Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorder. Worldwide prevalence of the disease is estimated at more than 24 million cases. With aging of populations, this number will likely increase to more than 80 million cases by the year 2040. The annual incidence worldwide is estimated at 4.6 million cases which is the equivalent of one new case every seven seconds! The pathophysiology of AD is complex and largely misunderstood. It is thought to start with the accumulation of beta-amyloid (Aβ) that leads to deposition of insoluble neuritic or senile plaques. Secondary events in this “amyloid cascade” include hyperphosphorylation of the protein tau into neurofibrillary tangles, inflammation, oxidation, and excitotoxicity that eventually cause activation of apoptotis, cell death and neurotransmitter deficits. This review will briefly summarize recent advances in the pathophysiology of AD and focus on the pharmacological treatment of the cognitive and functional symptoms of AD. It will discuss the roles of vascular prevention, cholinesterase inhibitors and an NMDA-antagonist in the management of AD. It will address the issues thought to be related to the lack of persistence or discontinuation of therapy with cholinesterase inhibitors shown in recent studies and some of the solutions proposed. These include setting realistic expectations in light of a neurodegenerative condition and available symptomatic treatments, slowly titrating medications, and using alternate routes of administration. Finally, it will introduce future therapeutic options currently under study.
Keywords: Dementia, Alzheimer’s, therapy, pharmacological, cholinesterase inhibitor, memantine.
INTRODUCTION
The year 2006 marked the centennial of the description by Alois Alzheimer of the first case of the disease that will eventually hold his name. Alzheimer’s disease (AD) is the most common neurodegenerative disorder. Worldwide prevalence of dementia is estimated at more than 24 million cases [44]. The annual incidence worldwide is estimated at 4.6 million cases which is the equivalent of one new case every seven seconds ! [44]. With aging of populations, this number will double every twenty years, likely increasing to more than 80 million cases by the year 2040 [44]. Aging is amongst the major risk factors for the disease. For example, in the Canadian Study on Health and Aging, close to 10% of all individuals aged 65 and older were affected by dementia and this rose to 35% in individuals aged 85 and older [21]. Most cases of dementia are due to AD with or without a cerebrovascular contribution [23]. The global burden of disability associated with dementia in the elderly is believed to be higher than in stroke, musculoskeletal disease, heart disease and cancer. Finally, the annual cost of dementia in the UK (17 billion pounds) is estimated to be higher than the costs of heart disease, stroke and cancer combined [19]. This review will briefly summarize recent advances in understanding the pathophysiology of AD and focus on the pharmacological treatment of cognitive and functional symptoms of the disease. It will discuss the roles of vascular prevention, cholinesterase inhibitors, and an NMDA-antagonist in the management of AD. It will address the issues thought to be related to the lack of persistence or discontinuation of therapy with cholinesterase inhibitors shown in recent studies and some of the solutions proposed. These include setting realistic expectations, slow titration of the medications, and alternate routes of administration. It will introduce future therapeutic options currently under study.
PATHOPHYSIOLOGY
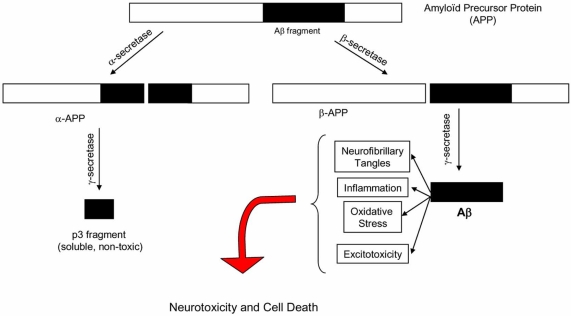
The pathophysiology of AD is complex and incompletely understood. Several hypotheses have been suggested to explain the series of events that eventually lead to the clinical manifestations of the disease. Presently, the two main competing hypotheses are the amyloid-cascade hypothesis and the hyperphosphorylation of protein tau hypothesis, which are ardently supported by the so-called “baptists” and “tauists” respectively [92]. These hypotheses are represented histologically by the two hallmarks that are still used today to reach a definite diagnosis of AD: amyloid plaques and neurofibrillary tangles (NFT) [16, 17]. In AD, amyloid plaques are found in the entorhinal cortex, the hippocampus and neocortical areas [37, 111, 131]. These plaques are also observed in the brains of normal aged individuals and do not correlate with progression of dementia. Neurofibrillary tangles are deposited in a hierarchical and systematic fashion that correlates closely with cognitive decline in AD [7, 18, 67]. They start in the entorhinal cortex and sequentially spread to the hippocampus, the rest of the temporal lobe, the association areas of the prefrontal and parietal cortices, and eventually reach all neocortical areas [35]. Neurofibrillary tangles are also observed in other neurodegenerative disorders. Several arguments support the amyloid-cascade hypothesis as the predominant one. In humans, amyloid precursor protein (APP) found on chromosome 21undergoes cleavage by three enzymatic complexes: α-secretase, β-secretase, and γ-secretase [111, 112] (Fig. 1). Sequential cleavage of APP by α-secretase and γ-secretase leads to the production of a small, non-toxic, and soluble peptide, referred to as p3. The sequential cleavage of APP by β-secretase and γ-secretase leads to the production of the insoluble beta-amyloid protein that deposits into plaques. Beta-amyloid (Aβ) is neurotoxic in vitro [27, 81, 89]. The mouse model of AD over-expressing the Amyloid Precursor Protein (APP) gene shows amyloid plaques similar to the ones found in the brains of AD patients. These mice also display short-term memory and learning deficits on specifically designed tests [66]. In humans, all individuals with clinical AD have abnormal deposits of Aβ (diffuse or neuritic plaques) [59]. Individuals with Down’s syndrome, who have three copies of chromosome 21, almost invariably express clinical and pathological features of AD by mid-life [62]. All mutations described in the rare autosomal-dominant forms of AD affect APP and γ-secretase and are associated with increased deposition of Aβ [3, 75, 83, 107]. Apolipoprotein E4, the main genetic risk factor for sporadic AD, is associated with increased production and deposition of Aβ [25, 108]. Generation of specific antibodies against Aβ in individuals with AD has been associated with mild and inconsistent clinical improvement and clearing of amyloid plaques in some patients [55, 62]. According to the amyloid-cascade hypothesis, abnormal generation (or insufficient clearance of Aβ) leads to several secondary events including hyperphosphorylation of the protein tau and generation of neurofibrillary tangles, inflammation, oxidation, and excitotoxicity [60, 84, 86]. These events lead in turn to the activation of the apoptotic cascade, neuronal cell death and neurotransmitter deficits. It is the deficit in acetylcholine, and to a lesser extent in norepinephrin and serotonin, that is thought to be responsible for the clinical manifestations of the disease [51].
Fig. (1).
Pathophysiology of Alzheimer ’s Disease.
The main alternate hypothesis to the amyloid cascade lends a central role to hyperphosphorylation of the protein tau. This protein normally binds and stabilizes microtubules, the main component of the cellular cytoskeleton. In AD, tau is hyperphosphorylated leading to the formation of paired helical filaments (PHF) and eventually NFTs [56, 74, 125]. These structures disrupt neuronal transport and eventually lead to cell death. The specific timing and chronology of the interaction between deposition of Aβ and hyperphosphorylation of tau is still controversial. Animal studies show that the presence of abnormal tau potentiates Aβ toxicity [2, 102]. This is supported by clinicopathological data suggesting that NFTs mediate the effect of amyloid deposition on clinical disease [5].
