47. The method employed in the current study did not utilize such hydrolysis techniques for concern over AH-GDM degradation. Therefore, the analytical method used to detect drug in the tumor was only capable of measuring passively absorbed drug from AH-GDM administration or the free drug released from copolymers following cellular internalization and subsequent drug release. As a result the reported tumor drug concentrations in polymer treatments are probably an under estimation of total drug in tumor that would also include the fraction still bound to the copolymer backbone. Emerging evidence indicates that release from backbone is not always necessary for efficacy48 and drug not measured in the tumor through this assay may be present potentially increasing the efficacy of the conjugates. However, if in fact AH-GDM requires polymer release to exert a therapeutic effect then results of the current study comparing only free drug concentrations are more appropriate. In either case, it appears that the increase in localized dose of drug delivered by the conjugates can effectively address dose-limiting cytotoxicity issues that exist for current 17-AAG and 17-DMAG therapies. Additionally, when exploring the tumor drug concentrations achieved for the targeted conjugate P1 versus the non-targeted conjugate P2, the increase resulting from targeted conjugates is further emphasized because a previous drug release study demonstrated that the targeted conjugate containing RGDfK had overall lower drug release capacity21.
Administration of two increasing polymer doses was also designed to provide initial information on the possibility of reaching maximum drug concentrations in the tumor, effectively saturating drug delivery processes. Results following the single i.v. injections possibly indicate that tumor drug saturation was not achieved and the potential exists for higher tumor drug concentrations by increasing the polymer dose. Up to 80 mg/kg drug equivalent polymer formulation suitable for i.v. injection in mice was achieved and tolerated in vivo21. An alternative to increasing the dose of a single injection would be to administer multiple 60 mg/kg drug equivalent doses. The current study shows that repeated administration of the conjugates every 24h may be warranted to further increase the tumor drug concentration. However, consideration of multiple doses must also include how this may affect potential toxic accumulation in sensitive normal organs, especially the liver and kidney.
The current study demonstrates the utility of targeted HPMA copolymer-RGDfK conjugates for the delivery of geldanamycin and other chemotherapeutic agents. Important attention must be paid to select drug candidates that are highly potent upon release from the copolymer backbone. Testing the polymeric nanomedicines in sensitive tumor models expressing the αvβ3 integrin on angiogenic blood vessels and/or tumor cells is necessary to demonstrate their full potential. It is easily conceivable that under the correct therapeutic conditions the ability to simultaneously increase tolerated drug doses as well as localized delivery of an agent is certain to bolster the chemotherapeutic armamentarium currently used for cancer therapy.
CONCLUSIONS
Targeted HPMA copolymer-AH-GDM-RGDfK conjugates showed higher accumulation in tumor than non-targeted conjugates. Administration of the conjugates resulted in increased tumor concentrations of free drug. Together, these copolymers have demonstrated an increased tolerability and a higher amount of drug delivered to the tumor. These properties can be used to surmount the dose limiting cytotoxicity issues present for current geldanamycin therapy while at the same time improving efficacy through increased localized concentrations of the drug.
ACKNOWLEDGEMENTS
The authors would like to thank the Translational Core Laboratory at the University of Maryland Greenebaum Cancer Center including Dr. Joseph Bryant, Dr. Mariola Sadowska, Dr. Eugene Ateh and Lanea George for assistance with drug accumulation studies. The authors would also like to thank Dr. Natalie Eddington and Dr. Hazem Hassan for their assistance with pharmacokinetic analysis. This research was supported by the National Institutes of Health grant R01 EB007171. MB was supported in part by the American Foundation for Pharmaceutical Education.
