Abstract
Visceral fat (VF) increases with the menopause and is an independent predictor of the metabolic syndrome, diabetes, and cardiovascular disease (CVD) in women. Little is known about how hormonal changes during the menopausal transition are related to the increase in VF. We aimed to determine the relationship between bioavailable testosterone and VF in middle-aged women at various stages of the menopausal transition and whether this relationship is independent of age and other CVD risk factors. The Study of Women’s Health Across the Nation (SWAN) is a longitudinal, community-based study. This report uses baseline data from a population-based longitudinal ancillary study at the Chicago site to examine the cross-sectional relationship between testosterone and computed tomography (CT)–assessed VF in women at different stages of the menopausal transition. Included are 359 women (47.2% black), aged 42–60 years, who were randomly selected from a complete community census in which a 72% participation rate was achieved. In multivariate models, bioavailable testosterone was associated with VF independent of age, race, percent total body fat, and other cardiovascular risk factors. Bioavailable testosterone was a stronger predictor than estradiol and was interchangeable in its strength of association with sex hormone–binding globulin (SHBG). As bioavailable testosterone was associated with VF even after adjusting for insulin resistance, this suggests that it plays an important role in regional fat distribution. Our findings may have direct implications in explaining the effect of menopause-related testosterone predominance on VF accumulation and subsequent cardiovascular risk.
INTRODUCTION
Cardiovascular risk increases in women after menopause (1–3). Among the pathways for this higher risk is an increase in visceral fat (VF). VF is an independent predictor of the metabolic syndrome, especially in normal weight women (4), diabetes, and cardiovascular disease (CVD) (5–9). VF has also been associated with the menopausal transition in cross-sectional (10,11) and longitudinal studies (12–14). This effect of menopause appears to be independent of age and total body fat (14,15). The role of VF in the menopause-related increase in CVD risk is biologically plausible. VF is metabolically and functionally different from subcutaneous fat tissue (16), and is a preferential source of inflammatory factors which contribute to premature atherosclerosis and risk of acute coronary syndrome (17,18).
We have shown previously that bioavailable testosterone is related to the development of the metabolic syndrome (19) in a 12-year longitudinal study of a multiethnic cohort undergoing the menopausal transition. When we examined components of the metabolic syndrome separately, we observed that bioavailable testosterone, but not estradiol, was strongly related to waist circumference, a marker of VF. Furthermore, in multivariate models, the relationship of sex hormone–binding globulin (SHBG) to waist circumference was similar to that of bioavailable testosterone. The role of testosterone in the development of visceral adiposity was supported by an investigation of healthy middle-aged Australian white women which found that baseline bioavailable testosterone, and change in bioavailable testosterone, predicted accumulation of VF 5 years later (20). Estradiol or its change were not significantly related to VF (20). The impact of bioavailable testosterone on VF was further supported by a clinical trial, where administration of a weak androgen (nandrolone decanoate) resulted in an increase of VF in obese white women (21).
The Study of Women’s Health Across the Nation (SWAN) is a seven-site longitudinal, multicultural investigation of the menopausal transition. The Chicago site of the SWAN study features a population-based ancillary study examining change in computed tomography (CT)–assessed VF as women traverse the menopause. This provides an opportunity to study further the relationship between testosterone and VF. We hypothesized that both VF and bioavailable testosterone are higher in postmenopausal women than in pre- or perimenopausal women. We further hypothesized that the association of bioavailable testosterone is interchangeable with that of SHBG, an indirect marker of the balance between testosterone and estradiol (22), and that both of these relationships are stronger than the relationship between estradiol and VF.