Recent data suggest that cerebrovascular disease plays an important role in the pathophysiology of AD. There are epidemiological, clinical and pathological similarities between AD and vascular dementia. Also, regional brain hypoperfusion leading to hypometabolism, degenerative changes and cognitive impairment have been described in early stages of AD. These principles are known as the Critically Attained Threshold of Cerebral Hypoperfusion (CATCH) theory [30]. According to this controversial hypothesis, regional hypoperfusion is the primary trigger of the degenerative cascade described earlier [1, 31, 32, 33]. Clinicopathological studies published recently show that most cases of dementia are due to a contribution of degenerative and cerebrovascular lesions (so-called “mixed dementia”) [91, 109]. In fact, some studies suggest that as individuals age, the contribution of amyloid plaques and NFTs to clinical dementia decreases [42, 106], and that concomitant cerebrovascular lesions play a crucial role in modulation of the clinical expression of cognitive impairment [118, 126].
PHARMACOLOGICAL TREATMENT
Clinical Outcomes in Clinical Trials
Alzheimer’s disease progressively impairs cognitive abilities and behaviour, leading to gradual functional decline. Outcome measures used in clinical trials are selected to mirror clinical hallmarks of the disease. Typically, these trials include a combination of measures evaluating cognition, clinicians’ and caregivers’ impression of change, functional abilities and behaviour. Pharmacological treatment of behavioural and psychological symptoms of dementia (BPSD) have been recently reviewed in this journal [77]. We will focus on cognitive, global, and functional outcomes. Cognitive outcome measures include the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog - scored from 0-70, a higher number indicating worse performance) [105], the Mini-Mental Status Examination (MMSE – scored from 0-30, a higher number indicating better performance) [49], and the Severe Impairment Battery (SIB – scored 0-100, a higher number indicating better performance) [97]. Global measures include the Clinical Global Impression of Change (CGIC – scored from 1 to 7, 4 indicating no change, 1 and 7 indicating significant improvement and significant deterioration respectively)[110], and the Clinician Interview-Based Impression of Change (CIBIC – scored from 1 to 7 similarly to the CGIC) [72]. Functional measures include the Progressive Deterioration Scale (PDS – scored from 0-100, a higher number indicating better performance) [34], the Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL – scored 0-78, a higher score indicating worse performance) [52], and the Disability Assessment for Dementia scale (DAD – scored 0-100%, a higher number indicating better performance) [54].
Vascular Prevention
Results of clinical trials in primary prevention of AD are contradictory. Three studies have shown that optimal use of antihypertensive agents in systolic and diastolic hypertension as well as after a cerebrovascular event is associated with a reduction in the incidence of cognitive impairment [50, 123, 127]. The recently completed Hypertension in the Very Elderly Study (HYVET) however showed that treatment of individuals 80 years of age or older is associated with a reduction in cardiovascular and cerebrovascuar end-points, but not in cognitive impairment [4, 98]. In two major primary prevention trials, cholesterol-lowering agents in individuals at risk lead to a reduction in cardiovascular and cerebrovascular events but had no benefit on cognitive outcomes [24, 114]. A secondary prevention trial in individuals with mild cognitive deficits (loosely defined as a MMSE between 20-28) comparing two treatments of systolo-diastolic hypertension did not show any difference between the drugs in terms of cognitive outcomes. Individuals with the best improvements in blood pressure measures benefited the most on working memory measures [119]. A prospective observational study shows that treatment of vascular risk factors in individuals with AD is associated with a slower cognitive decline [36]. There are no published intervention trials in tertiary vascular prevention in AD. These results have led the Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD) to recommend treatment of high blood pressure as an option in the prevention of cognitive decline (Grade B, Level I recommendation) [12].
Cholinesterase Inhibitors
Neurochemical studies of neurons from the basal forebrain of individuals with AD have shown a deficit in choline acetyl-transferase leading to a decrease in the production of acetylcholine and cortical cholinergic dysfunction [128]. These studies suggested that acetylcholine replacing or enhancing therapies might be of benefit in individuals with AD. Acetylcholine precursors and cholinergic receptor agonists were used in clinical trials, but abandoned for lack of effectiveness or intolerable side effects [13, 41, 124]. Cholinesterase inhibitors (ChEI) act by inhibiting the enzymes (acetyl- and butyryl-cholinesterase) responsible for the breakdown of acetylcholine hence increasing its availability at the synaptic cleft. In a within-subject pilot study including 13 individuals with Alzheimer’s disease and 18 healthy controls, physostigmine improved memory by optimizing extrastriate selectivity as detected by functional magnetic resonance imaging [6]. Three ChEI’s are widely available and will be discussed in this paper: donepezil, rivastigmine, and galantamine. They are considered symptomatic treatments as they improve symptoms without modifying the course of the disease.
Donepezil
Donepezil is a piperidine derivative that reversibly inhibits acetylcholinesterase [113]. It is very well absorbed after oral administration and reaches peak plasmatic concentration (Cmax) in 3-4 hours. Elimination half-life of donepezil is approximately 70 hours allowing once daily administration. It binds to plasma proteins in a proportion of 96%, and is metabolised by isoenzyme 2D6 and 3A4 of cytochrome P450. Starting and minimal effective dose is 5 mg once daily. Maximal recommended dose is 10 mg daily.
A recently published Cochrane review evaluating donepezil in AD included 24 trials and 5796 participants in mild to severe stages of the disease [9]. It showed statistically significant improvement versus placebo at 24 weeks (ADAS-Cog and SIB) and 52 weeks (MMSE). There was statistically significant improvement versus placebo on the CIBIC-plus at 24 weeks. There were also statistically significant benefits on the DAD at 24 weeks, the ADCS-ADL (version for moderate to severe AD) at 24 weeks, and PDS at 52 weeks. Overall, both the doses of 5 mg and 10 mg were beneficial, with the higher dose being marginally more effective. More side-effects were reported with donepezil than with placebo. Most common side-effects were nausea, vomiting, diarrhoea, muscle cramps, dizziness, fatigue, and anorexia, and they were dose-dependent. The authors conclude: “People with mild, moderate or severe dementia due to AD treated for periods of 12, 24, 52 weeks with donepezil experienced benefits in cognitive function, activities of daily living…Study clinicians rated global clinical state more positively in treated patients, and measured less decline in measures of global disease severity.” A positron emission tomography (PET) evaluated acetylcholinesterase (AChE) activity in 14 individuals with AD before and after treatment with donepezil [14]. The results show a modest inhibition of AChE with treatment that is most apparent in the cingulated cortex, and correlates with performance on tests of executive function and attention. A single photon emission computerized tomography (SPECT) study in 51 patients with AD before and after a 10-14 month treatment period with donepezil showed differential perfusion patterns in stabilized and non-stabilized individuals (based on their MMSE scores) [115]. In non-stabilized patients, there was lower cerebral blood flow on SPECT in the lateral and medial frontal lobes, limbic lobe, lower lateral temporal lobe, and cingulated gyrus compared to the stabilized patients. The authors conclude that SPECT may be useful in evaluating the brain function response to ChEI therapy.