ABBREVIATIONS
- 17-AAG
17-allylamino-17-demethoxygeldanamycin
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- AH
6-aminohexylamino
- AH-GDM
17-(6-aminohexylamino)-17-demethoxygeldanamycin
- EtOAc
ethyl acetate
- GDM
geldanamycin (GDM)
- HPLC
high performance liquid chromatography
- HPMA
N-(2-hydroxypropyl) methacrylamide
- MA-GFLG-AH-GDM
N-methacryloylglycylphenylalanylleucylglycl-17-(6-aminohexylamino)-17-demethoxygeldanamycin
- RGDfK
cyclo-(arginine- glycine- aspartic acid- D-phenylalanine- lysine)
- SEC
size exclusion chromatography
REFERENCES
- 1.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008;60:886–98. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 4.Line BR, Mitra A, Nan A, Ghandehari H. Targeting tumor angiogenesis: comparison of peptide and polymer-peptide conjugates. J. Nucl. Med. 2005;46:1552–60. [PubMed] [Google Scholar]
- 5.Mitra A, Coleman T, Borgman M, Nan A, Ghandehari H, Line BR. Polymeric conjugates of mono- and bi-cyclic alphaVbeta3 binding peptides for tumor targeting. J. Controlled Release. 2006;114:175–83. doi: 10.1016/j.jconrel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR. Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J. Controlled Release. 2005;102:191–201. doi: 10.1016/j.jconrel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Mitra A, Nan A, Papadimitriou JC, Ghandehari H, Line BR. Polymer-peptide conjugates for angiogenesis targeted tumor radiotherapy. Nucl. Med. Biol. 2006;33:43–52. doi: 10.1016/j.nucmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alphaVbeta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 9.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat. Rev. Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 10.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42:273–9. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- 12.Lattouf JB, Srinivasan R, Pinto PA, Linehan WM, Neckers L. Mechanisms of disease: the role of heat-shock protein 90 in genitourinary malignancy. Nat. Clin. Pract. Urol. 2006;3:590–601. doi: 10.1038/ncpuro0604. [DOI] [PubMed] [Google Scholar]
- 13.Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, Hurtado-Coll A, Yamanaka K, Gleave M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64:6595–602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin. Oncol. 2003;30:709–16. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 15.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother. Pharmacol. 1995;36:305–15. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 16.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 17.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 2005;23:1078–87. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 18.Nowakowski GS, McCollum AK, Ames MM, Mandrekar SJ, Reid JM, Adjei AA, Toft DO, Safgren SL, Erlichman C. A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin. Cancer. Res. 2006;12:6087–93. doi: 10.1158/1078-0432.CCR-06-1015. [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan RK, Egorin MJ, Eiseman JL, Ramalingam S, Friedland D, Agarwala SS, Ivy SP, Potter DM, Chatta G, Zuhowski EG, Stoller RG, Naret C, Guo J, Belani CP. Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin. Cancer Res. 2007;13:1769–74. doi: 10.1158/1078-0432.CCR-06-2233. [DOI] [PubMed] [Google Scholar]
- 20.Ronnen EA, Kondagunta GV, Ishill N, Sweeney SM, Deluca JK, Schwartz L, Bacik J, Motzer RJ. A phase II trial of 17-(Allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. Invest. New Drugs. 2006;24:543–6. doi: 10.1007/s10637-006-9208-z. [DOI] [PubMed] [Google Scholar]
- 21.Borgman MP, Ray A, Kolhatkar RB, Sausville EA, Burger AM, Ghandehari H. Targetable HPMA copolymer-aminohexylgeldanamycin conjugates for prostate cancer therapy. Pharm. Res. 2009;26:1407–18. doi: 10.1007/s11095-009-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strohalm J, Kopecek J. Poly N-(2-hydroxypropyl) methacrylamide: 4. Heterogenous polymerization. Angew. Makromol. Chem. 1978;70:109–118. [Google Scholar]
- 23.Lee JH, Kopeckova P, Kopecek J, Andrade JD. Surface properties of copolymers of alkyl methacrylates with methoxy (polyethylene oxide) methacrylates and their application as protein-resistant coatings. Biomaterials. 1990;11:455–64. doi: 10.1016/0142-9612(90)90058-x. [DOI] [PubMed] [Google Scholar]
- 24.Rejmanova P, Labsky J, Kopecek J. Aminolyses of monomeric and polymeric p-nitrophenyl esters of methacryloylated amino acids. Makromol. Chem. 1977;178:2159–2168. [Google Scholar]
- 25.Ulbrich K, Subr V, Strohalm J, Plocova D, Jelinkova M, Rihova B. Polymeric drugs based on conjugates of synthetic and natural macromolecules. I. Synthesis and physico-chemical characterisation. J. Controlled Release. 2000;64:63–79. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 26.Kasuya Y, Lu ZR, Kopeckova P, Minko T, Tabibi SE, Kopecek J. Synthesis and characterization of HPMA copolymer-aminopropylgeldanamycin conjugates. J. Controlled Release. 2001;74:203–11. doi: 10.1016/s0168-3659(01)00318-2. [DOI] [PubMed] [Google Scholar]
- 27.Kasuya Y, Lu ZR, Kopecková P, Tabibi SE, Kopecek J. Influence of the structure of drug moieties on the in vitro efficacy of HPMA copolymer-geldanamycin derivative conjugates. Pharm. Res. 2002;19:115–23. doi: 10.1023/a:1014216712820. [DOI] [PubMed] [Google Scholar]