METHODS AND PROCEDURES
Participants
Participants were women who enrolled in an ancillary study of the SWAN at the Chicago site. This study (the “SWAN Fat Patterning Study”) was designed to investigate the impact of the menopausal transition on the accumulation of VF. SWAN is a seven-site multiethnic longitudinal study of women transitioning through menopause, featuring ongoing annual interviews. Women were eligible for SWAN if they were between the ages of 42 and 52, not pregnant or breastfeeding, had an intact uterus and at least one ovary, had menstruated within 3 months, and were not using oral contraceptives or hormone therapy. By design, the Chicago site recruited only non-Hispanic white and black women. A unique feature of the Chicago SWAN site is that it is a population-based design, which drew on a complete community census to recruit a sample of black and white women with a 72% participation rate. These women were recruited in a way that featured comparability on socioeconomic status within the black and white women, thus minimizing any confound between ethnicity and socioeconomic status. Details of SWAN recruitment and the study protocol have been reported elsewhere (23).
Women enrolled in the Chicago SWAN Fat Patterning Study between August 2002 and December 2005 coincident with their annual SWAN follow-up visit. They were eligible if they did not have a history of diabetes, chronic liver disease, renal disease, anorexia nervosa, alcohol or drug abuse, were not currently pregnant or planning to become pregnant, and had not undergone surgical menopause (hysterectomy and/or bilateral oophorectomy). Because of equipment limitations, women with breast implants, hip replacements, or weight exceeding 299 pounds could not participate.
Of the 386 eligible Chicago SWAN participants, 77% enrolled in the Fat Patterning Study. Because many SWAN participants were postmenopausal by the time the Fat Patterning Study began, we recruited additional pre- and perimenopausal women (65% of those eligible from the census) who were screened as part of the original SWAN recruitment effort but were too young to participate in 1996. These women were younger than the previously recruited women were, but did not differ in BMI, age-adjusted total fat or VF, education, or depressive symptoms. The final cohort consisted of 435 women (200 black and 235 white). Due to missing data on VF (due to equipment malfunction) (n = 3), new surgical menopause (n = 23), new hormone use (n = 45), or missing covariates (n = 5), 359 women (166 black, 193 white) were included in the current analyses.
Procedures
At entry into the parent SWAN study and at each annual assessment, all SWAN participants completed a standard protocol; full details are provided elsewhere (23). Covariates of interest for the present analyses were measured as part of the annual SWAN assessment coincident with recruitment to the SWAN Fat Patterning Study for the 297 SWAN participants. The 138 women recruited uniquely to the Fat Patterning Study completed the same protocol as the SWAN participants. For all women, data presented here represent the baseline visit for the SWAN Fat Patterning Study.
All aspects of the study were approved by the Rush University Medical Center’s institutional review board, and all women provided written, informed consent for their participation.
Assessment of VF
VF in the abdominal cavity was assessed by CT at High Tech Medical Park located within 9 miles of the Chicago SWAN study site. All abdominal CT scans were conducted by a trained technician using a General Electric Lightspeed VCT scanner (General Electric Medical Systems, Milwaukee, WI), with the participant in the supine position and arms folded across her chest. Following a scout view, a single 10-mm thick image of the abdomen at the L4-L5 vertebral space was obtained. Images were stored on optical disks and transferred to the reading center at the University of Colorado Health Sciences Center for analysis. Scans were read using a software developed by the reading center (RSI, Boulder, CO) and used in large cohort studies (24,25). Scans were read by a trained radiologist, blind to the participants’ clinical or demographic characteristics. The radiologist defined total abdominal fat area using a cursor to delineate the area within the muscle wall surrounding the abdominal cavity (26). VF was defined as all adipose tissue within this area with an attenuation range between −190 and −30 Hounsfeld units (26).