Rivastigmine
Rivastigmine is a carbamate derivative that reversibly inhibits both acetyl- (AChE) and butyryl- (BuChE) cholinesterase [95]. It is the only ChEI with significant inhibition of BuChE. Butyrylcholinesterase is widely distributed in the central nervous system and may play a role in cholinergic function and neurodegeneration [29]. It is unclear how specific BuChE inhibition relates to rivastigmine’s clinical effect. Rivastigmine is well absorbed after oral administration and reaches Cmax in one hour. Its elimination half-life is approximately 1 to 2 hours. It binds to proteins in a proportion of 40%, is hydrolysed by esterases (including cholinesterases), and is excreted in the urine. Cytochrome P450 isoenzymes are not involved in the metabolism of rivastigmine hence minimizing drug-drug interactions. Starting dose of rivastigmine is 1.5 mg twice a day and can be gradually titrated to the maximal dose of 6 mg twice a day. The minimal effective dose is 3mg twice a day. A transdermal form of rivastigmine has been developed and is available on most markets since 2008 [10, 130]. The main objective of transdermal rivastigmine is to allow titration to the highest (and most therapeutic) doses of the medication while minimizing side effects. This is achieved by slow release of the medication into the circulation as demonstrated by a Cmax of 8 hours by transdermal route. Starting dose of transdermal rivastigmine is 5 cm2, and the effective and maximal dose is 10 cm2.
A Cochrane review evaluating rivastigmine in AD has recently been updated and includes 9 trials and 4775 patients [8]. It shows that rivastigmine at high doses (6 to 12 mg daily) is associated with statistically significant improvement versus placebo at 26 weeks on cognitive (ADAS-Cog), global (CIBIC-plus) and functional outcomes (PDS). The results are more subtle on lower doses (4 mg daily or lower), reaching statistical significance for cognitive outcomes only. More side-effects were reported with rivastigmine than with placebo and they were dose-dependent. Most common side-effects were nausea, vomiting, diarrhoea, anorexia, headache, syncope, abdominal pain and dizziness. The recently published Investigation of transDermal Exelon in Alzheimer’s disase (IDEAL) study compared two doses of transdermal rivastigmine with the oral form [130]. The higher 20 cm2 dose (not commercially available) was not associated with more clinical benefit and led to more side-effects. The smaller 10 cm2 dose which is the highest commercially available dose was associated with similar clinical benefit than the maximal oral dose, but with significantly less gastrointestinal side-effects. Neither dose of transdermal rivastigmine led to behavioural benefits compared to placebo. The authors of the Cochrane review conclude: “ Rivastigmine appears to be beneficial for people with mild to moderate Alzheimer’s disease…improvements were seen in the rate of decline of cognitive function, activities of daily living, and severity of dementia…”. A plasma and Cerebrospinal fluid (CSF) biomarker study evaluated the long-term ChEI inhibitory effect of rivastigmine in eleven patients with AD treated for 12 months. This uncontrolled study showed persistent inhibition of both AChE and BuChE in plasma as well as CSF [28]. This contrasts with studies with other ChEI’s which are usually associated with up-regulation of cholinesterases. This up-regulation is sometimes invoked to explain the relatively short duration of effect of these medications. However, the relative “lack” of up-regulation of ChE with rivastigmine has not been unequivocally shown to relate to more sustained clinical benefit.
Galantamine
Galantamine is a tertiary alkaloid agent that reversibly inhibits AChE [103]. It also binds allosterically to nicotinic receptors enhancing cholinergic function. The clinical relevance of allosteric binding to nicotinic receptors in humans is unclear. Galantamine is rapidly absorbed after oral administration and reaches Cmax in approximately 1 hour. Elimination half-life is between 7 to 8 hours. It binds to plasma proteins in a proportion of 18% and is metabolized by isoenzyme 2D6 and 3A4 of cytochrome P450. Galantamine is commercialized as an extended-release formulation allowing once-daily dosing. Starting dose of galantamine ER is 8 mg once daily. Minimal effective dose is 16 mg daily, and maximal dose is 24 mg daily.
A recently published Cochrane review evaluating galantamine in mild to moderate AD and Mild Cognitive Impairment (prodrome of dementia) included 10 trials and a total of 6805 participants [80]. It showed statistically significant improvement versus placebo at 24 weeks on the ADAS-Cog, and the CIBIC-plus. Functional measures (ADCS-ADL and DAD) were seldom included in these clinical trials, but they showed statistical significance in individual trials. In contrast to other clinical trials with ChEI, there was no consistent dose-response effect in doses above 8mg daily. Galantamine’s side effects are comparable to other ChEI’s and consist mainly of cholinergic gastrointestinal symptoms. The authors conclude: “…this review shows consistent positive effects for galantamine for trials of three to six months’ duration. Although there was not a statistically significant dose-response effect, doses above 8mg/d were, for the most part, consistently statistically significant.” A randomized trial of galantamine in individuals with vascular dementia and AD with a cerebrovascular contribution (so-called “mixed dementia”) showed statistically significant benefits versus placebo on the ADAS-Cog, the CIBIC-plus, and the DAD [40]. The results of this trial has led a group of experts to recommend galantamine as “a treatment option for mixed AD with cerebrovascular disease” (Grade B, Level I recommendation) [12]. A PET study evaluated the effects of galantamine treatment on AChE and nicotinic receptor binding in 18 patients with mild AD over 12 months [68]. It shows sustained cortical inhibition of AChE. Overall, there was no statistically significant change in nicotine binding. However, both cholinesterase inhibition and nicotine binding correlated positively with performance on a test of attention in these patients. A recently published study used functional magnetic resonance imaging in two tasks of visual perception in 8 individuals with AD before and after a three-month trial of galantamine [15]. It showed a decrease in the activation of the dorsal visual pathway with treatment suggestive of a more efficient processing of visual stimuli or a modification in the compensatory changes observed in AD.
Comparative Studies of Cholinesterase Inhibitors
Four clinical trials compared the ChEI’s in AD and led to conflicting results [58]. A careful review of the methodology of three of these trials using the Consolidated Standards of Reporting Trial (CONSORT) checklist concluded that 27% to 55% of these criteria per study were inadequately reported [64]. Considering these serious methodological limitations in comparison studies, one cannot favour one ChEI over the other at this point. Nevertheless, differences in tolerability were noted in one meta-analysis and led authors to conclude that “ across studies, the frequency in which these (adverse) events were reported was generally lowest for donepezil and highest for rivastigmine” [58]. On this issue, the CCCDTD concludes: “Although all three ChEI’s available in Canada have efficacy for mild to moderate AD, equivalency has not been established in direct comparison. Selection of which agent to be used will be based on adverse effect profile, ease of use, familiarity, and beliefs about the importance of the differences between the agents in their pharmacokinetics and other mechanisms of action.” [63].
Memantine
Studies have shown that enhancement of the excitatory effects of the neurotransmitter glutamate may play a role in the pathogenesis of AD (theory of “excitotoxicity”) [120]. Memantine is a N-methyl-D-aspartate (NMDA) non-competitive glutatmate receptor antagonist [70]. It is well absorbed after oral administration and reaches Cmax in 3 to 8 hours. Elimination half-life is 60-80 hours. It binds to proteins in a proportion of 45%, and is almost completely excreted unchanged in the urine. Starting dose is 5 mg daily (in one or two doses). Minimal therapeutic dose is 10 mg daily, and maximal dose is 20 mg daily.
