Abstract
General anesthetic drugs interact with many receptors in the nervous system, but only a handful of these interactions are critical for producing anesthesia. Over the last 20 years, neuropharmacologists have revealed that one of the most important target sites for general anesthetics is the GABAA receptor. In this review we will discuss what is known about anesthetic – GABAA receptor interactions.
Keywords: GABAA Receptor, General Anesthetic, Isoflurane, Desflurane, Sevoflurane, Propofol, Etomidate, Barbiturates.
INTRODUCTION
General anesthetics have been used to relieve pain and suffering in surgical patients for almost 170 years. Prior to their discovery, surgery was a traumatic and barbaric affair, yet today, it is accepted as a routine and essential part of modern medicine. It is curious therefore, that despite the technological advances that have been made in perioperative medicine and surgical techniques, the pharmacologic therapeutics administered by anesthesiologists to render patients unconscious, continue to be used without a precise understanding of how they generate the anesthetized state.
Fortunately, over the last two decades, the intensive use of the tools of modern molecular pharmacology and neuroscience has enabled investigators to understand better than ever before how anesthetic chemicals can alter the function of the nervous system. In this review, we will carefully define what we mean by “anesthesia” and then we will focus on how the currently used anesthetic agents modulate the function of the GABAA receptor (GABAAR), the most abundant fast inhibitory neurotransmitter receptor in the CNS.
At the outset, we would like to emphasize that this review is neither historical nor is it exhaustive. We do not intend to review the interaction of every drug used by anesthesiologists in the Operating Room, past or present, with every critical binding site in the body. The purpose of this review is to summarize some of the key findings that we feel have advanced our understanding of how the currently used general anesthetic drugs interact with their neuronal targets. The history of anesthetic pharmacology is rich and has played a critical role in our understanding of the anesthetized state and the reader is encouraged to read previous reviews if they are interested in reviewing the action of disused anesthetics such as halothane, chloroform or ether. Neither shall we dwell on discarded theories of anesthetic action. It is the fervent view of the authors that general anesthesia is no different from any other pharmacological process: exogenously administered drugs interact with key sites on cellular proteins in the body which results directly in the alteration in the function of these proteins, in the present case, neuronal proteins that control how information is conveyed through the nervous system.
It is noteworthy that the study of general anesthesia as a whole, has not immediately lent itself to being accessible to traditional pharmacology; anesthesia is a clinical state where a myriad of behavioral endpoints are caused by a group of drugs that bear no physical resemblance to one another. Nevertheless, it appears that GABAARs are incredibly accommodating and possess several binding sites that interact with many of the currently used general anesthetics. Moreover, these interactions obey many of the basic principles of rational pharmacology; the effects of drug on protein are concentration dependent, they are subject to the “ceiling effect”, they are allosteric in nature, the effects are dependent on the temperature of the preparation, some compounds exhibit stereoselectivity and depending on the anesthetic, they appear to modulate the efficacy and/or the apparent affinity of the receptor for its endogenous ligand.
In the following sections, we will define what we understand to be “general anesthesia” and how anesthetic drugs interact with native and mutant GABAARs.
WHAT IS GENERAL ANESTHESIA?
When Oliver Wendell Holmes first coined the term anæsthesia in 1846, the word, which literally means a lack of sensation, was used to describe a patient who after inhaling ether vapor, underwent surgery without any apparent suffering. Widespread use of the technique and this term followed, describing a state where one or more chemicals were systemically administered to “anesthetize” patients, permitting humane surgery to occur. Arguably one of the greatest breakthroughs in medical history, it is interesting that this essential component of modern medicine has developed without a rational theory consistent with modern pharmacology that accounts for the molecular mechanisms of these drugs in the human body.
One reason for this is that after more than 160 years of routine clinical use, there is still no consensus on what singularly defines the anesthetized state. During general anesthesia, a myriad of events occur in the body that alter motor responses, cognition, sensation and autonomic control. A patient will go from being anxious, to unconscious and immobile and back to awake and in light of this, there continues to be a debate on which of these effects, or what combination of these effects are the critical determinants of general anesthesia.
The seminal work by Eger et al. [64], set the benchmark in understanding anesthetic actions for almost 50 years. The definition of the Minimum Alveolar Concentration (MAC) places great emphasis on anesthetic induced immobility, as a read-out for sensation, and less emphasis on processes not directly linked to the sensation of acute pain and their associated motor reflexes. Subsequent research has demonstrated, perhaps not unsurprisingly, that the key sites in the nervous system that determine MAC are found in the spinal cord [3] and interestingly, may not be critically dependent on GABAARs [73]. Nevertheless, although MAC may not have revealed information on the cerebral sites of anesthetic action and anesthetic actions on sensory processing, one of the great benefits of the MAC studies was to define a set of drug concentrations that are appropriate for use in in vitro studies on potential molecular targets, a giant leap forward for molecular pharmacologists interested in understanding how these drugs induce anesthesia.