Assessment of total body fat
Total body fat mass was assessed with whole body dual-energy X-ray absorptiometry scans using a General Electric Lunar Prodigy scanner (GE-Lunar, Madison, WI). Dual-energy X-ray absorptiometry scans use two X-ray energy sources, which enable separation of body mass into fat mass, lean tissue mass, and bone mineral content. Dual-energy X-ray absorptiometry scans, completed the same day as the CT scans at High Tech Medical Park, were performed with a participant in the supine position, arms by her side, wearing only a hospital gown. Scans were analyzed using GE-Lunar enCORE software (GE-Lunar). For data analyses, total body fat was quantified as the percent of fat in the total body habitus to represent the amount of fat for a given body size; total percent fat was calculated as total fat mass divided by total mass (total fat mass, total lean mass, and bone mineral content). Due to equipment malfunction, five women were unable to complete a dual-energy X-ray absorptiometry scan as part of the baseline study visit. Because BMI and total fat are very highly correlated (r = 0.83) in our sample, a regression equation was estimated to predict total body fat from BMI, and this value was then used in analyses.
Assessment of covariates
Menopausal status
Bleeding criteria were used to characterize menopausal status as premenopausal (normal cycling), early perimenopausal (irregular cycles but bleeding within the past 3 months), late perimenopausal (irregular cycles with bleeding in the past 11 months but not within the last 3 months), and postmenopausal (no menses for at least 12 months). This self-reported definition was validated against follicle-stimulating hormone levels in that postmenopausal women who had higher follicle-stimulating hormone levels than peri- and premenopausal women (geometric mean = 86.3, 29.7, and 14.7, respectively; all pairwise comparisons P < 0.001).
Age was calculated as the difference between exam date and self-reported date of birth. Race was self-reported as black or white. The highest educational degree was self-reported at the screening visit: high school or less, some college, college degree, or graduate school.
All participants underwent exams, which included interviews, anthropometry, questionnaires, and a blood draw for the assessment of sociodemographic factors, cardiovascular risk factors, and reproductive hormones. Due to budgetary constraints, the blood assays, including high-density lipoprotein, were analyzed every other year only.
The Framingham risk score (FRS) was calculated using data on age, smoking status, total serum cholesterol and high-density lipoprotein cholesterol, resting blood pressure, and use of antihypertensive medication (27). Total cholesterol and high-density lipoprotein cholesterol were analyzed on EDTA-treated plasma using standard methods, previously described (28,29). Resting blood pressure was measured with a mercury sphygmomanometer, using a standard protocol following at least a 5-min rest with participants seated. Blood pressure was measured in the right arm, using an appropriately sized cuff. Two sequential blood pressure readings were obtained, 2 min apart. Use of antihypertensive medication was self-reported annually in SWAN and confirmed via medication review. For the calculation of the FRS, lipids were taken from the previous year, if necessary.
Depressive symptoms were assessed in SWAN with the 20-item Center for Epidemiological Studies Depression Scale (CES-D) (30), a validated scale with good test–retest reliability in ethnically diverse samples (31), and used extensively in epidemiological studies (32). A score of ≥16 on the CES-D is indicative of clinically significant symptomatology (32); therefore, we modeled the CES-D as a dichotomous predictor, CES-D score ≥16 vs. <16 as the referent category.
Hormones
Phlebotomy was performed in the morning following an overnight fast. Subjects were scheduled for venipuncture on days 2–5 of a spontaneous menstrual cycle (in cycling women) within 60 days of the anniversary of the baseline examination date. All assays were performed on the ACS-180 automated analyzer (Bayer Diagnostics, Tarrytown, NY) using a double-antibody chemiluminescent immunoassay with a solid phase anti-immunoglobulin-G conjugated to paramagnetic particles, anti-ligand antibody, and competitive ligand labeled with dimethylacridinium ester. The estradiol (E2) assay modifies the rabbit anti-E2–6 ACS-180 immunoassay to increase sensitivity, with a lower limit of detection of 1.0 pg/ml. Serum testosterone (T) concentrations were determined by competitive binding of a dimethylacridinium ester–labeled T derivative to a rabbit polyclonal anti-T antibody premixed with monoclonal anti-rabbit immunoglobulin-G antibody immobilized on the solid phase paramagnetic particles. Inter- and intra-assay coefficients of variation were 10.5% and 8.5%, respectively, with a limit of detection of 2.19 ng/dl. The two-site chemiluminescent assay for serum SHBG concentrations involved competitive binding of dimethylacridinium ester–labeled SHBG to a commercially available rabbit anti-SHBG antibody and a solid phase of goat anti-rabbit immunoglobulin-G conjugated to paramagnetic particles. Inter- and intra-assay coefficients of variation for SHBG were 9.9% and 6.1%, respectively, with a limit of detection of 1.95 nmol/l. Serum E2 concentrations were measured with a modified, off-line ACS-180 (E2–6) immunoassay. Inter- and intra-assay coefficients of variation averaged 10.6% and 6.4%, respectively, over the assay range, and the lower limit of detection was 1 pg/ml. Duplicate E2 assays were conducted with results reported as the arithmetic mean for each subject, with a coefficient of variation of 3–12%. All other assays were single determinations. Bioavailable testosterone was calculated as T (ng/dl) × 100/(28.84 × SHBG (nmol/l)).