The Cochrane review evaluating memantine included 3 clinical trials in moderate to severe AD and three unpublished studies in mild to moderate AD [90]. In moderate to severe AD, pooled data show statistically significant benefits versus placebo at 6 months on measures of cognition (SIB), function (ADCS-ADL severe version), and global impression (CIBIC-plus). In mild to moderate AD, analyses show statistically significant benefits on measures of cognition (ADAS-cog) that do not translate into significant differences on measures of global impression and function. Memantine is generally well tolerated. Some studies suggest a reduced frequency of agitation and agressivity versus placebo, although the meta-analysis suggests an overall increased risk of agitation compared to placebo. The authors conclude: “Memantine has a small beneficial effect at six months in moderate to severe AD.” In most countries where it is available, memantine is approved for treatment of moderate to severe AD. A clinical trial compared the added efficacy of memantine in patients with moderate to severe AD receiving stable doses of donepezil [122]. It shows additional benefits of memantine versus placebo on the SIB, ADCS-ADL, and CIBIC-plus. Theses results suggest that memantine has an additive effect on ChEI’s in moderate to severe AD. A randomized placebo-controlled study is under way in Canada to determine if memantine reduces the incidence and severity of agitation and agressivity.
Cost-Effectiveness of Symptomatic Treatments
Relatively few clinical trials have evaluated cost-effectiveness of pharmacological treatment in AD. The Cochrane review on donepezil considered the results of the two trials allowing cost-benefit evaluation and concluded: “There is some evidence that use of donepezil is neither more nor less expensive compared with placebo when assessing total health care resource cost.”[9, 46]. Several cost-effectiveness modeling studies have shown results in favour of ChEI [45]. These analyses are based mainly on the cognitive, functional and behavioural benefits provided by these medications that eventually allow delaying long-term institutionalisation. A significant portion of excess cost associated with AD is due to hospitalisations and post-acute care and this is difficult to include in cost-effectiveness models. The AD 2000 study aimed at evaluation pharmacoeconomic benefits of ChEI’s in AD. The main objectives of this non-industry sponsored clinical trial were to assess the effects of donepezil treatment on delaying institutionalisation and functional deterioration in mild to moderate AD [26]. It showed statistically significant benefits on cognitive and functional measures that did not lead to a delay in institutionalisation and functional deterioration. The authors of this study concluded: “donepezil is not cost-effective, with benefits below minimally relevant thresholds.” Unfortunately, this study suffers from several serious methodological limitations including a small number of individuals at the start of the trial compromising the power of analyses, repeated washout periods during follow-up, and significant attrition of patients with follow-up. Secondary analyses of other clinical trials and observational data have also shown results favouring pharmacological treatment. For example, secondary analyses of clinical trials with donepezil and galantamine show that the magnitude of “exposure” to the drug in terms of doses and duration is associated with a delay in institutionalisation [43, 53]. Two observational studies of patients evaluated and treated in AD research centers show that treatment with ChEI’s delays institutionalisation [78,79]. Memantine provides an additional benefit to ChEI in one study [78]. These results are encouraging but need to be validated in well-designed randomised clinical trials prospectively considering all aspects of resource utilization and indirect costs leading to a reliable and satisfying cost-effectiveness analysis.
Summary Of Clinical Benefit From Symptomatic Treatments and Recommendations
Overall, the benefits of symptomatic treatments in AD are modest. Some authors argue that the magnitude of this benefit, though statistically significant, is marginal at best and may even be difficult to detect, measure and quantify clinically [69, 100]. A metanalysis of 20 studies with ChEI’s specifically evaluated measurability and quantification of clinical benefit [104]. This study considered two measures of Effect Size (ES) as indicators of the magnitude of response leading it to be clinically detectable. Effect Size is determined by calculating the absolute difference in effect between the treatment and placebo groups, in relation to the variance of the given measure. Effect size is usually considered mild if between 0.2 and 0.4 and moderate when it falls between 0.41 and 0.7. The analyses show that median ES for ADAS-Cog varies from 0.15 to 0.47 depending on doses of ChEI. The same values were obtained for ES on the CIBIC-plus. The author concludes that ChEI’s produce benefits that are mild to moderate, which are dose-dependent, and more importantly, reproducible across clinical trials. Another metanalysis of 16 studies evaluated Numbers Needed to Treat (NNT) for clinical benefits and Numbers Needed to Harm (NNH) for side-effects of ChEI’s [73]. This study shows that 12 patients would need to be treated to observe minimal improvement or better on measures of cognition and global evaluation. The NNT for stabilisation or improvement is 7. NNH for one additional patient to experience an adverse event is 12.These figures compare very favourably to other well accepted interventions in medicine [47]. How do these results translate into clinical practice? In mild to moderate AD, clinical trials usually produce maximal improvement on cognitive measures after three months of treatment, but allow stabilisation of cognition for a period varying between 9 and 12 months. On measures of global impression, maximal improvement is observed at 3 months, and stabilisation usually lasts for about 6 months. On measures of function, the response usually observed is stabilisation rather than improvement. Functional capacities lost (for example managing finances or driving) are seldom recuperated with symptomatic treatment.
Published guidelines on symptomatic treatment for AD vary in the strength of their recommendations. Certain consensus guidelines consider at least a trial of ChEI and/or memantine as a standard approach in the pharmacological treatment of AD. These include the recommendations from the American Academy of Neurology [39] , the British Association of Psychopharmacology [20], the American Association for Geriatric Psychiatry [82], Recommendations for Best Practices in the Treatment of AD in Managed Care [47], and the Canadian Consensus Conference of Diagnosis and Treatment dementia [63]. For example, the CCCDTD states that “all three cholinesterase inhibitors available in Canada are modestly efficacious for mild to moderate AD. They are all viable options for most patients with mild to moderate AD” (Grade A, Level I recommendations). The AAN recommends that: “cholinesterase inhibitors should be considered in patients with mild to moderate AD (Standard), although studies suggest a small average degree of benefit.” The consensus statement from the British Association for Psychopharmacology states: “There is type 1a evidence for the efficacy of memantine in the treatment of moderate to severe Alzheimer’s disease”. Conversely, the National Institute for Health and Clinical Excellence (NICE) in the UK made the following recommendations based on a cost-effectiveness evaluation of ChEI’s in AD: “The three acethylcholinesterase inhibitors donepezil, galantamine, and rivastigmine are recommended as options in the management of patients with Alzheimer’s disease of moderate severity only.” [93]. They add: “Memantine is not recommended as a treatment option for patients with moderately severe to severe Alzheimer’s disease except as part of well designed clinical studies”. The clinical practice guidelines from the American College of Physicians and the American Academy of Family Physicians conclude : “Clinicians should base the decision to initiate a trial of therapy with a cholinesterase inhibitor or memantine on individualized assessment.” [99, 100]. This assessment should take into consideration the “clinically marginal” benefits, the heterogeneous and unpredictable response in an individual patient, the potential for side-effects, and stage of the disease.
Drug Utilisation Studies of Pharmacological Treatments in AD
Two observational administrative database studies evaluated persistence with the three available ChEI in two Canadian provinces [61, 85]. The study from Quebec included 18748 individuals and the study from Ontario 5622 individuals, all aged 65 and older. Persistence rates at one year varied between 40% and 54% among individual ChEI’s in the Ontario study. In the Quebec study, the overall persistence rate for the drug class was 40% at one year. Both studies showed better persistence with galantamine as compared to donepezil and rivastigmine. The reason for this finding is unclear, but the reproducibility of this result across two different provinces is intriguing. Two studies from the US evaluated persistence with donepezil and rivastigmine [117, 121]. While mean duration of use were well below one year for both drugs, there was no difference in persistence between the two. Among the hypothesis evoked to explain these disappointingly low rates of persistence with ChEI’s are: tolerance, lack of familiarity with the drugs by primary care physicians, and unrealistic expectations by physicians, patients and their caregivers. Tolerance to these medications can be optimized by starting at low doses and slowly and individually titrating in no less than four-week interval periods. Alternate routes of administration such as the transdermal formulation of rivastigmine can significantly enhance tolerance by minimizing usual cholinergic side-effects. Alzheimer’s disease is a progressive neurodegenerative disease and available treatments do not modify progression of the process. Expectations with ChEI treatment need to be adapted to these realities. In that sense, even stabilisation of clinical symptoms for 6 to 12 months is considered an acceptable response to treatment. Slower deterioration for individual patients is more challenging to establish in clinical practice than in clinical trials where data from placebo groups are readily available for comparison. Clinically, an individual patient’s deterioration can be compared to published data on the natural history of progression of untreated AD [47]. Published recommendations are vague as to the determinants of the duration of pharmacological treatment in AD and the specific criteria for discontinuation.
