Abstract
There is no effective treatment for prostate cancer arising after androgen ablation. Previous studies have analyzed the short-term effects of androgen ablation on the immune system and suggest an abatement of immune suppression by hormone removal. Since castration-resistant disease can arise years after treatment, it is crucial to determine the duration of immune potentiation by castration. Because immunotherapeutic efficacy is determined by the balance of immune cell subsets and their location within the tumor, we assessed the acute and chronic effect of androgen ablation on the localization of T cell subsets within castration-resistant murine prostate cancer. We observed a transient increase in CD4+ and CD8+ T cell numbers at the residual tumor after androgen ablation. More than two months later, regulatory T cells were increasingly within prostate epithelium, while cytolytic T cells (CTL), which were evenly distributed prior to androgen ablation, became sequestered within stroma. Anti-CD25 antibody administration along with castration enhanced CTL access to cancerous glands, but did not increase effector function. Intra-prostatic injection of LIGHT-expressing tumor cells increased the proportion of CD8+ T cells with functional capacity within the cancerous gland. In addition, Treg depletion within the tumor was enhanced. Together these manipulations significantly reduced castration-resistant tumor burden. Thus, our results indicate that immune modulations which prevent Treg accumulation and augment effector cell infiltration of prostatic epithelium may be effective in reducing tumor burden or preventing tumor recurrence after androgen ablation therapy.
Keywords: castration, prostate cancer, T cells, regulatory T cell depletion, stroma, LIGHT, vaccination
Introduction
Androgen ablation therapy of prostate cancer causes apoptotic cell death, significantly reducing tumor burden (1). However, the benefit is often short-lived and many patients develop fatal androgen-independent disease (2).
Systemic androgen removal modulates T cell number and function, increasing peripheral T lymphocyte numbers (3, 4) and reducing Treg numbers (5). Androgen ablation caused an early and transient increase in T cell infiltration of human prostate tumors (6) and removed tolerance to prostate cancer antigens in a transgenic mouse model (7), suggesting that systemic removal of androgen can enhance prostate anti-tumor immunity.
The presence of lymphocytes within prostate tumors (8, 9) and circulating tumor-reactive T cells in the peripheral blood of prostate cancer patients (10-14) have been reported. Effector CD8+ T cells (6, 15), regulatory FoxP3+ cells (16) and Th17 cells (17) have been observed within prostate tumors. Whether the composition of lymphocytes favors effector or regulatory cells serves as a prognostic indicator of disease outcome (18-20).
In mouse models, dysfunctional T cells both systemically and at the tumor site (21-25) indicate that CTL may fail to become fully functional, or may receive one or more suppressive signals. The presence of Tregs at the tumor site can inhibit effector cell trafficking and function. However, studies depleting Tregs have had mixed results in mouse models (26, 27). Human and animal studies suggest that androgen ablation may overcome some suppressive signals, resulting in increased effector cell presence at the tumor site. In order for the post-castration influx of lymphocytes to be advantageous, the balance should favor effector cells, and be maintained over time. However, immune potentiation must be insufficient, since the tumor recurs.
Malignant cells within prostate glands are surrounded by stroma which impedes lymphocyte infiltration of the tumor and presents a significant barrier (28), which functional CTL must cross to access the tumor. While a study in patients with non-small cell lung cancer showed high levels of epithelial infiltration by CD8+ cells and stromal infiltration by CD4+ cells, and suggested both as independent positive prognostic indicators of patient survival (18), such a study has not been reported for prostate cancer.
We hypothesized that androgen ablation enhances anti-tumor immunity by transiently shifting the balance of lymphocyte subsets to favor effector CD8+ T cells and that over time, the lymphocyte composition changes to favor cells which inhibit tumor rejection. We investigated this hypothesis in a prostate-specific Pten (phosphatase and tensin homolog on chromosome 10) knockout mouse model of prostate cancer, a model that recapitulates human prostate cancer development and progression (29). Androgen ablation in this model leads to apoptotic death in the primary tumor with persistence of invasive disease. We characterized the T lymphocyte infiltrate within these androgen independent prostate tumors and found an overall increase in both the number of T cells within the prostate and the ratio of CD8+:FoxP3+ T cells.