Physical activity
Physical activity was measured at the SWAN baseline visit and follow-ups 3, 5, 6, and 9 via self-report with an adapted version of the Kaiser Physical Activity Survey, which was initially adapted from the Baecke physical activity questionnaire (33), assessing frequency of sports, nonsports leisure time, and household/child-care activities. An activity score was created by summing across domains, with a higher score indicating greater activity. For the current analyses, data from the Kaiser Physical Activity Survey was administered at the concurrent SWAN visit except for ~20% of the sample for whom Kaiser Physical Activity Survey values were obtained from their most recently available prior visit.
Insulin resistance (homeostasis model assessment for insulin resistance) was calculated from glucose and insulin using the homeostasis model (34).
We present additional variables for descriptive purposes. Smoking was assessed in SWAN by questions on ever smoking, amount smoked and quit date. BMI was calculated as weight in kilograms divided by height in meters squared. Standardized protocols were used to measure weight, height and waist circumference. Height was measured without shoes using a stadiometer. Weight was measured without shoes and in light indoor clothing using scales that were calibrated on a monthly basis to a standard. Waist circumference was measured with the respondent in nonrestrictive undergarments.
Data analyses
All analyses were conducted using PC-SAS, version 9.1 (SAS Institute, Cary, NC). We used descriptive statistics to characterize participants on fat measurements, reproductive hormones, and all other covariates included in our analytic models. For reproductive hormones, SHBG, and insulin resistance, we report median and interquartile range, because the distributions are skewed. To assess the bivariate relation of hormones to VF, we present Spearman’s rank correlations without and with adjustment for percent body fat. We used linear models to examine independent correlates of VF. First, we developed a base model by including all covariates significantly correlated with either VF or bioavailable testosterone, using a liberal P value of 0.2. We did not include insulin resistance in this model, because it is not in the causal pathway from testosterone to VF. Initially, we included smoking in the models, but it did not survive in the base or in any subsequent model and was thus excluded in the final presentation. Inspection of residual plots revealed that a transformation of VF was necessary, and we chose to use the logarithm (to base 10). Therefore, we present geometric means in the subgroup comparisons. Our final base model included percent body fat, age, race, FRS, dichotomous depression, and physical activity. To test our hypothesis that postmenopausal women have more VF, we added an indicator for postmenopausal status to this base model. To test hypotheses regarding the association of testosterone and estradiol with VF, we added both hormones to the base model. To evaluate the possible role of insulin resistance in mediating the association between bioavailable testosterone and VF, we included homeostasis model assessment for insulin resistance as a covariate. To test the hypothesis that postmenopausal women have more bioavailable testosterone, we used a linear model adjusting for all covariates in the base model. Age, FRS, homeostasis model assessment for insulin resistance, physical activity, and hormone measures were modeled continuously in the current analyses whereas race (with black ethnicity as the referent) and depression (CES-D, referent <16) were modeled as binary variables.