- 28.Kasuya Y, Lu Z, Kopeckova P, Kopecek J. Improved synthesis and evaluation of 17-substituted aminoalkylgeldanamycin derivatives applicable to drug delivery systems. Bioorg. Med. Chem. Lett. 2001;11:2089–91. doi: 10.1016/s0960-894x(01)00374-2. [DOI] [PubMed] [Google Scholar]
- 29.Salacinski PR, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen) Anal. Biochem. 1981;117:136–46. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- 30.Rhim JS, Tsai WP, Chen ZQ, Chen Z, Van Waes C, Burger AM, Lautenberger JA. A human vascular endothelial cell model to study angiogenesis and tumorigenesis. Carcinogenesis. 1998;19:673–81. doi: 10.1093/carcin/19.4.673. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. microPET imaging of glioma integrin alphaVbeta3 expression using (64)Culabeled tetrameric RGD peptide. J. Nucl. Med. 2005;46:1707–18. [PubMed] [Google Scholar]
- 32.Kumar CC, Nie H, Rogers CP, Malkowski M, Maxwell E, Catino JJ, Armstrong L. Biochemical characterization of the binding of echistatin to integrin alphaVbeta3 receptor. J. Pharmacol. Exp. Ther. 1997;283:843–53. [PubMed] [Google Scholar]
- 33.Borgman MP, Coleman T, Kolhatkar RB, Geyser-Stoops S, Line BR, Ghandehari H. Tumor-targeted HPMA copolymer-(RGDfK)-(CHX-A”-DTPA) conjugates show increased kidney accumulation. J. Controlled Release. 2008;132:193–99. doi: 10.1016/j.jconrel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egorin MJ, Lagattuta TF, Hamburger DR, Covey JM, White KD, Musser SM, Eiseman JL. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother. Pharmacol. 2002;49:7–19. doi: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 35.Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, Siahaan TJ. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. 1999;53:530–41. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 36.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 1995;13:265–70. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 37.Seymour LW, Duncan R, Strohalm J, Kopecek J. Effect of molecular weight (Mw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J. Biomed. Mater. Res. 1987;21:1341–58. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 38.Boswell CA, Eck P, Regino CA, Bernardo M, Wong K, Milenic D, Choyke P, Brechbiel M. Synthesis, characterization, and biological evaluation of integrin alphaVbeta3-targeted PAMAM dendrimers. Mol. Pharm. 2008;5:527–39. doi: 10.1021/mp800022a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi M, Sancey L, Briat A, Riou L, Boturyn D, Dumy P, Fagret D, Ghezzi C, Vuillez JP. Chemical and biological evaluations of an (111)In-labeled RGD-peptide targeting integrin alphaVbeta3 in a preclinical tumor model. Cancer Biother. Radiopharm. 2008 doi: 10.1089/cbr.2008.0528. [DOI] [PubMed] [Google Scholar]
- 40.Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, Rajopadhye M, Boonstra H, Corstens FH, Boerman OC. Tumor targeting with radiolabeled alphaVbeta3 integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–51. [PubMed] [Google Scholar]
- 41.Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H, Liu Z, Wang F, Chen X, Liu S. Improving tumor-targeting capability and pharmacokinetics of (99m)Tc-labeled cyclic RGD dimers with PEG(4) linkers. Mol. Pharm. 2008 doi: 10.1021/mp800150r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan H, Sima M, Kopeckova P, Wu K, Gao S, Liu J, Wang D, Miller SC, Kopecek J. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl)methacrylamide copolymer-alendronate conjugates. Mol. Pharm. 2008;5:548–58. doi: 10.1021/mp800003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 44.Seymour LW, Miyamoto Y, Maeda H, Brereton M, Strohalm J, Ulbrich K, Duncan R. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur. J. Cancer. 1995;31A:766–70. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 45.Yuan F, Qin X, Zhou D, Xiang QY, Wang MT, Zhang ZR, Huang Y. In vitro cytotoxicity, in vivo biodistribution and antitumor activity of HPMA copolymer-5-fluorouracil conjugates. Eur. J. Pharm. Biopharm. 2008;70:770–6. doi: 10.1016/j.ejpb.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Fraier D, Frigerio E, Pianezzola E, Strolin Benedetti M, Cassidy J, Vasey P. A sensitive procedure for the quantitation of free and N-(2-hydroxypropyl) methacrylamide polymer-bound doxorubicin (PK1) and some of its metabolites, 13-dihydrodoxorubicin, 13-dihydrodoxorubicinone and doxorubicinone, in human plasma and urine by reversed-phase HPLC with fluorimetric detection. J. Pharm. Biomed. Anal. 1995;13:625–33. doi: 10.1016/0731-7085(95)01301-z. [DOI] [PubMed] [Google Scholar]
- 47.Julyan PJ, Seymour LW, Ferry DR, Daryani S, Boivin CM, Doran J, David M, Anderson D, Christodoulou C, Young AM, Hesslewood S, Kerr DJ. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J. Controlled Release. 1999;57:281–90. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 48.Rihova B, Strohalm J, Hovorka O, Subr V, Etrych T, Chytil P, Pola R, Plocova D, Boucek J, Ulbrich K. Doxorubicin release is not a prerequisite for the in vitro cytotoxicity of HPMA-based pharmaceuticals: in vitro effect of extra drug-free GlyPheLeuGly sequences. J. Controlled Release. 2008;127:110–20. doi: 10.1016/j.jconrel.2008.01.003. [DOI] [PubMed] [Google Scholar]