Using immunohistochemical analysis (IHC), we determined that although Treg depletion did not increase the percentage of functional CD8+ T cells, it increased their access to cancerous glands. Vaccination with tumor cells expressing the TNF superfamily member LIGHT, (lymphotoxin-like exhibits inducible expression and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes) (30) along with castration and anti-CD25 administration reduced tumor volume, and sustained Treg depletion within the tumor. Thus, CTL infiltration of prostatic epithelium and in situ vaccination were crucial to an effective anti-tumor response.
Materials and Methods
Mice and cell lines
All animal work followed WFUHS Institutional Animal Care and Use Committee (IACUC) regulations. PtenloxP/loxP mice were obtained from Dr. Yong Chen, WFUHS with permission of Dr. Hong Wu, UCLA (29). A PB-Cre4 transgenic mouse breeding pair (31) was obtained from the NCI Mouse Models of Human Cancer Consortium, and the line was maintained and intercrossed with PtenloxP/loxP mice, to generate Pten-/- male mice as described previously (29).
Animals were killed at the indicated time points and wet weights of pooled prostate lobes were obtained. Tumors and spleens were embedded in OCT medium. H&E stained sections were evaluated for presence of tumor by MCW in a blinded fashion.
UV-8101-RE sarcoma cells (32) were maintained in DMEM + 10% fetal bovine serum (both from Lonza, Hopkinton, MA). Pten CaP8 cells were maintained in Pten medium as described previously (33). TRAMP C-1 cells were maintained in TRAMP medium (34).
Castration
Mice were anesthetized with an intraperitoneal injection of 100μl/25g of a Ketamine/Xylazine mixture (23.75mg/ml Ketamine + 1.25mg/ml Xylazine) and castrated by surgical removal of both testicles.
Regulatory T cell depletion
Tregs were depleted by a single i.p. injection of 0.5 mg anti-CD25 antibody (35);clone PC61, BioXCell, West Lebanon, NH).
Antibody sources
Anti-CD4 (clone GK1.5), anti-FoxP3 (clone FJK-16s), and IgG2b isotype control (eBioscience, San Diego, CA), polyclonal rabbit anti-granzyme B, and rabbit IgG (Abcam, Cambridge, MA), anti-CD8 (clone 53-6.7), IgG2a isotype control, goat anti-rat IgG (BD Biosciences, San Jose, CA), biotinylated donkey anti-rabbit, and goat anti-mouse Fab (Jackson ImmunoResearch, West Grove, PA), goat anti-rat-AF546 and donkey anti-rat-AF488 (Invitrogen, Carlsbad CA).
Immunohistochemistry and immunofluorescence
Eight micrometer cryosections were stained using standard immunohistochemical techniques. For single staining, slides were incubated with anti-CD4 (5μg/ml) anti-CD8 (5μg/ml) or anti-Foxp3 (10μg/ml) antibody for 1 hour and biotinylated goat anti-rat Ig (1:300). Signal was amplified using ABC kit (Vector Labs; Burlingame, CA), developed with DAB and mounted using Permount (Fisher; Pittsburgh, PA).
Granzyme B+/CD8+ cells were detected by IHC or IF double staining. CD8 staining was developed with DAB + nickel, followed by anti-granzyme B (5μg/ml, 30 minutes), donkey anti-rabbit-biotin (1:250) and ABC, developed with AEC (Vector Labs; Burlingame, CA). For immunofluorescence, granzyme B was detected with red substrate 1 kit (Vector Labs, Burlingame CA) and CD8 was detected with Alexafluor-488 (1:100, Molecular Probes, Invitrogen Corp., Carlsbad, CA).
For double staining of CD4/FoxP3, slides were fixed in ice-cold methanol, washed in PBS, and blocked with 1% BSA/PBS. Endogenous mouse Ig was blocked with goat anti-mouse Fab (100μg/ml, 30 minutes) washed, followed with rat anti-mouse FoxP3 (40μg/ml, overnight, room temperature). Slides were developed with goat anti-rat-AF546 (25μg/ml), washed, blocked, and anti-CD4 (25μg/ml, 30 minutes) was followed by donkey anti-rat-AF488 (25μg/ml), counter-stained with DAPI and mounted in 90% glycerol/PBS.