RESULTS
This large cohort of middle-aged women (age 50.6 ± 3.9) undergoing the menopausal transition (13.4% pre-, 49.3% peri-, and 37.3% postmenopausal) consisted of 193 white and 166 black women. Table 1 shows the characteristics of the cohort. The average BMI was in the overweight range (29.3 kg/m2). Of the total sample, 40.7% were obese.
Table 1.
Characteristics of the cohort
| All | |
|---|---|
| N | 359 |
| White, N (%) | 193 (53.8) |
| Age, years, mean (s.d.) | 50.6 (3.9) |
| Menopausal status, N (%) | |
| Premenopausal | 48 (13.4) |
| Early perimenopausal | 139 (38.7) |
| Late perimenopausal | 38 (10.6) |
| Postmenopausal | 134 (37.3) |
| Depressed (CES-D), N (%) | 45 (13.2) |
| Education ≤high school, N (%) | 41 (11.4) |
| Current smoker, N (%) | 72 (20.1) |
| Unmarried, N (%) | 122 (34.0) |
| Obese, N (%) | 146 (40.7) |
| BMI, kg/m2, mean (s.d.) | 29.3 (6.4) |
| Visceral fat, cm2, mean (s.d.) | 96.1 (52.8) |
| Percent body fat, mean (s.d.) | 43.4 (8.6) |
| Insulin resistance (HOMA-IR), median (IQR) | 1.27 (0.92–1.83) |
| Framingham risk score, mean (s.d.) | 10.6 (4.3) |
| Physical activity (KPAS), mean (s.d.) | 7.7 (1.6) |
| Estradiol, pg/ml, median (IQR) | 28.5 (17.0–62.5) |
| Testosterone, ng/dl, median (IQR) | 39.8 (29.0–51.3) |
| SHBG, µg/ml, median (IQR) | 5.11 (3.66–7.53) |
| Bioavailable testosterone, median (IQR) | 2.87 (1.90–4.71) |
CES-D, Center for Epidemiological Studies Depression Scale; HOMA-IR, homeostasis model assessment for insulin resistance; IQR, interquartile range; KPAS, Kaiser Physical Activity Survey.
Table 2 shows that in unadjusted analyses, VF was significantly positively related to percent body fat, age, FRS, and bioavailable testosterone, and significantly negatively related to physical activity, total estradiol, and SHBG. Most of these relationships were attenuated when adjusted for total body fat. Bioavailable testosterone was significantly related to age, FRS, depression, and SHBG.
Table 2.
Risk factor correlations with visceral fat and bioavailable testosterone: unadjusted and adjusted for % body fat
| IAF | Bioavailable testosterone | |||
|---|---|---|---|---|
| Variable | Unadjusted | Adjusted for % body fat | Unadjusted | Adjusted for % body fat |
| % Body fat | 0.644*** | 0.250*** | ||
| White | 0.045 | −0.115* | 0.001 | −0.057 |
| Depression (CES-D) | 0.092 | 0.117* | −0.100* | −0.115* |
| Smoking | 0.081 | 0.121* | 0.007 | 0.002 |
| Unmarried | −0.017 | −0.032 | −0.089 | −0.103* |
| Education ≤high school | 0.074 | 0.076 | 0.020 | 0.025 |
| Income | −0.030 | −0.045 | 0.034 | 0.043 |
| Age | 0.273*** | 0.183*** | 0.139** | 0.091 |
| Framingham risk score | 0.435*** | 0.350*** | 0.205*** | 0.163*** |
| Insulin resistance (HOMA-IR) | 0.514*** | 0.258*** | 0.405*** | 0.192*** |
| Physical activity (KPAS) | −0.244*** | −0.117* | −0.033 | 0.026 |
| Estradiol | −0.117* | −0.111* | −0.033 | −0.014 |
| Testosterone | 0.088 | 0.062 | 0.623*** | 0.633*** |
| SHBG | −0.460*** | −0.378*** | −0.745*** | −0.721*** |
| Bioavailable testosterone | 0.420*** | 0.345*** | ||
CES-D, Center for Epidemiological Studies Depression Scale; HOMA-IR, homeostasis model assessment for insulin resistance; KPAS, Kaiser Physical Activity Survey.