For many decades after the inception of general anesthesia, through the lack of available drugs, it was usual for only one general anesthetic drug to be used to induce immobility, analgesia, muscle relaxation, amnesia and unconsciousness all at once. It turns out that anesthesia generated in this way was a dangerous practice. The therapeutic index for these early anesthetic drugs was in the low single digits. Modern anesthesia on the other hand makes use of much safer drugs with higher therapeutic indices and is very rarely achieved with a single drug. Anesthesiologists several devices to measure a patient’s vital signs and actively balance cocktails of drug arrays, with the effect of one drug sparing the patient exposure to higher concentrations of another and in some cases, making use of the synergistic combination of some drug pairs [15, 23, 30, 61].
Therefore, to recap: under conditions of optimal monitoring, sedation and anesthesia, it is possible for a patient to undergo surgery and have no recall of intra-operative events. With the safer drugs in clinical practice today, we acknowledge that modest movements may persist if neuromuscular blockade is not employed yet the patient is senseless of their surroundings and surgery can be completed without any adverse events. This satisfies the original definition of anesthesia – no sensation accompanied by a successful surgery. Therefore, a review of the current neuropharmacology of general anesthetic drugs should discuss the non-immobilizing sedative drugs as well as general anesthetics. We acknowledge the important role benzodiazepines play in the perioperative setting but would like to point the reader to an accompanying review in this volume that extensively reviews the interaction of benzodiazepines with GABAARs and the importance of the these interactions for anxiolysis and sedation.
THEORY OF GENERAL ANESTHETIC ACTION
At the cellular level, anesthetics alter the behavior of neurons, by interacting directly with a small number of ion channels. Under normal conditions, these specialized membrane proteins are activated by chemical signals or changes in the membrane environment. Upon activation, channels change the electrical excitability of neurons by controlling the flow of depolarizing (excitatory) or hyperpolarizing (inhibitory) ions across the cell membrane via an ion channel that is integral with the receptor that senses the initial signal. General anesthetics primarily act by either enhancing inhibitory signals or by blocking excitatory signals. It is important to note that none of the current clinical general anesthetics are selective for a single ion channel. At clinical concentrations, every anesthetic modulates the function of two or more types of channels in the central nervous system. Thus, each different anesthetic agent alters neuronal activity by acting in differing degrees at multiple sites.
GENERAL ANESTHETICS AND THEIR TARGETS
Currently, there are 5 inhalational and 5 intravenous anesthetics used to induce or maintain general anesthesia: Inhalational: Nitrous Oxide, Isoflurane, Sevoflurane, Desflurane and Xenon. Intravenous: Propofol, Etomidate, Ketamine, Methohexital and Thiopental. These 10 general anesthetic drugs are often accompanied by sedative benzodiazepines: midazolam, diazepam and lorazepam.
Of these 10 general anesthetics, ketamine, nitrous oxide and xenon inhibit ionotropic glutamate receptors, with the strongest effects being seen on the NMDA receptor subtype. These anesthetics also have modest effects on many other receptors, including GABAARs, but their primary action is the blockade of NMDA receptors and will not be covered further in this review.
The other 7 general anesthetics and 3 sedatives share a common target and mechanism of action, they all enhance the function of GABAARs, the most abundant fast inhibitory neurotransmitter receptor in the CNS. These 7 general anesthetics also have a spectrum of modest to strong effects on other ion channels, including glycine receptors, neuronal nicotinic receptors, 5-HT3 receptors, glutamate receptors and the two pore potassium channels [1, 17], with each drug differing in its array of effects, but in order to focus on the GABAAR effects, the effects on these other ion channels will also not be considered further in this review.
THE GABAA RECEPTOR
Receptor Diversity and Anatomical Expression
Along with the nicotinic acetylcholine receptor (nAChR), the glycine receptor, and the serotonin type 3 (5-hydroxytryptamine type 3) receptor, GABAARs are members of the Cys-loop superfamily of ligand-gated ion channels. Each of these receptors is formed as a pentameric combination of transmembrane polypeptide subunits. The name of the superfamily comes from the characteristic “cys-loop” found in all subunits, defined by a disulfide bond between two cysteine residues separated by 13 amino acid residues. Neurotransmitter binding sites are located at two or more extracellular interfaces and in the case of GABAARs, the endogenous ligand binds between the α and β subunits. So far, nineteen genes have been identified for GABAAR subunits (α1 – 6, β1 – 3, γ1 – 3, δ, ε, θ, π, ρ1 – 3)[62]. Despite nearly 2.5 million different possible permutations of subunits, only nine different receptor configurations are unequivocally expressed in significant amounts in the brain [53]. If one includes GABAARs that can be classified as “tentative” or “existence with high probability”, the number of receptor combinations increases to 26 [53]. Preferred subunit combinations are specifically distributed among different brain regions [69] and even demonstrate different subcellular localization [reviewed in 47]. Because each type of GABAAR exhibits distinct biophysical and pharmacologic properties, these receptors are capable of diverse influences on regulating synaptic transmission and synaptic integration and since these receptors are expressed in parts of the nervous system which process higher-order brain functions, it is not surprising that GABAAR function is central to memory, awareness and consciousness.