FUTURE TREATMENTS
Dimebon (Latrepirdine)
Latrepirdine is a non-selective anti-histaminic agent that has been used as an anti-allergic medication in Eastern Europe. In vitro and in animal models of AD, it has been shown to have mitochondrial stabilizing properties leading to improvement in neuronal function and inhibition of cell death [38]. In a single-country multi-site randomised clinical trial, latrepirdine was shown to be significantly superior to placebo on measures of cognition (ADAS-cog and MMSE), global evaluation (CIBIC), function (ADCS-ADL) and behaviour (NPI) at 26 weeks. It was relatively well tolerated, the most common adverse events being dry mouth and depressed mood. Several large scale phase III randomised clinical trials with latrepirdine are ongoing. If these trials replicate the findings of the initial study, latrepirdine may very well be the next pharmacological agent to be commercialized for symptomatic treatment of AD.
Other Treatments
All currently available treatment options for AD are symptomatic and do not modify natural progression of the disease. Disease-modifying approaches are constantly being explored. Several agents under study target the amyloïd cascade. They can be divided into three general categories: agents aiming at decreasing the production of Aβ, at reducing its aggregation, or at increasing its clearance [57, 71]. Agents that can decrease production of Aβ include gamma-secretase inhibitors [48, 116] or modulators [129] as well as alpha-secretase activators [76]. For Aβ to aggregate into insoluble toxic plaques, it needs to bind to metallic ions such as copper and zinc. Chelators inhibiting this binding such as clioquinol [101] have been proposed and studied in AD. Cyclohexanehexol [88] inhibits aggregation of Aβ into high molecular weight toxic oligomers and has been shown to reverse pathological and clinical hallmark of AD in animal models. Recently completed, but yet unpublished, phase III clinical trials with the gamma-secretase modulator flurizan and the amyloid-binding inhibitor tramiprosate showed largely negative results on primary end-points. Optimizing clearance of Aβ and its oligomers by immunization seemed very promising in animal models of AD [87]. A pilot study in patients with AD lead to an unexpected high rate of meningoencephalitis leading to interruption of the trial [96]. Neuropathologic findings in some patients enrolled in this study show a clear association between high pre-mortem serum antibody response and clearance of amyloid deposits in the brain cortex. However, there was no association between antibody response, amyloid deposition, and clinical findings [11, 65]. The immunologic approach in AD is still being tested in trials of passive immunization with intravenous immunoglobulins and monoclonal antibodies against Aβ [132]. Several of the agents described are currently in phase II-III clinical trials.
Inhibition of protein tau phosphorylation is also being explored in animal models and pilot human studies in AD. Agents in this category include glycogen synthase kinase 3-β (GSK3-β) inhibitors such as lithium [94] and valproate [134], and microtubule stabilizing agents such as paclitaxel [133]. A recently completed but unpublished phase III clinical trial with valproate is negative.
Other agents being tested target oxidative stress in AD, neuroinflammation, cholesterol metabolism and the neuroendocrine pathways [22, 71].
Development of disease-modifying treatments in AD faces many challenges that will need to be considered in future research and clinical trials. Novel pharmacological agents will need to cross the blood-brain barrier and be readily bioavailable to the central nervous system. Several of the secretases being targeted have numerous substrates some of which are required for normal function. Hence, total inhibition rather than modulation may lead to unacceptable adverse events. Transferring results of animal model research to humans has been fraught with obstacles as demonstrated by the immunization trials. Finally, methodology in clinical trials will need to be adapted to detect disease-modifying effect, and this may lead to longer and more expensive studies.
CONCLUSIONS
In conclusion, AD is a common and costly disease. Currently several symptomatic treatments are available that provide mild benefits that are nevertheless dose-dependent, clinically detectable, and reproducible across clinical trials. Sustained use of these medications needs to be improved for optimal benefit. This can be achieved by tailoring doses and titration to the individual patients to enhance tolerance, and mostly, setting realistic expectations in face of a neurodegenerative disease and symptomatic treatments. Several agents under study are potentially disease-modifying and may significantly alter the otherwise inexorable deterioration in AD. It is such treatments that will significantly improve clinical symptoms of the disease and lead to robust pharmacoeconomic benefits such as the reduction of resource utilisation. However, when expensive disease-modifying agents will become available, they will present the clinician with many clinical and ethical challenges as to their indications, decisions to combine or discontinue treatments. These challenges will need to be considered in future clinical trials.
Table 1.
Pharmacologic Properties of Widely Approved Alzheimer’s Disease Treatments
| Name | Mechanism of Action | Elimination Half-life | Protein Binding | Metabolism | Starting Dose | Effective Dose | Maximal Dose |
|---|---|---|---|---|---|---|---|
| Donepezil | .Acetylcholinesterase inhibitor .Piperidine derivative |
70 hours | 96% | .Hepatic .Cytochrome P450 – 2D6 and 3A4 |
5mg once daily | 5mg once daily | 10 mg once daily |
| Rivastigmine (Oral) | .Acetylcholinesterase inhibitor .Butyrylcholinesterase inhibitor .Carbamate derivative |
1-2 hours | 40% | Urinary | 1.5 mg twice daily | 3.0 mg twice daily | 6 mg twice daily |
| Rivastigmine (Transdermal) | .Acetylcholinesterase inhibitor .Butyrylcholinesterase inhibitor .Carbamate derivative |
1-2 hours | 40% | Urinary | 5 cm2 | 10 cm2 | 10 cm2 |
| Galantamine ER | .Acetylcholinesterase inhibitor .Nicotinic allosteric agonist .Tertiary alkaloid |
7-8 hours | 18% | .Hepatic .Cytochrome P450 – 2D6 and 3A4 |
8 mg once daily | 16 mg once daily | 24 mg once daily |
| Memantine | .N-Methyl-D-Aspartate (NMDA) non-competitive receptor antagonist | 60-80 hours | 45% | Urinary | 5 mg daily (in 1 or 2 doses) | 10 mg daily (in 1 or 2 doses) | 20 mg daily (in 1 or 2 doses) |
DISCLOSURES
Fadi Massoud and Serge Gauthier have received speaker’s honoraria, consultant fees, and research support from Pfizer, Janssen Ortho Inc., Novartis and Lundbeck.