Enumeration of T cell subsets
Pictures of 3-4 fields per prostate were quantified using the ImageJ Cell Counter Plug-in (National Institutes of Health). The total number of positive cells per field as well as the number of positive cells in the glands was determined. Pictures were taken by EJA or PD. Cell counts were blinded or independently verified by PD.
Statistical analysis
One to four 8μm sections of tissue were measured on each mouse. The analysis used all of the measurements and accounted for the correlation of having repeated measures in a mouse by using a mixed effects model, with a random mouse effect. Differences in prostate tumor weight and the number of cells at each time point and treatment were compared using pairwise comparisons from the mixed effects ANOVA model. Differences within mice in the percentage of cells expressing GzB, GzB percentage in stroma versus gland, and the density (cells/mm2) of CD4+, CD8+, FoxP3+ in stroma versus gland, were compared to 0, using a t-test contrast in the mixed effects ANOVA model. All analysis was performed in SAS (v 9.2, Cary, NC), with a two-sided alpha level of 0.05. Data are shown as mean +/- SEM and are compiled from at least 5 animals per group.
Flow cytometry
Splenocytes were dissociated into a single cell suspension and stained using the Mouse Regulatory T cell staining kit (eBioscience), following the manufacturer’s instructions. Cells were analyzed on a FACSCalibur using Cellquest PRO® software (BD Biosciences).
MLTC and 51Cr release assay
MLTC cultures were set up and lytic activity was assayed as described previously (32), and assessed on day 6-7 of culture. Supernatant was harvested and measured after 4.5 hours.
Results
Deletion of exon 5 of the Pten tumor suppressor gene in the prostate causes invasive prostate adenocarcinoma in 100% of the mice (29) by 9 weeks of age. Although castration at 16 weeks of age induced apoptosis within the primary tumor, invasive adenocarcinoma was maintained in the residual tissue. We analyzed animals between 2.5 weeks and 10 weeks post-castration to span different stages of androgen independent prostate growth. Prostate weights did not change significantly over time (Figure 1A) and carcinoma was detected at all time points by H&E staining (Figure S1).
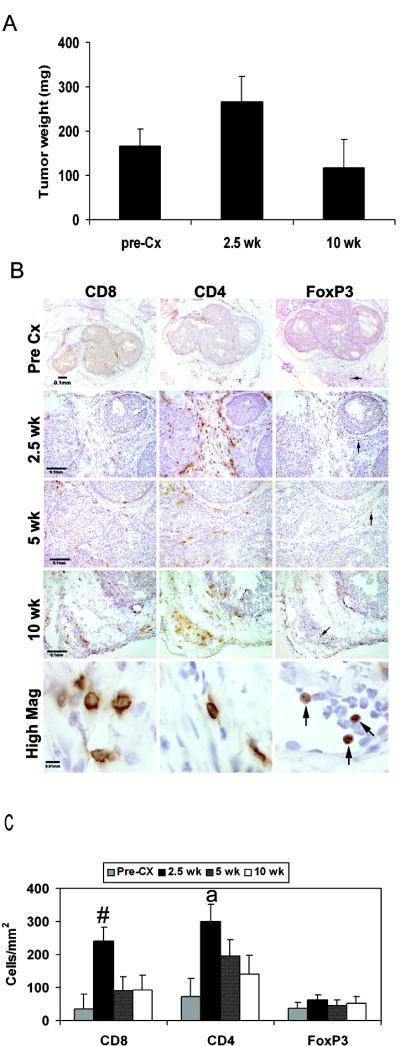
FIGURE 1. Castration induces a transient increase in T cell localization to the prostate without altering prostate tumor weight.
(A) Prostate wet weights at indicated time points are shown. (B) CD8, CD4 and FoxP3 staining at pre-castration (pre-Cx), 2.5, 5 and 10 weeks after castration. Scale bar indicates 100 μm (pre-Cx-10 weeks) and 10 μm (high mag). Arrows mark FoxP3+ cells. (C) CD8+ and CD4+ T cell numbers increased at 2.5 weeks post-castration. Quantification was performed as described in Materials and Methods.  , Pre-Cx; ■, 2.5 weeks;
, Pre-Cx; ■, 2.5 weeks;  5 weeks; □ 10 weeks; #;p< 0.05 versus pre-Cx, 5 weeks and 10 weeks a; p<0.05 versus pre-castration and 10 weeks.