P ≤ 0.05,
P ≤ 0.01,
P ≤ 0.001.
Table 3 presents the results of the multivariate models. The base model includes the significant or marginally significant covariates of percent body fat, age, race, FRS, depression, and physical activity.
Table 3.
Multivariate correlates of visceral fat
| Base model | Model 1: Base + postmenopause |
Model 2: Base + hormones |
Model 3: Base + hormones + HOMA |
|
|---|---|---|---|---|
| Parameter | Estimate (s.e.)a | Estimate (s.e.)a | Estimate (s.e.)a | Estimate (s.e.)a |
| % Body Fat | 0.608 (0.038)*** | 0.607 (0.038)*** | 0.570 (0.036)*** | 0.523 (0.036)*** |
| Age | 0.067 (0.037) | 0.024 (0.042) | 0.048 (0.036) | 0.047 (0.036) |
| White race | 0.301 (0.072)*** | 0.306 (0.072)*** | 0.279 (0.067)*** | 0.322 (0.068)*** |
| Framingham risk score | 0.233 (0.039)*** | 0.231 (0.039)*** | 0.214 (0.036)*** | 0.202 (0.036)*** |
| Depression (dichotomous) | 0.194 (0.106) | 0.200 (0.106) | 0.260 (0.098)* | 0.180 (0.098)* |
| Physical activity | −0.073 (0.037)* | −0.075 (0.037)* | −0.074 (0.034)* | −0.055 (0.034) |
| Postmenopause indicator | 0.174 (0.084)* | |||
| Insulin resistance (HOMA-IR)b | 0.207 (0.035)*** | |||
| Bioavailable testosteroneb | 0.202 (0.033)*** | 0.166 (0.034)*** | ||
| Estradiolb | −0.007 (0.034) | 0.024 (0.034) | ||
| R2 | 0.574 | 0.579 | 0.614 | 0.649 |
HOMA-IR, homeostasis model assessment for insulin resistance.
Estimates are standardized β-coefficients and standard errors.
Transformed by logarithm to base 10.
P ≤ 0.05,
P ≤ 0.01,
P ≤ 0.001.
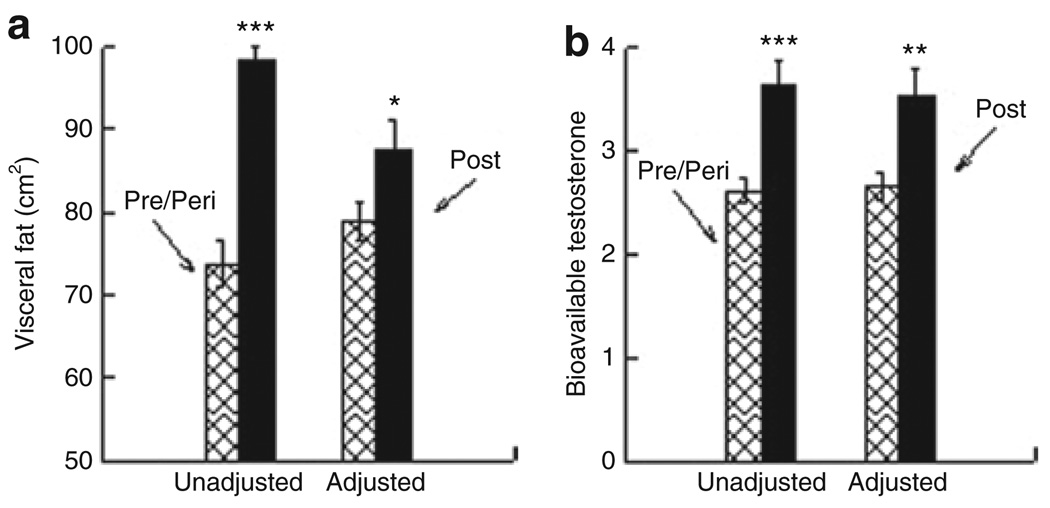
Figure 1a presents VF by menopausal status before and after adjustment for all covariates in the base model. Postmenopausal women had significantly more VF than women who were still menstruating before (98.3 cm2 vs. 73.8 cm2, P < 0.001) and after adjustment (87.6 cm2 vs. 79.0 cm2, P = 0.04). Figure 1b shows that postmenopausal women have higher values of bioavailable testosterone compared to women who have not reached their final menstrual period before and after adjustment (3.64 vs. 2.61, P < 0.001, and 3.53 vs. 2.66, P = 0.001).