ANESTHETIC ACTION ON NATIVE RECEPTORS
Anesthetic drugs allosterically modulate GABAARs and disrupt normal physiologic circuits which require precise timing of GABA-ergic input. There exists strong evidence that GABAARs are involved in mediating some of the classical components of general anesthesia: hypnosis [2], depression of spinal reflexes [13], and amnesia [43]. The contribution of GABAARs in mediating immobility and analgesia is less clear (see mutagenesis section). Through experimental investigation it is becoming increasingly clear that not only are the different classical components of general anesthesia mediated by different pathways in the nervous system, but different classes of anesthetics can have differing effects within these pathways.
For example, specific behavioral effects by pharmacologic therapeutics have been linked to different GABAAR assemblies present in different brain regions; sedation produced by benzodiazepines has been associated with the α1 containing GABAARs [44] and anxiolysis appears to mediated by α2 containing GABAARs in the limbic system but not the GABAARs in the reticular activating system; which primarily consists of α3 containing GABAARs [42].
Moreover, manipulation of hippocampal GABAARs by the selective intrasynaptic GABAAR blocker, gabazine, revealed that isoflurane selectively enhances intrasynaptic GABAA mediated currents (associated with α1 –2, β2 –3, and γ2 subunits) in comparison to thiopental which is less selective and augmented both intrasynaptic and extrasynaptic (via the α5 subunit) GABAA mediated currents [9]. Not only can anesthetics of different classes be selective in their neurophysiological effects, but so can drugs of the same class, for example, synaptic input (frequency and decay time constant) onto hippocampal interneurons is differentially modulated by sevoflurane versus isoflurane [52]. From this handful of examples, it is clear that the anesthetized state produced by each anesthetic is subtly unique and dependent on each drug’s particular pharmacological characteristics in different brain regions. These microscopic observations are supported by autoradiographical measurements of cerebral blood flow and glucose utilization in anesthetized rodent brains which revealed that specific brain structures could be either hypometabolic or hypermetabolic depending on whether isoflurane or sevoflurane were used [41]. However, the specific effect of each individual volatile anesthetic on specific GABAAR subunit assemblies has yet to be systematically examined.
VOLATILE ANESTHETICS
Nakahiro et al. [49] were the first to directly record the enhancement of GABA responses by isoflurane. By patch clamping dorsal root ganglion neurons in primary culture, they were able to enhance the amplitude of currents activated by low concentrations of GABA. Our understanding of the molecular pharmacology of this anesthetic was further enhanced when it was shown that the stereoselectivity for GABAAR modulation paralleled its selectivity as an anesthetic [21], strongly implicating the GABAAR as a critical target site for isoflurane. However, our understanding of which receptor subunits are critical for general anesthetic action remains incomplete, despite an enormous effort over the last 15 years using the appropriate concentrations of drug (the EC50s for general anesthesia translate to the following micromolar concentrations that should be used for in vitro experiments at room temperature: 280 µM isoflurane, 330 µM, sevoflurane, 280 µM desflurane [18]) on recombinant receptors in heterologous expression systems [22] and cells that express unique GABAAR isoforms. But in general, what appears to be clear is as follows: isoflurane, desflurane and sevoflurane all enhance the amplitude of responses to low concentrations of GABA and prolong the duration of GABA mediated synaptic inhibition. At supraclinical concentrations, they are able to open the receptor’s anion channel in the absence of GABA, a process known as “direct activation”.
All GABAARs containing an α subunit are sensitive to at least one volatile general anesthetic drug. The co-transfection of HEK293 cells with β1 subunit cDNAs with either an α1 or an α2 subunit cDNA is sufficient for the expression of GABAA Rs that are sensitive to isoflurane [22]. The addition of the γ subunit does not abolish isoflurane sensitivity, thus we can safely conclude that the binding site(s) for inhaled anesthetics are located, at the very least, within alpha and beta subunits. This is in contrast to homomeric receptors that are constructed from rho subunits and whose responses are inhibited by volatile anesthetics [45].