5 weeks; □ 10 weeks; #;p< 0.05 versus pre-Cx, 5 weeks and 10 weeks a; p<0.05 versus pre-castration and 10 weeks.
T lymphocyte ratios favor CD8+ T cells early after castration
Previous studies (6, 15) reported an increase in lymphocyte numbers at the prostate one to four weeks after hormone ablation. In order to determine the effect of castration on the number and proportion of T lymphocytes, we analyzed the prostates of Pten knockout mice by IHC (Figure 1B). Quantitative analysis showed that compared to intact 8-16 week old mice, 2.5 weeks after castration there was an approximately 6-fold and 4-fold increase in the number of CD8+ and CD4+ T cells in the prostate, respectively (Figure 1C). However, by 5 weeks post castration, the numbers of both subsets were not significantly different from pre-castration numbers (Figure 1C), suggesting that the effects of hormone removal on the immune system are acute. The small number of FoxP3+ T cells present did not change significantly throughout tumor progression (Figure 1C).
The ratio of CD8+ to FoxP3+ T cells within the tumor is a prognostic indicator of immune efficacy (20, 36, 37). Although not statistically significant, we observed an approximately 7-fold increase in the CD8+:FoxP3+ T cell ratio at 2.5 weeks after castration (data not shown) demonstrating that androgen ablation had an immune potentiating effect which favored effector cells.
Excluded from this analysis were distinct lymphoid aggregates which were found in approximately one-third of the samples (Figure S2) since their functional status may be different from tumor-infiltrating lymphocytes (38-40).
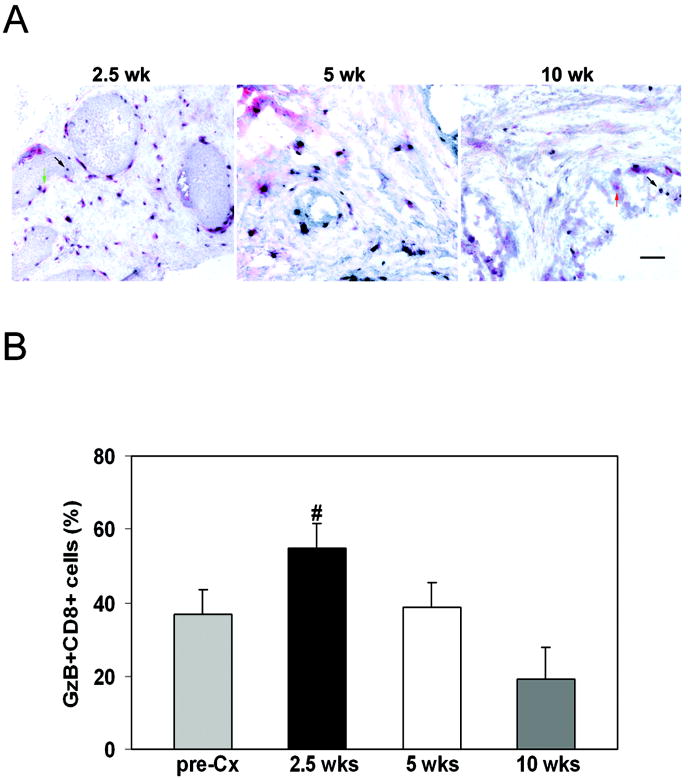
For most tumors, rejection is mediated by effector CTL. Therefore, we evaluated the functional status of the CD8+ T cells infiltrating the prostate gland after castration, to assess whether there was an increase in functional cells. The proportion of CD8+ T cells which expressed granzyme B, a marker of lytic capacity, was evaluated (Figure 2A) and was not significantly different before castration and at 2.5 weeks following castration (Figure 2B). Since CD8+ T cell numbers increased, the number of granzyme B+ cells increased approximately 10-fold (data not shown). However there was no effect on tumor volume. By 10 weeks post-castration, the percentage of Granzyme B+ cells in the tumor had declined significantly (Figure 2B), indicating that enhancement of immune function was not sustained. Notably, about one-third of the CD8+ T cells at the tumor site before castration expressed granzyme B, suggesting that the growing tumor may have elicited a cytolytic T cell response.