Figure 1.
Association between menopausal status and (a) visceral fat (b) bioavailable testosterone. Unadjusted and adjusted for covariates in base model from Table 3. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
In Table 3, model 2 shows that bioavailable testosterone was significantly positively related to VF, independent of covariates. As expected, body fat, depression, and FRS remained significantly associated with VF, although age and estradiol did not. Higher physical activity was inversely related to VF. Blacks had significantly less VF than whites after adjustment (75.3 cm2 vs. 88.4 cm2, P < 0.0001). We explored an interaction term for race with bioavailable testosterone, but it was not significant. Thus, we can conclude that the association of testosterone with VF is similar in black and white women. Figure 2 illustrates the association between bioavailable testosterone and VF adjusted for the covariates in the base model.
Figure 2.
Visceral fat and bioavailable testosterone. Adjusted for % body fat, age, ethnicity, Framingham risk score, depression, and physical activity.
In Table 3, model 3 shows that adding homeostasis model assessment for insulin resistance did not change the effect of the hormones on VF. The influence of estradiol on VF was minimal, whereas the parameter estimate for bioavailable testosterone was lower but still highly significant.
When we replaced bioavailable testosterone with SHBG in the above models (data not shown), the results were essentially the same as those with bioavailable testosterone. This suggests that bioavailable testosterone and SHBG are similar in their predictive capability.
DISCUSSION
In this large biracial sample of middle-aged women, we found that postmenopausal women have significantly more VF than women who are still menstruating. These effects were independent of sociodemographic and standard cardiovascular risk factors.
The association between bioavailable testosterone and VF was similar in black and white women. These results were similar to previous findings in white women (20,35). However, they are inconsistent with a small sample of postmenopausal black women (35) where no association between VF and bioavailable testosterone or SHBG was found.
Traditionally, estrogen was thought to protect premenopausal women from CVD, and that the increased cardiovascular risk in women after menopause was related to the loss of the protective effect of estrogen (36). Indeed, we found a negative association between total estradiol and VF. Our data suggest that testosterone is a strong predictor of VF and, as such, associated with higher cardiovascular risk in women transitioning through menopause, similar to premenopausal women whose estrogen production may be intact (37).
There is biologic plausibility for a menopause-related link between testosterone and VF. Estrogen stimulates the production of SHBG (38), a protein that regulates reproductive hormone bioavailability by binding testosterone and estradiol. As such, it determines the amount of androgen available for action on cells. As estradiol production decreases with the menopause, SHBG levels decline, and thus the proportion of bioavailable testosterone increases. Because androgen receptors have been identified in adipocytes, particularly preadipocytes in VF (39), it is plausible that this bioavailable testosterone regulates VF accumulation. However, alternatively it may be that the accumulation of VF from another cause will be associated with lower SHBG levels due to insulin resistance and higher bioavailable testosterone (40). When both estradiol and insulin resistance were included in the model, the association between bioavailable testosterone and VF was attenuated but persisted. Therefore, we conclude that the effect of bioavailable testosterone on VF is only partially mediated by insulin resistance.