REFERENCES
- 1.Aliev G, Smith M. A., Obrenovich M. E., de la Torre J. C., Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox. Res. 2003;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- 2.Ashe K. H. A tale about tau. N. Engl. J. Med. 2007;357:933–935. doi: 10.1056/NEJMcibr073552. [DOI] [PubMed] [Google Scholar]
- 3.Barton A. J., Crook B. W., Karran E. H., Brown F, Dewar D, Mann D. M., Pearson R C, Graham D. I., Hardy J, Hutton M, Duff K, Goate A. M., Clark R. F., Roberts G. W. Alteration in brain presenilin 1 mRNA expression in early onset familial Alzheimer's disease. Neurodegeneration. 1996;5:213–218. doi: 10.1006/neur.1996.0029. [DOI] [PubMed] [Google Scholar]
- 4.Beckett N. S., Peters R, Fletcher A. E., Staessen J. A., Liu L, Dumitrascu D, Stoyanovsky V, Antikainen R. L., Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt C. J. Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 5.Bennett D. A., Schneider J. A., Wilson R. S., Bienias J. L., Arnold S. E. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch. Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 6.Bentley P, Driver J, Dolan R. J. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain. 2009;132:2356–2371. doi: 10.1093/brain/awp176. [DOI] [PubMed] [Google Scholar]
- 7.Billingsley M. L., Kincaid R. L. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birks J, Grimley Evans J, Iakovidou V, Tsolaki M, Holt F E. Rivastigmine for Alzheimer's disease. Cochrane Database. Syst. Rev. 2009;2:CD001191. doi: 10.1002/14651858.CD001191. [DOI] [PubMed] [Google Scholar]
- 9.Birks J, Harvey R J. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst. Rev. 2009:CD001190. doi: 10.1002/14651858.CD001190. DOI:10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Blesa R, Ballard C, Orgogozo J M, Lane R, Thomas S K. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology. 2007;69:S23–S28. doi: 10.1212/01.wnl.0000281848.25142.11. [DOI] [PubMed] [Google Scholar]
- 11.Boche D, Zotova E, Weller R O, Love S, Neal J W, Pickering R M, Wilkinson D, Holmes C, Nicoll J A. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131:3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 12.Bocti C, Black S, Frank C. Management of dementia with a cerebrovascular component. Alzheimers. Dement. 2007;3:398–403. doi: 10.1016/j.jalz.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Bodick N C, Offen W W, Levey A I, Cutler N R, Gauthier S G, Satlin A, Shannon H E, Tollefson G D, Rasmussen K, Bymaster F P, Hurley D J, Potter W Z, Paul S M. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 14.Bohnen N I, Kaufer D I, Hendrickson R, Ivanco L S, Lopresti B J, Koeppe R A, Meltzer C C, Constantine G, Davis J G, Mathis C A, Dekosky S T, Moore R Y. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bokde A L, Karmann M, Teipel S J, Born C, Lieb M, Reiser M F, Moller H J, Hampel H. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease: a functional magnetic resonance imaging study. J. Clin. Psychopharmacol. 2009;29:147–156. doi: 10.1097/JCP.0b013e31819a8f2e. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 18.Brion J P, Anderton B H, Authelet M, Dayanandan R, Leroy K, Lovestone S, Octave J N, Pradier L, Touchet N, Tremp G. Neurofibrillary tangles and tau phosphorylation. Biochem. Soc. Symp. 2001;67:81–88. doi: 10.1042/bss0670081. [DOI] [PubMed] [Google Scholar]
- 19.Burns A, Iliffe S. Dementia. BMJ. 2009;338:b75. doi: 10.1136/bmj.b75. [DOI] [PubMed] [Google Scholar]
- 20.Burns A, O'Brien J, Auriacombe S, Ballard C, Broich K, Bullock R, Feldman H, Ford G, Knapp M, McCaddon A, Iliffe S, Jacova C, Jones R, Lennon S, McKeith I, Orgogozo J M, Purandare N, Richardson M, Ritchie C, Thomas A, Warner J, Wilcock G, Wilkinson D. Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J. Psychopharmacol. 2006;20:732–755. doi: 10.1177/0269881106068299. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Study of Health and Aging. Canadian study of health and aging: study methods and prevalence of dementia. CMAJ. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Zhang X J, Li L, Le W D. Current experimental therapy for Alzheimer's disease. Curr. Neuropharmacol. 2007;5:127–134. doi: 10.2174/157015907780866901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertkow H. Diagnosis and treatment of dementia: introduction. CMAJ; Introducing a series based on the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia; 2008. pp. 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 25.Corder E H, Saunders A M, Strittmatter W J, Schmechel D E, Gaskell P C, Small G W, Roses A D, Haines J L, Pericak-Vance M A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 26.Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, Edwards S, Hardyman W, Raftery J, Crome P, Lendon C, Shaw H, Bentham P. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 2004;363:2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 27.Cummings J L. Alzheimer's disease. N. Engl. J. Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 28.Darreh-Shori T, Almkvist O, Guan Z Z, Garlind A, Strandberg B, Svensson A L, Soreq H, Hellstrom-Lindahl E, Nordberg A. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002;59:563–572. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]
- 29.Darvesh S, Hopkins D A, Geula C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 30.de la Torre J C. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer's pathogenesis. Neurobiol. Aging. 2000;21:331–342. doi: 10.1016/s0197-4580(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 31.de la Torre J C. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 32.de la Torre J C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 33.de la Torre J C. Pathophysiology of neuronal energy crisis in Alzheimer's disease. Neurodegener. Dis. 2008;5:126–132. doi: 10.1159/000113681. [DOI] [PubMed] [Google Scholar]
- 34.DeJong R, Osterlund O W, Roy G W. Measurement of quality-of-life changes in patients with Alzheimer's disease. Clin. Ther. 1989;11:545–554. [PubMed] [Google Scholar]
- 35.Delacourte A, David J P, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 36.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–680. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- 37.Dickson D W. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Doody R S, Gavrilova S I, Sano M, Thomas R G, Aisen P S, Bachurin S O, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 39.Doody R S, Stevens J C, Beck C, Dubinsky R M, Kaye J A, Gwyther L, Mohs R C, Thal L J, Whitehouse P J, Dekosky S T, Cummings J L. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 40.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju C V. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359:1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 41.Etienne P, Dastoor D, Gauthier S, Ludwick R, Collier B. Alzheimer disease: lack of effect of lecithin treatment for 3 months. Neurology. 1981;31:1552–1554. doi: 10.1212/wnl.31.12.1552. [DOI] [PubMed] [Google Scholar]
- 42.Ewbank D C, Arnold S E. Cool with plaques and tangles. N. Engl. J. Med. 2009;360:2357–2359. doi: 10.1056/NEJMe0901965. [DOI] [PubMed] [Google Scholar]
- 43.Feldman H H, Pirttila T, Dartigues J F, Everitt B, Van Baelen B, Schwalen S, Kavanagh S. Treatment with galantamine and time to nursing home placement in Alzheimer's disease patients with and without cerebrovascular disease. Int. J. Geriatr. Psychiatry. 2009;24:479–488. doi: 10.1002/gps.2141. [DOI] [PubMed] [Google Scholar]
- 44.Ferri C P, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes P R, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fillit H, Hill J. The economic benefits of acetylcholine-sterase inhibitors for patients with Alzheimer disease and associated dementias. Alzheimer Dis. Assoc. Disord. 2004;18(Suppl 1):S24–S29. doi: 10.1097/01.wad.0000127492.65032.d3. [DOI] [PubMed] [Google Scholar]
- 46.Fillit H, Hill J. Economics of dementia and pharmacoeconomics of dementia therapy. Am. J. Geriatr. Pharmacother. 2005;3:39–49. doi: 10.1016/j.amjopharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Fillit H M, Doody R S, Binaso K, Crooks G M, Ferris S H, Farlow M R, Leifer B, Mills C, Minkoff N, Orland B, Reichman W E, Salloway S. Recommendations for best practices in the treatment of Alzheimer's disease in managed care. Am. J. Geriatr. Pharmacother. 2006;4(Suppl A):S9–S24. doi: 10.1016/j.amjopharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Fleisher A S, Raman R, Siemers E R, Becerra L, Clark C M, Dean R A, Farlow M R, Galvin J E, Peskind E R, Quinn J F, Sherzai A, Sowell B B, Aisen P S, Thal L J. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch. Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folstein M F, Folstein S E, McHugh P R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 50.Forette F, Seux M L, Staessen J A, Thijs L, Birkenhager W H, Babarskiene M R, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 51.Francis P T, Palmer A M, Snape M, Wilcock G K. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S, Gelinas I, Gauthier L, McIntyre M, Gauthier S. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 53.Geldmacher D S, Provenzano G, McRae T, Mastey V, Ieni J R. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. J. Am. Geriatr. Soc. 2003;51:937–944. doi: 10.1046/j.1365-2389.2003.51306.x. [DOI] [PubMed] [Google Scholar]