FIGURE 2. Androgen ablation does not alter granzyme B+, CD8+ T cell proportions.
(A) Representative sections of granzyme B+ cells (red) and CD8+ cells (black) are shown at each time point. (B) The percentage of granzyme B+ CD8+ cells was calculated (#; p<0.05 versus 10 weeks).  , Pre-Cx; ■, 2.5 weeks; □, 5 weeks;
, Pre-Cx; ■, 2.5 weeks; □, 5 weeks;  , 10 weeks. Scale bar is 100 μm.
, 10 weeks. Scale bar is 100 μm.
Effector T cells localized to the prostate do not efficiently access tumor epithelium
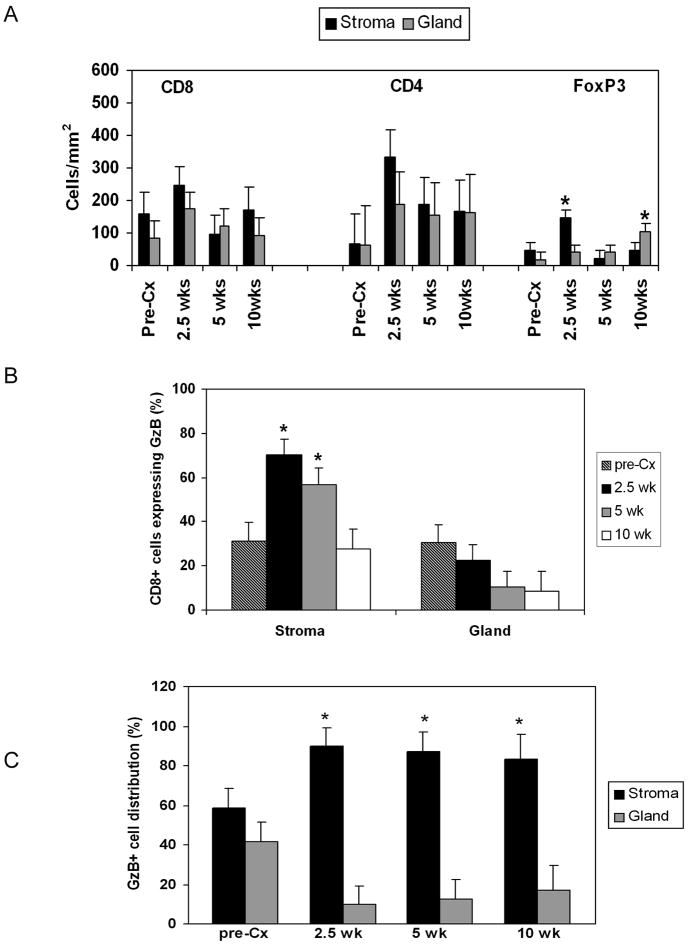
Random distribution of lymphocytes within a tissue may indicate that they are not actively involved in immune responses (41). There was no preferential localization of CD8+ and CD4+ cells within the prostate at any time point (Figure 3A). In contrast to the CD4+ population, FoxP3+ cells were predominantly located in the tumor stroma early after castration (Figure 3A). However, as the tumor progressed, Tregs were increasingly detected in the epithelium, suggesting that glandular localization of Tregs may be one hallmark of prostate cancer progression.
FIGURE 3. Both effector CD8+ T cells and FoxP3+ T cells are localized to prostate tumor stroma after androgen ablation.
(A) FoxP3+ cells are preferentially localized to tumor stroma after androgen ablation and shift to the cancerous glands over time. (*; p < 0.05 compared to gland or stroma at the same time point). (B) The percentage of CD8+ cells in stroma or gland expressing granzyme B was determined (*; p < 0.05 versus gland at the same time point). ■ Pre-Cx; ■ 2.5 weeks;  5 weeks; □ 10 weeks. (C) The distribution of granzyme B+/CD8+ T cells in stroma or gland was determined (*= p ≤ 0.05 versus gland at the same time point). ■ stroma; □ gland.
5 weeks; □ 10 weeks. (C) The distribution of granzyme B+/CD8+ T cells in stroma or gland was determined (*= p ≤ 0.05 versus gland at the same time point). ■ stroma; □ gland.
