Abstract
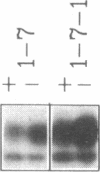
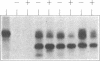
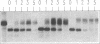
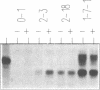
Two related polypeptides, H1 and H2, comprise the human asialoglycoprotein receptor (ASGP-R). Stable lines of murine NIH 3T3 fibroblasts expressing H1 alone or H2 alone do not bind or internalize the ligand asialoorosomucoid (ASOR), which contains triantennary oligosaccharides. In contrast, cells expressing H1 and H2 together bind and degrade ASOR with properties indistinguishable from those of the ASPG-R in human hepatoma HepG2 cells. Whether or not H2 is coexpressed, H1 is synthesized as a 40-kDa precursor bearing high-mannose oligosaccharides, processed to its mature 46-kDa form, and transported to the cell surface. In cells expressing only H1, homodimers and -trimers of H1 are formed. In contrast, when expressed in 3T3 cells without H1, H2 is synthesized as its 43-kDa precursor, bearing high-mannose oligosaccharides, but is rapidly degraded. When H1 and H2 are coexpressed in the same cell, the H1 polypeptide “rescues” the H2 polypeptide; H2 is processed to its characteristic 50-kDa mature form and is transported to the surface. We conclude that the human ASGP-R is a multichain heterooligomer, probably a trimer of H1 molecules in noncovalent association with one, two, or three H2 molecules, and that the two polypeptides normally interact early in biosynthesis.
Keywords: galactose lectin, subunit assembly, receptor biosynthesis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Libresco S., Shia M. A., Lodish H. F. The H1 and H2 polypeptides associate to form the asialoglycoprotein receptor in human hepatoma cells. J Cell Biol. 1988 Apr;106(4):1067–1074. doi: 10.1083/jcb.106.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J., Lodish H. F. Two asialoglycoprotein receptor polypeptides in human hepatoma cells. J Biol Chem. 1987 Aug 25;262(24):11825–11832. [PubMed] [Google Scholar]
- Breitfeld P. P., Simmons C. F., Jr, Strous G. J., Geuze H. J., Schwartz A. L. Cell biology of the asialoglycoprotein receptor system: a model of receptor-mediated endocytosis. Int Rev Cytol. 1985;97:47–95. doi: 10.1016/s0074-7696(08)62348-7. [DOI] [PubMed] [Google Scholar]
- Connolly D. T., Townsend R. R., Kawaguchi K., Bell W. R., Lee Y. C. Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem. 1982 Jan 25;257(2):939–945. [PubMed] [Google Scholar]
- Deitcher D. L., Neutra M. R., Mostov K. E. Functional expression of the polymeric immunoglobulin receptor from cloned cDNA in fibroblasts. J Cell Biol. 1986 Mar;102(3):911–919. doi: 10.1083/jcb.102.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg D. F., Wager R. E., Farrell D. C., Hildreth J., 4th, Quesenberry M. S., Loeb J. A., Holland E. C., Drickamer K. Major and minor forms of the rat liver asialoglycoprotein receptor are independent galactose-binding proteins. Primary structure and glycosylation heterogeneity of minor receptor forms. J Biol Chem. 1987 Jul 15;262(20):9828–9838. [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Parkhurst S. M., Lee Y. C. Different modes of ligand binding to the hepatic galactose/N-acetylgalactosamine lectin on the surface of rabbit hepatocytes. Biochemistry. 1985 Jan 1;24(1):22–28. doi: 10.1021/bi00322a004. [DOI] [PubMed] [Google Scholar]
- Hsueh E. C., Holland E. C., Carrera G. M., Jr, Drickamer K. The rat liver asialoglycoprotein receptor polypeptide must be inserted into a microsome to achieve its active conformation. J Biol Chem. 1986 Apr 15;261(11):4940–4947. [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Lee Y. C. Affinity labeling of the galactose/N-acetylgalactosamine-specific receptor of rat hepatocytes: preferential labeling of one of the subunits. Biochemistry. 1987 Oct 6;26(20):6320–6329. doi: 10.1021/bi00394a005. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Lin P., Lee Y. C. New synthetic cluster ligands for galactose/N-acetylgalactosamine-specific lectin of mammalian liver. Biochemistry. 1984 Aug 28;23(18):4255–4261. doi: 10.1021/bi00313a037. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Townsend R. R., Hardy M. R., Lönngren J., Arnarp J., Haraldsson M., Lönn H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J Biol Chem. 1983 Jan 10;258(1):199–202. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McPhaul M., Berg P. Formation of functional asialoglycoprotein receptor after transfection with cDNAs encoding the receptor proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8863–8867. doi: 10.1073/pnas.83.23.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. A fibronectin matrix is required for differentiation of murine erythroleukemia cells into reticulocytes. J Cell Biol. 1987 Dec;105(6 Pt 2):3105–3118. doi: 10.1083/jcb.105.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. T., Sanford J. P., Doyle D. Identification of a complex of the three forms of the rat liver asialoglycoprotein receptor. J Biol Chem. 1988 Jul 25;263(21):10534–10538. [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Schwartz A. L., Steer C. J., Kempner E. S. Functional size of the human asialoglycoprotein receptor as determined by radiation inactivation. J Biol Chem. 1984 Oct 10;259(19):12025–12029. [PubMed] [Google Scholar]
- Schwartz A. L. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem. 1984;16(3):207–233. doi: 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Schwartz A. L., Lodish H. F. Sequence of human asialoglycoprotein receptor cDNA. An internal signal sequence for membrane insertion. J Biol Chem. 1985 Feb 25;260(4):1979–1982. [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]