- 54.Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am. J. Occup. Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 55.Gilman S, Koller M, Black R S, Jenkins L, Griffith S G, Fox N C, Eisner L, Kirby L, Rovira M B, Forette F, Orgogozo J M. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 56.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 57.Golde T E. Disease modifying therapy for AD? . J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 58.Hansen R A, Gartlehner G, Webb A P, Morgan L C, Moore C G, Jonas D E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. Clin. Interv. Aging. 2008;3:211–225. [PMC free article] [PubMed] [Google Scholar]
- 59.Hardy J, Selkoe D J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 60.Harris M E, Hensley K, Butterfield D A, Leedle R A, Carney J M. Direct evidence of oxidative injury produced by the Alzheimer's beta-amyloid peptide (1-40) in cultured hippocampal neurons. Exp. Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 61.Herrmann N, Binder C, Dalziel W, Smyth S, Camacho F. Persistence with cholinesterase inhibitor therapy for dementia: an observational administrative health database study. Drugs Aging. 2009;26:403–407. doi: 10.2165/00002512-200926050-00004. [DOI] [PubMed] [Google Scholar]
- 62.Hock C, Konietzko U, Streffer J R, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer M A, Umbricht D, de Quervain D J, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch R M. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 63.Hogan D B, Bailey P, Carswell A, Clarke B, Cohen C, Forbes D, Man-Son-Hing M, Lanctot K, Morgan D, Thorpe L. Management of mild to moderate Alzheimer's disease and dementia. Alzheimers. Dement. 2007;3:355–384. doi: 10.1016/j.jalz.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Hogan D B, Goldlist B, Naglie G, Patterson C. Comparison studies of cholinesterase inhibitors for Alzheimer's disease. Lancet Neurol. 2004;3:622–626. doi: 10.1016/S1474-4422(04)00883-X. [DOI] [PubMed] [Google Scholar]
- 65.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones R W, Bullock R, Love S, Neal J W, Zotova E, Nicoll J A. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 66.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 67.Iqbal K, Alonso A C, Gong C X, Khatoon S, Pei J J, Wang J Z, Grundke-Iqbal I. Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J. Neural Transm. Suppl. 1998;53:169–180. doi: 10.1007/978-3-7091-6467-9_15. [DOI] [PubMed] [Google Scholar]
- 68.Kadir A, Darreh-Shori T, Almkvist O, Wall A, Grut M, Strandberg B, Ringheim A, Eriksson B, Blomquist G, Langstrom B, Nordberg A. PET imaging of the in vivo brain acetylcholinesterase activity and nicotine binding in galantamine-treated patients with AD. Neurobiol. Aging. 2008;29:1204–1217. doi: 10.1016/j.neurobiolaging.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H P, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kavirajan H. Memantine: a comprehensive review of safety and efficacy. Expert Opin. Drug Saf. 2009;8:89–109. doi: 10.1517/14740330802528420. [DOI] [PubMed] [Google Scholar]
- 71.Klafki H W, Staufenbiel M, Kornhuber J, Wiltfang J. Therapeutic approaches to Alzheimer's disease. Brain. 2006;129:2840–2855. doi: 10.1093/brain/awl280. [DOI] [PubMed] [Google Scholar]
- 72.Knopman D S, Knapp M J, Gracon S I, Davis C S. The Clinician Interview-Based Impression (CIBI): a clinician's global change rating scale in Alzheimer's disease. Neurology. 1994;44:2315–2321. doi: 10.1212/wnl.44.12.2315. [DOI] [PubMed] [Google Scholar]
- 73.Lanctot K L, Herrmann N, Yau K K, Khan L R, Liu B A, LouLou M M, Einarson T R. Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. CMAJ. 2003;169:557–564. [PMC free article] [PubMed] [Google Scholar]
- 74.Lee V M. Disruption of the cytoskeleton in Alzheimer's disease. Curr. Opin. Neurobiol. 1995;5:663–668. doi: 10.1016/0959-4388(95)80073-5. [DOI] [PubMed] [Google Scholar]
- 75.Lemere C A, Lopera F, Kosik K S, Lendon C L, Ossa J, Saido T C, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, Hincapie L, Arango J C, Anthony D C, Koo E H, Goate A M, Selkoe D J, Arango J C. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat. Med. 1996;2:1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- 76.Lichtenthaler S F, Haass C. Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J. Clin. Invest. 2004;113:1384–1387. doi: 10.1172/JCI21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liperoti R, Pedone C, Corsonello A. Antipsychotics for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD) Curr. Neuropharmacol. 2008;6:117–124. doi: 10.2174/157015908784533860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez O L, Becker J T, Wahed A S, Saxton J, Sweet R A, Wolk D A, Klunk W, Dekosky S T. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J. Neurol. Neurosurg. Psychiatry. 2009;80:600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez O L, Becker J T, Wisniewski S, Saxton J, Kaufer D I, Dekosky S T. Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2002;72:310–314. doi: 10.1136/jnnp.72.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loy C, Schneider L. Galantamine for Alzheimer's disease and mild cognitive impairment. Cochrane. Database. Syst. Rev. 2006:CD001747. doi: 10.1002/14651858.CD001747.pub3. DOI: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lue L F, Kuo Y M, Roher A E, Brachova L, Shen Y, Sue L, Beach T, Kurth J H, Rydel R E, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyketsos C G, Colenda C C, Beck C, Blank K, Doraiswamy M P, Kalunian D A, Yaffe K. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am. J. Geriatr. Psychiatry. 2006;14:561–572. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 83.Mann D M, Iwatsubo T, Cairns N J, Lantos P L, Nochlin D, Sumi S M, Bird T D, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor M N, Haltia M. Amyloid beta protein (Abeta) deposition in chromosome 14-linked Alzheimer's disease: predominance of Abeta42(43) Ann. Neurol. 1996;40:149–156. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- 84.Markesbery W R, Mattson M P, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel R E. The role of oxidative stress in Alzheimer disease beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. Arch. Neurol. 1999;56:1449–1452. [Google Scholar]
- 85.Massoud F, Dorais M, Charbonneau C, Lescrauwaet B, Boucher J M, LeLorier J. Drug utilization review of cholinesterase inhibitors in Quebec. Can. J. Neurol. Sci. 2008;35:508–509. doi: 10.1017/s0317167100009215. [DOI] [PubMed] [Google Scholar]
- 86.Mattson M P, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel R E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McLaurin J, Cecal R, Kierstead M E, Tian X, Phinney A L, Manea M, French J E, Lambermon M H, Darabie A A, Brown M E, Janus C, Chishti M A, Horne P, Westaway D, Fraser P E, Mount H T, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 88.McLaurin J, Kierstead M E, Brown M E, Hawkes C A, Lambermon M H, Phinney A L, Darabie A A, Cousins J E, French J E, Lan M F, Chen F, Wong S S, Mount H T, Fraser P E, Westaway D, St George-Hyslop P. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 89.McLean C A, Cherny R A, Fraser F W, Fuller S J, Smith M J, Beyreuther K, Bush A I, Masters C L. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 90.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane. Database. Syst. Rev. 2009:CD003154. doi: 10.1002/14651858.CD003154.pub5. DOI:10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 91.MRC Pathology Group. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 92.Mudher A, Lovestone S. Alzheimer's disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 93.National Institute for Health and Clinical Excellence (NICE) (Alzheimer's disease - donepezil, rivastigmine, galantamine and memantine (review) - final appraisal. Available at: http://www.nice.org.uk/page.aspx?o=322952 . 2009. [Accessed August 31].