To assess whether functional CD8+ T cells were able to overcome the stromal barrier, we calculated the percentage of CD8+ T cells within stroma and gland which expressed granzyme B. While there was no difference in the location of granzyme B+CD8+ cells in non-castrated mice, at both 2.5 and 5 weeks following castration there was a significantly higher proportion of functional cells in the tumor-associated stroma than in the glands (Figure 3B). In addition, the majority of functional cells were excluded from the prostate glands at all time points examined (Figure 3C). Thus, while androgen ablation induced a substantial increase in lymphocyte numbers in the prostate, their location within the organ was not conducive for targeting of malignant epithelial cells.
Systemic PC61 administration does not deplete regulatory T cells within the prostate tumor
Persistence of regulatory T cells may be an immune suppression mechanism critical to prostate cancer progression. Therefore, we hypothesized that depletion of regulatory T cells at the time of castration would enhance T cell localization to and function within the prostate epithelium.
In a melanoma model, prophylactic anti-CD25 treatment promoted tumor rejection, while therapeutic anti-CD25 treatment did not increase CD8+ T cell:Treg ratio or enhance tumor infiltration by effector T cells (42). Since androgen ablation results in death of the majority of existing tumor cells, we reasoned that concurrent PC61 treatment would mimic the prophylactic phenomenon; therefore, we administered the anti-CD25 antibody, PC61, intraperitoneally two days prior to castration (35). Flow cytometry analysis of splenocytes showed that Tregs were depleted systemically 48 hours later (Figure 4A).
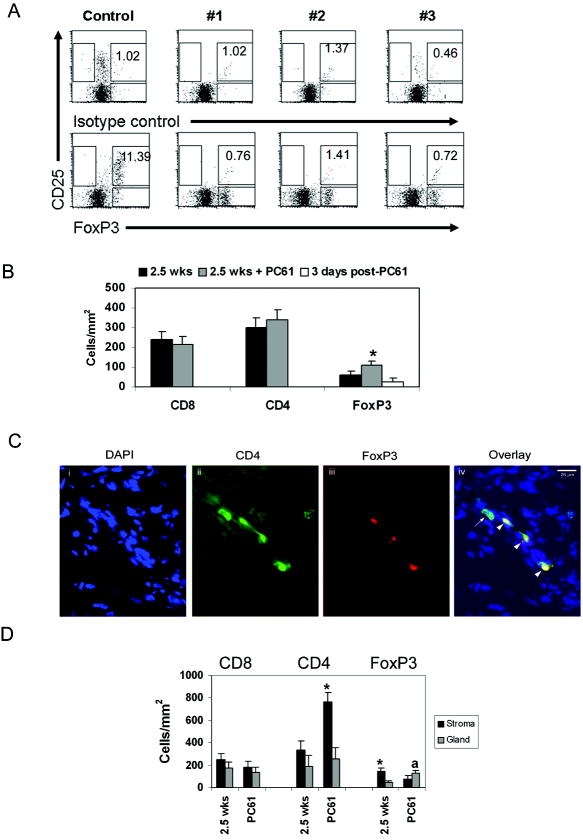
FIGURE 4. Treg depletion changes T cell distribution within the prostate tumor microenvironment.
(A) Tregs were detected by flow cytometry as described in Materials and Methods. CD25+FoxP3+ T cells (%) is shown in the upper right quadrant. (B) FoxP3+ cells are not depleted in the prostate after systemic PC61 administration, while CD8+ and CD4+ T cell numbers are unchanged. ■ 2.5 weeks;  2.5 weeks + PC61 administration; □ 3 days post-administration of PC61. * = p<0.05 compared to 2.5 weeks, and 3 days post-PC61. (C) Fluorescent double staining for CD4 and FoxP3. One representative sample from the 2.5 weeks + PC61 treatment group is shown. Arrowheads show CD4/FoxP3 double-positive cells, while arrow indicates single CD4+ cell. (D) CD4+ T cells are preferentially localized to prostate stroma while FoxP3+ cells are within cancerous glands. ■ Stroma;
2.5 weeks + PC61 administration; □ 3 days post-administration of PC61. * = p<0.05 compared to 2.5 weeks, and 3 days post-PC61. (C) Fluorescent double staining for CD4 and FoxP3. One representative sample from the 2.5 weeks + PC61 treatment group is shown. Arrowheads show CD4/FoxP3 double-positive cells, while arrow indicates single CD4+ cell. (D) CD4+ T cells are preferentially localized to prostate stroma while FoxP3+ cells are within cancerous glands. ■ Stroma;  Gland. * = p<0.05 compared to gland at the same time point; a = p=0.05 compared to stroma at the same time point. Data for 2.5 weeks time points in (B) and (D) are the same as in Figure 1C, and 3A respectively.