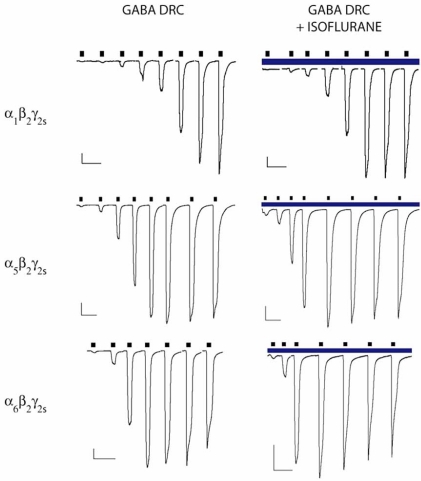
Research on the effects of volatiles at extrasynaptic GABAARs in thalamic [8] and hippocampal [4] brain slice preparations suggest that the extrasynaptic GABAARs exhibit higher affinities for GABA and exhibit decreased desensitization. At present, there is great interest in the relevance of extrasynaptic receptors in generating the anesthetized state, with many laboratories investigating the importance of the anesthetic enhancement of CSF-activated tonic responses that are associated with extrasynaptic sites (reviewed in [6]). According to our own measurements, CSF contains ~150 nM GABA (A.J & P.S.G., data not shown). The resulting anion shunt is currently thought to be critical to the behavior of neurons in different brain regions. Similarly, the molecular actions of inhaled anesthetics on recombinant subunit combinations (α4—6, and δ- containing GABAARs) thought to be associated with these extrasynaptic receptors are also being intensively investigated. Recent results in our laboratory using HEK293 cells expressing either α1β2γ2s, α5β2γ2s, or α6β2γ2s GABAARs have demonstrated that each of these subunit combinations exhibits enhanced GABA sensitivity with isoflurane in a dose-dependent fashion, but the α5- and α6- containing subunit combinations had a heightened GABA sensitivity and the α6β2γ2s combination demonstrated decreased desensitization, together with modest differences in isoflurane induced enhancement (see Table 1 and Fig. 1). Evidence such as this effectively closes the loop on the hypotheses of extrasynaptic GABA-ergic transmission garnered in brain slice preparations. Our data is consistent with the observation that low, sub-anesthetic concentrations of isoflurane potentiate the Cl- current at α5β3γ2L GABAARs but not at α1β3γ2L GABAARs in hippocampal neurons [11]. It is important to recognize that dose can play an important role in characterizing the effects of anesthetics on these receptors, either alone or in combination with other anesthetics because drug interactions vary considerably at different time points during surgery and along a response surface [59]. Similarly, we feel that systematic investigations of drug actions on subunit combinations provides a clearer picture as to the effects of those drugs even if some of those subunit combinations have not been identified yet in the mammalian CNS. As it stands today, the literature is lacking a complete survey of the effect of isoflurane, sevoflurane and desflurane on the 26 GABAAR populations found in the CNS of a single species, using comparable methodologies, be it the kinetics of ion channel function or shifts of the GABA concentration response.
Table 1.
| Isoflurane 140 µM | Isoflurane 280 µM | Isoflurane 560 µM | Isoflurane 1400 µM | |
|---|---|---|---|---|
| α1β2γ2s | 0.35 ± 0.06 | 0.54 ± 0.06 | 0.56 ± 0.02 | 0.58 ± 0.03 |
| α5β2γ2s | 0.28 ± 0.05 | 0.54 ± 0.03 | 0.57 ± 0.05 | 0.75 ± 0.03 |
| α6β2γ2s | 0.44 ± 0.09 | 0.57 ± 0.12 | n.d. | 0.60 ± 0.08 |
Fractional Effect of Isoflurane on GABA EC50 for α1β2γ2s, α5β2γ2s, and α6β2γ2sGABAA receptors. Values are mean ± SEM and are determinations of at least 4 concentration-response shifts from at least 3 cells, as determined by the fractional change in the effective GABA concentration for 50% of maximal activation (EC50). Isoflurane concentrations are reported as molar concentrations. These concentrations equate to 0.5, 1, 2 and 5 times isoflurane minimum alveolar concentration (MAC). n.d. (not determined).
Fig. (1).
Examples of chloride currents for α1β2γ2s, α5β2γ2s, and α6β2γ2s GABAA receptors. Left panels show representative whole-cell recordings achieved by applications of increasing concentrations of GABA (black bars). Right panels show paired recordings with the same concentrations of GABA in the presence of 2 MAC isoflurane (blue bar). GABA concentrations (all in µM) for α1β2γ2s GABAA receptors (0.3, 1, 3, 10, 30, 100, 300, 1000), for α5β2γ2s GABAA receptors (0.1, 0.3, 1, 3, 10, 30, 100, 300), and for α6β2γ2s GABAA receptors (0.1, 0.3, 1, 3, 10, 30, 100). Vertical scale bars 100 pA; Horizontal scale bars 10 s. The data indicate that α6 containing receptors are more sensitive to low concentrations of anesthetic than α1β2γ2s, or ;5β2γ2s receptors and that isoflurane is more effective at reducing the apparent affinity of GABA at α5 containing receptors than at α1β2γ2s, or α6β2γ2s GABAA receptors. The methods used to collect these results have been described previously [59].
Desflurane and sevoflurane are both known to have similar actions on GABAARs as isoflurane; both drugs increase the apparent affinity of the receptor for GABA. However, much less is known about their relative effects on different GABAA R subunits. Synaptic receptors containing α1, α2, β1 and β2 containing receptors are sensitive to at least one of these drugs, but their relative effects on extrasynaptic receptors has yet to be studied [28, 51, 59].
Finally, there has yet to be published an unambiguous demonstration of volatile anesthetic action on GABAARs at the single channel level. While many studies using fast solution exchange have inferred that volatile anesthetics alter one or more rate constants that govern channel function [31], the drug effects on the lifetimes of specific states remain unknown.
INTRAVENOUS ANESTHETICS
In common with the inhaled anesthetics, the intravenous anesthetics (thiopental, etomidate and propofol) enhance the amplitude of responses to low concentrations of GABA at clinically relevant concentrations (2 µM propofol, 3 µM etomidate, 25 µM thiopental and 10 µM methohexital) and prolong the duration of GABA mediated synaptic inhibition. At supraclinical concentrations, they also directly activate the receptor’s anion channel.