- 94.Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Onor M L, Trevisiol M, Aguglia E. Rivastigmine in the treatment of Alzheimer's disease: an update. Clin. Interv. Aging. 2007;2:17–32. doi: 10.2147/ciia.2007.2.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orgogozo J M, Gilman S, Dartigues J F, Laurent B, Puel M, Kirby L C, Jouanny P, Dubois B, Eisner L, Flitman S, Michel B F, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 97.Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch. Neurol. 1994;51:41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- 98.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 99.Qaseem A, Snow V, Cross J T, Jr., Forciea M A, Hopkins R, Jr., Shekelle P, Adelman A, Mehr D, Schellhase K, Campos-Outcalt D, Santaguida P, Owens D K. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann. Intern. Med. 2008;148:370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 100.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann. Intern. Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 101.Ritchie C W, Bush A I, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li Q X, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi R E, Masters C L. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch. Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 102.Roberson E D, Scearce-Levie K, Palop J J, Yan F, Cheng I H, Wu T, Gerstein H, Yu G Q, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 103.Robinson D M, Plosker G L. Galantamine extended release in Alzheimer's disease: profile report. Drugs Aging. 2006;23:839–842. doi: 10.2165/00002512-200623100-00006. [DOI] [PubMed] [Google Scholar]
- 104.Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:677–685. doi: 10.1136/jnnp.2003.029074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosen W G, Mohs R C, Davis K L. A new rating scale for Alzheimer's disease. Am. J. Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 106.Savva G M, Wharton S B, Ince P G, Forster G, Matthews F E, Brayne C. Age, neuropathology, and dementia. N. Engl. J. Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 107.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 108.Schmechel D E, Saunders A M, Strittmatter W J, Crain B J, Hulette C M, Joo S H, Pericak-Vance M A, Goldgaber D, Roses A D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schneider J A, Arvanitakis Z, Bang W, Bennett D A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 110.Schneider L S, Olin J T, Doody R S, Clark C M, Morris J C, Reisberg B, Schmitt F A, Grundman M, Thomas R G, Ferris S H. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 111.Selkoe D J. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 112.Selkoe D J. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann. Intern. Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 113.Seltzer B. Donepezil: an update. Expert Opin. Pharmacother. 2007;8:1011–1023. doi: 10.1517/14656566.8.7.1011. [DOI] [PubMed] [Google Scholar]
- 114.Shepherd J, Blauw G J, Murphy M B, Bollen E L, Buckley B M, Cobbe S M, Ford I, Gaw A, Hyland M, Jukema J W, Kamper A M, Macfarlane P W, Meinders A E, Norrie J, Packard C J, Perry I J, Stott D J, Sweeney B J, Twomey C, Westendorp R G. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 115.Shimizu S, Hanyu H, Iwamoto T, Koizumi K, Abe K. SPECT follow-up study of cerebral blood flow changes during Donepezil therapy in patients with Alzheimer's disease. J. Neuroimaging. 2006;16:16–23. doi: 10.1177/1051228405001468. [DOI] [PubMed] [Google Scholar]
- 116.Siemers E R, Quinn J F, Kaye J, Farlow M R, Porsteinsson A, Tariot P, Zoulnouni P, Galvin J E, Holtzman D M, Knopman D S, Satterwhite J, Gonzales C, Dean R A, May P C. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 117.Singh G, Thomas S K, Arcona S, Lingala V, Mithal A. Treatment persistency with rivastigmine and donepezil in a large state medicaid program. J. Am. Geriatr. Soc. 2005;53:1269–1270. doi: 10.1111/j.1532-5415.2005.53384_9.x. [DOI] [PubMed] [Google Scholar]
- 118.Snowdon D A, Greiner L H, Mortimer J A, Riley K P, Greiner P A, Markesbery W R. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 119.Starr J M, Whalley L J, Deary I J. The effects of antihypertensive treatment on cognitive function: results from the HOPE study. J. Am. Geriatr. Soc. 1996;44:411–415. doi: 10.1111/j.1532-5415.1996.tb06412.x. [DOI] [PubMed] [Google Scholar]
- 120.Sucher N J, Awobuluyi M, Choi Y B, Lipton S A. NMDA receptors: from genes to channels. Trends Pharmacol. Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- 121.Suh D C, Thomas S K, Valiyeva E, Arcona S, Vo L. Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer's disease. Drugs Aging. 2005;22:695–707. doi: 10.2165/00002512-200522080-00006. [DOI] [PubMed] [Google Scholar]
- 122.Tariot P N, Farlow M R, Grossberg G T, Graham S M, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 123.Tedesco M A, Ratti G, Mennella S, Manzo G, Grieco M, Rainone A C, Iarussi D, Iacono A. Comparison of losartan and hydrochlorothiazide on cognitive function and quality of life in hypertensive patients. Am. J. Hypertens. 1999;12:1130–1134. doi: 10.1016/s0895-7061(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 124.Thal L J, Rosen W, Sharpless N S, Crystal H. Choline chloride fails to improve cognition of Alzheimer's disease. Neurobiol. Aging. 1981;2:205–208. doi: 10.1016/0197-4580(81)90022-1. [DOI] [PubMed] [Google Scholar]
- 125.Trojanowski J Q, Lee V M. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- 126.Troncoso J C, Zonderman A B, Resnick S M, Crain B, Pletnikova O, O'Brien R J. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann. Neurol. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 128.Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, Delon M R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 129.Wilcock G K, Black S E, Hendrix S B, Zavitz K H, Swabb E A, Laughlin M A. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial. Lancet Neurol. 2008;7:483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 130.Winblad B, Grossberg G, Frolich L, Farlow M, Zechner S, Nagel J, Lane R. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology. 2007;69:S14–S22. doi: 10.1212/01.wnl.0000281847.17519.e0. [DOI] [PubMed] [Google Scholar]
- 131.Wisniewski T, Ghiso J, Frangione B. Biology of A beta amyloid in Alzheimer's disease. Neurobiol. Dis. 1997;4:313–328. doi: 10.1006/nbdi.1997.0147. [DOI] [PubMed] [Google Scholar]
- 132.Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer's disease. Lancet Neurol. 2008;7:805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee E B, Xie S X, Joyce S, Li C, Toleikis P M, Lee V M, Trojanowski J Q. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl. Acad. Sci. USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X Z, Li X J, Zhang H Y. Valproic acid as a promising agent to combat Alzheimer's disease. Brain Res. Bull. 2010;81:3–6. doi: 10.1016/j.brainresbull.2009.09.003. [DOI] [PubMed] [Google Scholar]