Strengths of the study include the precise assessment of fat and the large, representative, biethnic cohort of middle-aged women where the socioeconomic status of the black and white participants is similar. A weakness of the study is the cross-sectional design. Thus, we cannot conclude that higher testosterone causes more VF. However, evidence does exist that at least one direction of causality is from testosterone to VF (20,21). Additional longitudinal research is needed to establish temporal relationships. With repeat assessments of VF over 4 years in the SWAN Fat Patterning Study, we hope to address this issue in our future work.
In summary, VF is significantly associated with the menopause, independent of aging. This menopause effect is likely due to higher bioavailable testosterone (or lower SHBG) and is independent of age, total body adiposity, and cardiovascular risk factors. Because abdominal adiposity is one of the major predictors of CVD (5–9), menopause-related testosterone predominance in the reproductive hormonal milieu may be an important target for CVD prevention during the menopausal transition. In light of influential clinical trials (41,42), estrogen therapy does not appear to be a route to the reduction of this potential target.
ACKNOWLEDGMENTS
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health, US Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Fat Patterning Study is supported by the National Heart, Lung and Blood Institute (grant HL067128) and the Charles J. and Margaret Roberts Trust. We thank the study staff at each site and all the women who participated in SWAN. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metab Clin Exp. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 2.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 6.Peiris AN, Sothmann MS, Hennes MI, et al. Relative contribution of obesity and body fat distribution to alterations in glucose insulin homeostasis: predictive values of selected indices in premenopausal women. Am J Clin Nutr. 1989;49:758–764. doi: 10.1093/ajcn/49.5.758. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Armellini F, Milani MP, et al. Body fat distribution in preand post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord. 1992;16:495–504. [PubMed] [Google Scholar]
- 8.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3) Suppl 1:S12–S18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 10.Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL. Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metab Clin Exp. 2003;52:186–191. doi: 10.1053/meta.2003.50024. [DOI] [PubMed] [Google Scholar]
- 11.Tchernof A, Desmeules A, Richard C, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie JR, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year prospective study. Climacteric. 1999;2:205–211. doi: 10.3109/13697139909038063. [DOI] [PubMed] [Google Scholar]
- 13.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews KA, Meilahn E, Kuller LH, et al. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 16.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 17.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 18.Després JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie JR, Dennerstein L, Taffe JR, et al. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil Steril. 2003;79:1335–1340. doi: 10.1016/s0015-0282(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 21.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. J Clin Endocrinol Metab. 1996;81:2198–2203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 22.Yen SSC, Jaffe RB, Barbieri RL. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 4th edn. Philadelphia: Saunders; 1999. [Google Scholar]
- 23.Sowers MF, Crawford SL, Sternfeld B. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA; London: Academic; 2000. pp. 175–188. [Google Scholar]
- 24.Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 25.Hill JO, Sidney S, Lewis CE, et al. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 28.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 29.Steiner PM, Freidel J, Bremner W, Stein JH. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipid clinics’ methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 30.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Roberts RE. Reliability of the CES-D Scale in different ethnic contexts. Psychiatry Res. 1980;2:125–134. doi: 10.1016/0165-1781(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 32.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 33.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 34.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 35.Berman DM, Rodrigues LM, Nicklas BJ, et al. Racial disparities in metabolism, central obesity, and sex hormone-binding globulin in postmenopausal women. J Clin Endocrinol Metab. 2001;86:97–103. doi: 10.1210/jcem.86.1.7147. [DOI] [PubMed] [Google Scholar]
- 36.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23:129–136. doi: 10.1016/0378-5122(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 37.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24:302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 38.Kalme T, Loukovaara M, Koistinen R, et al. Estradiol increases the production of sex hormone-binding globulin but not insulin-like growth factor binding protein-1 in cultured human hepatoma cells. Fertil Steril. 1999;72:325–329. doi: 10.1016/s0015-0282(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 39.Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998;274:C1645–C1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 40.Phillips GB, Jing T, Heymsfield SB. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metab Clin Exp. 2008;57:838–844. doi: 10.1016/j.metabol.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 42.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]