The interactions between GABAARs and the intravenous anesthetics are generally thought to occur within, or proximal to, the β subunits. Since the β subunit shows less specific subcellular localization than that of the α subunit, the intravenous anesthetics may not demonstrate rich physiological differences among intra- vs extra-synaptic GABAARs throughout the brain. Additionally, the distribution of the β subunit in mammalian brain does not share the same clear distinctions as the distribution of the α subunit[40, 69]. By examining in situ hybridization data, one clear trend emerges: the β1 subunit is largely confined to the hippocampus while the β2 and β3 subunits appear more widely distributed.
Propofol
The dialkylphenol, propofol (2,6, diisopropylphenol) potentiates GABA responses and directly activates GABAAR function [55]. Initially, only the property of direct activation of the GABA receptor by propofol was assumed to be dependent on the β subunit [55, 56] while the modulatory effects were considered to involve other subunits [56]. There is evidence that α, β and γ subunits all contribute to GABAAR sensitivity to propofol [20, 32, 39]. In particular, propofol was shown to be less efficacious at β1 containing receptors than at those containing β2 or β3 subunits [57]. The potency and the efficacy of the receptor – drug interaction is dependent on key propofol moieties, most notably, the phenolic hydroxyl group and the number and arrangement of methyl groups at the 2- and 6- positions that flank it [37]. Interestingly this study indicated that the 4-position played little role in modulation. The discovery of the essential pharmacophore for propofol’s action, it has recently led to the development of FOS-propofol, a new anesthetic that releases a propofol molecule after liver metabolism. This provides the anesthesiologist with a water soluble anesthetic that has slower induction kinetics than it’s active metabolite, which may be desirable in some surgical settings [48].
Finally, propofol may be actively involved in the recruitment of new subunits to the surface of the neuron; quantitative PCR revealed that during deep anesthesia propofol, increases α4 subunit mRNA occurred in comparison with midazolam, thiopental and isoflurane [60].
Etomidate
The effects of etomidate on GABA and benzodiazepine binding at the GABAAR have been known for over 25 years [65]. Like isoflurane, etomidate interacts with the GABAAR in a stereospecific manner [67]. Etomidate has smaller effects on receptors containing the β1 subunit [24]. Of all of the clinically used anesthetics, etomidate has the greatest selectivity for the GABAAR, and has the fewest relevant interactions with other ion channels in order to generate the anesthetized state.
Thiopental
Besides methohexital which has a special role in electroconvulsive therapy [26], thiopental is the only barbiturate routinely used by modern anesthesiologists. Like etomidate, it is known to enhance GABAAR function in a stereospecific manner [68]. This is also true for other barbiturate sedatives such as hexabarbital and pentobarbitial. The action of the latter drug has been confirmed at the single channel level, in what is arguably the most detailed study of a general anesthetic modulating the GABAAR. Steinbach and Akk [63] demonstrated the complexity of the anesthetic-receptor modulation, but were also able to clearly show that pentobarbital enhanced channel function by stabilizing one of the open states. In common with high concentrations of propofol and etomidate, thiopental can also directly gate GABAARs.
Thiopental appears to be selective for either tonic or phasic GABAAR activity in the hippocampus [9] suggesting a possible role for the δ subunit in controlling thiopental actions on GABAARs. This is interesting since the molecular site of action of barbiturates at GABAARs has long been thought to reside within one of the β subunits. Thus, it would appear that barbiturate actions on GABAARs are more complex than with other anesthetics [63]. This observation is supported by the fact that thiopental is a more effective agonist than GABA in receptors containing α6 subunits [14].
MUTANT RECEPTORS AND GENETICALLY MANIPULATED ANIMALS
In 1997, Neil Harrison and Adron Harris published the first report of GABAARs that had been designed to be insensitive to inhaled anesthetics [46]. The rationale behind the ground-breaking work was similar to the successful strategy that had revealed the functional target site of benzodiazepines 4 years earlier [35], taking advantage of the fact that the function of receptors constructed from the ρ1 subunit was not enhanced by inhaled anesthetics [45]. Through a series of receptor chimeras, they identified a pair of transmembrane amino acid substitutions that were sufficient to render α2β1 containing receptors insensitive to enflurane, an inhalational anesthetic (and a chemical isomer of isoflurane). By replacing the α2 residues serine270 and alanine291 with the corresponding residues in the rho1 subunit, an isoleucine and tryptophan respectively, the recombinant receptors were no longer enhanced by the anesthetic. One week later, it was reported that a homologous residue in the β3 subunit was critical for the action of intravenous anesthetics [7].
During the following decade, mutations have been introduced into many of the GABAAR subunit transmembrane domains, using a variety of rationales, to delineate the general anesthetic target sites in each receptor subunit (see Table 2). In all cases, the mutation of one or more transmembrane residues has resulted in the impairment or abolition of anesthetic modulation by either isoflurane, desflurane, sevoflurane, propofol or etomidate. Each of these studies concluded that amino acid substitutions alter the molecular environment in the anesthetic binding cavity, presumably by reducing the number of favorable interactions between the receptor and the anesthetic molecule and thus reduced the effect of the anesthetic on enhancing GABAAR function. In common with the work with the different subunit combinations, our understanding of the specific residue-anesthetic interactions is also far from complete.
Table 2.
| Transmembrane Domain | ||||
|---|---|---|---|---|
| Subunit | M1 | M2 | M3 | M4 |
| α1 | [5] | [25] | [33] | [27] |
| α2 | [29] | [46] | [46] | - |
| α3 | - | [58] | [58] | - |
| β1 | [19] | [66] | - | - |
| β2 | [12] | [7] | [38] | [54] |
Transmembrane domain mutations in most of the synaptic GABAA receptor subunits reduce modulation by general anesthetics. M1 denotes transmembrane domain number 1. References are from a mixture of species, comprising human, rat and mouse gene products. A dash denotes that to our knowledge, the domain has not yet been mutated to render a significant effect on the effect of a clinically used anesthetic.
For the inhaled anesthetics, the majority of the published studies have focused on the interactions of isoflurane with mutant receptors and in comparison, much less is know about the interaction of sevoflurane and desflurane with mutant receptors. That said, where comparisons are possible, it appears that all three inhaled anesthetics behave in qualitatively similar manners, but it is premature to say for certain whether all inhaled anesthetics interact with their binding sites identically. Only a complete survey of all the critical positions in all of the subunits will reveal if inhaled anesthetics preferentially target specific parts of a given receptor population. But at the current time, it is fair to say that these three anesthetics likely form weak bonds with several residues in the four transmembrane domains of GABAAR α subunits as their primary interaction with their critical neuronal targets.
For many years, a slightly clearer picture emerged for the intravenous anesthetics interacting with β subunits, in part due to the relative ease of conducting experiments with non-volatile compounds, the smaller number of β subunits and the smaller number of IV anesthetics. Again, residues in the 4 transmembrane domains appear to be critical in defining the actions of etomidate, propofol and barbiturates.
It is interesting to note that the mutation of many residues in the transmembrane domains have modest effects on anesthetic modulation of GABAARs. However, the strongest effects seem to be reserved for homologous positions in transmembrane domains M2, M3 and M4 respectively (see Table 2) and there is evidence to suggest that this pattern extends across several members of the LGIC superfamily [36].
The full potential of these studies was realized in 2003 and again in 2005 when the first reports of the incorporation of some of these mutations into the mouse genome were reported [25, 34]; the mouse lines generated exhibited a selective loss of sensitivity to intravenous and inhaled anesthetics respectively. These animals and their lines that they have inspired [10, 16, 70-72] are now being used extensively to understand the role different GABAAR subunits are playing in generating the anesthetized state.
CONCLUSIONS
Our current understanding of the molecular mechanisms of general anesthesia will not be complete until we understand how drug action at multiple sites alters the integration of information in the nervous system. Central to this will be an understanding of which circuits in the nervous system are most important for generating sensation and how perturbations at receptors in these circuits generates a state of anaisthesis. We have already seen how GABA receptor modulation leads to the sleep-like state associated with anesthesia [50]. Using the knowledge derived from the studies described here, we should learn in the coming years a great deal more about the molecular mechanisms of sleep, sensation, pain and perhaps one day, consciousness.
REFERENCES
- 1.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antkowiak B, Kirschfeld K. [Neural mechanisms of anesthesia] Anasthesiol. Intensivmed. Notfallmed Schmerzther. 2000;35:731–743. doi: 10.1055/s-2000-8935. [DOI] [PubMed] [Google Scholar]
- 3.Antognini JF, Carstens E. Macroscopic sites of anesthetic action: brain versus spinal cord. Toxicol. Lett. 1998;100(101):51–58. doi: 10.1016/s0378-4274(98)00164-7. [DOI] [PubMed] [Google Scholar]
- 4.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 5.Bali M, Jansen M, Akabas MH. GABA-induced intersubunit conformational movement in the GABAA receptor alpha 1M1-beta 2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site. J. Neurosci. 2009;29:3083–3092. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc. Natl. Acad Sci. U.S.A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J. Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieda MC, Su H, Maciver MB. Anesthetics discriminate between tonic and phasic gamma-aminobutyric acid receptors on hippocampal CA1 neurons. Anesth. Analg. 2009;108:484–490. doi: 10.1213/ane.0b013e3181904571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, 2nd Blednov YA, Saad A, Dai S, Pearce RA, Harris RA, Homanics GE, Harrison NL. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J. Pharmacol. Exp. Ther. 2006;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- 11.Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J. Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CS, Olcese R, Olsen RW. A single M1 residue in the beta2 subunit alters channel gating of GABAA receptor in anesthetic modulation and direct activation. J. Biol. Chem. 2003;278:42821–42828. doi: 10.1074/jbc.M306978200. [DOI] [PubMed] [Google Scholar]
- 13.Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995;18:549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- 14.Drafts BC, Fisher JL. Identification of structures within GABAA receptor alpha subunits that regulate the agonist action of pentobarbital. J. Pharmacol. Exp. Ther. 2006;318:1094–1101. doi: 10.1124/jpet.106.104844. [DOI] [PubMed] [Google Scholar]
- 15.Eger EI, 2nd Tang M, Liao M, Laster MJ, Solt K, Flood P, Jenkins A, Raines D, Hendrickx JF, Shafer SL, Yasumasa T, Sonner JM. Inhaled anesthetics do not combine to produce synergistic effects regarding minimum alveolar anesthetic concentration in rats. Anesth Analg. 2008;107:479–485. doi: 10.1213/01.ane.0000295805.70887.65. [DOI] [PubMed] [Google Scholar]
- 16.Elsen FP, Liljelund P, Werner DF, Olsen RW, Homanics GE, Harrison NL. GABA(A)-R alpha1 subunit knockin mutation leads to abnormal EEG and anesthetic-induced seizure-like activity in mice. Brain Res. 2006;1078:60–70. doi: 10.1016/j.brainres.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 18.Franks NP, Lieb WR. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology. 1996;84:716–720. doi: 10.1097/00000542-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield LJ, Jr, Zaman SH, Sutherland ML, Lummis SC, Niemeyer MI, Barnard EA, Macdonald RL. Mutation of the GABAA receptor M1 transmembrane proline increases GABA affinity and reduces barbiturate enhancement. Neuropharmacology. 2002;42:502–521. doi: 10.1016/s0028-3908(01)00196-4. [DOI] [PubMed] [Google Scholar]
- 20.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br. J. Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AC, Lieb WR, Franks NP. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br. J. Pharmacol. 1994;112:906–910. doi: 10.1111/j.1476-5381.1994.tb13166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol. Pharmacol. 1993;44:628–632. [PubMed] [Google Scholar]
- 23.Hendrickx JF, Eger EI, 2nd Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth. Analg. 2008;107:494–506. doi: 10.1213/ane.0b013e31817b859e. [DOI] [PubMed] [Google Scholar]
- 24.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br. J. Pharmacol. 1997;120:749–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homanics GE, Elsen FP, Ying SW, Jenkins A, Ferguson C, Sloat B, Yuditskaya S, Goldstein PA, Kralic JE, Morrow AL, Harrison NL. A gain-of-function mutation in the GABA receptor produces synaptic and behavioral abnormalities in the mouse. Genes Brain Behav. 2005;4:10–19. doi: 10.1111/j.1601-183X.2004.00090.x. [DOI] [PubMed] [Google Scholar]
- 26.Hooten WM, Rasmussen KG., Jr Effects of general anesthetic agents in adults receiving electroconvulsive therapy: a systematic review. J. ECT. 2008;24:208–223. doi: 10.1097/YCT.0b013e31815bfe2a. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins A, Andreasen A, Trudell JR, Harrison NL. Tryptophan scanning mutagenesis in TM4 of the GABA(A) receptor alpha1 subunit: implications for modulation by inhaled anesthetics and ion channel structure. Neuropharmacology. 2002;43:669–678. doi: 10.1016/s0028-3908(02)00175-2. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins A, Franks NP, Lieb WR. Effects of temperature and volatile anesthetics on GABA(A) receptors. Anesthesiology. 1999;90:484–491. doi: 10.1097/00000542-199902000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins A, Lobo IA, Gong D, Trudell JR, Solt K, Harris RA, Eger EI. 2nd (2008) General anesthetics have additive actions on three ligand gated ion channels. Anesth. Analg. 2008;107:486–493. doi: 10.1213/ane.0b013e31817b70c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones MV, Harrison NL. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J. Neurophysiol. 1993;70:1339–1349. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- 32.Jones MV, Harrison NL, Pritchett DB, Hales TG. Modulation of the GABAA receptor by propofol is independent of the gamma subunit. J. Pharmacol. Exp. Ther. 1995;274:962–968. [PubMed] [Google Scholar]
- 33.Jung S, Harris RA. Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABA receptors. J. Neurochem. 2006;96:885–892. doi: 10.1111/j.1471-4159.2005.03617.x. [DOI] [PubMed] [Google Scholar]
- 34.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 35.Kleingoor C, Wieland HA, Korpi ER, Seeburg PH, Kettenmann H. Current potentiation by diazepam but not GABA sensitivity is determined by a single histidine residue. Neuroreport. 1993;4:187–190. doi: 10.1097/00001756-199302000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol. Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J. Pharmacol. Exp. Ther. 2001;297:338–351. [PubMed] [Google Scholar]
- 38.Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001;41:952–964. doi: 10.1016/s0028-3908(01)00141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam DW, Reynolds JN. Modulatory and direct effects of propofol on recombinant GABAA receptors expressed in xenopus oocytes: influence of alpha- and gamma2-subunits. Brain Res. 1998;784:179–187. doi: 10.1016/s0006-8993(97)01334-6. [DOI] [PubMed] [Google Scholar]
- 40.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J. Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenz C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89:1480–1488. doi: 10.1097/00000542-199812000-00026. [DOI] [PubMed] [Google Scholar]
- 42.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 43.McGaugh JL, Izquierdo I. The contribution of pharmacology to research on the mechanisms of memory formation. Trends Pharmacol. Sci. 2000;21:208–210. doi: 10.1016/s0165-6147(00)01473-5. [DOI] [PubMed] [Google Scholar]
- 44.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 45.Mihic SJ, Harris RA. Inhibition of rho1 receptor GABAergic currents by alcohols and volatile anesthetics. J. Pharmacol. Exp. Ther. 1996;277:411–416. [PubMed] [Google Scholar]
- 46.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 47.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Moore GD, Walker AM, MacLaren R. Fospropofol: a new sedative-hypnotic agent for monitored anesthesia care. Ann. Pharmacother. 2009;43:1802–1808. doi: 10.1345/aph.1M290. [DOI] [PubMed] [Google Scholar]
- 49.Nakahiro M, Yeh JZ, Brunner E, Narahashi T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989;3:1850–1854. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- 50.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat. Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 51.Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003;99:678–684. doi: 10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa K, MacIver MB. Agent-selective effects of volatile anesthetics on GABAA receptor-mediated synaptic inhibition in hippocampal interneurons. Anesthesiology. 2001;94:340–347. doi: 10.1097/00000542-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 53.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson JE, Garcia PS, O'Toole KK, Derry JM, Bell SV, Jenkins A. A conserved tyrosine in the beta2 subunit M4 segment is a determinant of gamma-aminobutyric acid type A receptor sensitivity to propofol. Anesthesiology. 2007;107:412–418. doi: 10.1097/01.anes.0000278875.36639.2c. [DOI] [PubMed] [Google Scholar]
- 55.Sanna E, Garau F, Harris RA. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol. Pharmacol. 1995;47:213–217. [PubMed] [Google Scholar]
- 56.Sanna E, Mascia MP, Klein RL, Whiting PJ, Biggio G, Harris RA. Actions of the general anesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. J. Pharmacol. Exp. Ther. 1995;274:353–360. [PubMed] [Google Scholar]
- 57.Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at gamma-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol. Pharmacol. 1997;51:484–490. [PubMed] [Google Scholar]
- 58.Schofield CM, Jenkins A, Harrison NL. A highly conserved aspartic acid residue in the signature disulfide loop of the alpha 1 subunit is a determinant of gating in the glycine receptor. J. Biol. Chem. 2003;278:34079–34083. doi: 10.1074/jbc.M302416200. [DOI] [PubMed] [Google Scholar]
- 59.Sebel LE, Richardson JE, Singh SP, Bell SV, Jenkins A. Additive effects of sevoflurane and propofol on gamma-aminobutyric acid receptor function. Anesthesiology. 2006;104:1176–1183. doi: 10.1097/00000542-200606000-00012. [DOI] [PubMed] [Google Scholar]
- 60.Sekine S, Matsumoto S, Issiki A, Kitamura T, Yamada J, Watanabe Y. Changes in expression of GABAA alpha4 subunit mRNA in the brain under anesthesia induced by volatile and intravenous anesthetics. Neurochem. Res. 2006;31:439–448. doi: 10.1007/s11064-005-9024-4. [DOI] [PubMed] [Google Scholar]
- 61.Shafer SL, Hendrickx JF, Flood P, Sonner J, Eger EI. 2nd Additivity versus synergy: a theoretical analysis of implications for anesthetic mechanisms. Anesth. Analg. 2008;107:507–524. doi: 10.1213/ane.0b013e31817b7140. [DOI] [PubMed] [Google Scholar]
- 62.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABA(A) receptor genes in the human genome. J. Biol. Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 63.Steinbach JH, Akk G. Modulation of GABA(A) receptor channel gating by pentobarbital. J. Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoelting RK, Longnecker DE, Eger EI. 2nd Minimum alveolar concentrations in man on awakening from methoxyflurane, halothane, ether and fluroxene anesthesia: MAC awake. Anesthesiology. 1970;33:5–9. doi: 10.1097/00000542-197007000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Thyagarajan R, Ramanjaneyulu R, Ticku MK. Enhancement of diazepam and gamma-aminobutyric acid binding by (+)etomidate and pentobarbital. J. Neurochem. 1983;41:578–585. doi: 10.1111/j.1471-4159.1983.tb04778.x. [DOI] [PubMed] [Google Scholar]
- 66.Tierney ML, Birnir B, Pillai NP, Clements JD, Howitt SM, Cox GB, Gage PW. Effects of mutating leucine to threonine in the M2 segment of alpha1 and beta1 subunits of GABAA alpha1beta1 receptors. J. Membr. Biol. 1996;154:11–21. doi: 10.1007/s002329900128. [DOI] [PubMed] [Google Scholar]
- 67.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–717. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 68.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Preparation of barbiturate optical isomers and their effects on GABA(A) receptors. Anesthesiology. 1999;90:1714–1722. doi: 10.1097/00000542-199906000-00029. [DOI] [PubMed] [Google Scholar]
- 69.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying SW, Werner DF, Homanics GE, Harrison NL, Goldstein PA. Isoflurane modulates excitability in the mouse thalamus via GABA-dependent and GABA-independent mechanisms. Neuropharmacology. 2009;56:438–447. doi: 10.1016/j.neuropharm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J. Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeller A, Arras M, Jurd R, Rudolph U. Mapping the contribution of beta3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharmacol. 2007;7:2. doi: 10.1186/1471-2210-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Sonner JM, Eger EI, 2nd Stabernack CR, Laster MJ, Raines DE, Harris RA. Gamma-aminobutyric acidA receptors do not mediate the immobility produced by isoflurane. Anesth. Analg. 2004;99:85–90. doi: 10.1213/01.ANE.0000118108.64886.42. [DOI] [PubMed] [Google Scholar]