Gland. * = p<0.05 compared to gland at the same time point; a = p=0.05 compared to stroma at the same time point. Data for 2.5 weeks time points in (B) and (D) are the same as in Figure 1C, and 3A respectively.
Since changes in lymphocyte function and number were most evident at 2.5 weeks post-castration, we evaluated the effect of a single anti-CD25 treatment at that time point. The number of CD4+ and CD8+ T cells which infiltrated the prostate was not different from untreated animals (Figure 4B). Surprisingly, FoxP3+ T cell numbers were significantly higher than castrated animals which were not treated with antibody (Figure 4B), suggesting that PC61 administration did not deplete intra-tumoral Tregs, or that compensatory mechanisms within the tumor maintained Tregs at the tumor site. We analyzed prostate tumors 3 days after antibody administration and found that Treg numbers within the prostate tumor were similar to those of untreated animals (Figure 4B), suggesting that systemic antibody administration did not deplete intra-tumoral Tregs.
Fluorescent double staining (Figure 4C) showed that the FoxP3+ cells within the tumor were also CD4+, indicating the accumulation and maintenance of classical Tregs at the tumor site (43).
Treg depletion augments effector cell infiltration of tumor epithelium without increasing effector cell function
We also examined the effect of PC61 treatment on the localization of T cells within the prostate microenvironment. While the CD8+ cells continued to be randomly distributed throughout the organ, CD4+ cells were preferentially localized within tumor stroma (Figure 4D).
Interestingly, the FoxP3+ cells were enriched in the glands, in contrast to prior significant enrichment in the stroma (Figure 4D). Thus, PC61 treatment accelerated localization of Tregs to the epithelium, which occurred by 10 weeks post-castration in the absence of antibody treatment.
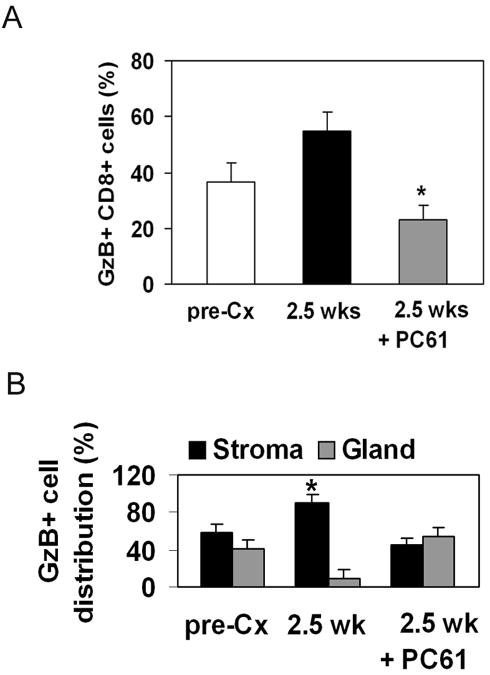
The percentage of Granzyme B+ CD8+ T cells was significantly lower in antibody treated mice (Figure 5A). Since CD8+ T cell numbers did not decrease after antibody treatment, systemic anti-CD25 administration may have prevented acquisition of full effector function.
FIGURE 5. Treg depletion alleviates sequestration of Granzyme B+ cells in the tumor stroma.
(A) Percentage of Granzyme B+ CD8+ cells in the prostate is reduced after PC61 administration. * = p < 0.05 compared to 2.5 weeks; □ pre-Cx; ■ 2.5 weeks;  2.5 weeks post-castration + PC61 administration. (B) Granzyme B+ cells are equally distributed between tumor stroma and gland after PC61 treatment. ■ stroma;
2.5 weeks post-castration + PC61 administration. (B) Granzyme B+ cells are equally distributed between tumor stroma and gland after PC61 treatment. ■ stroma;  gland. * = p<0.05 compared to gland at the same time point.
gland. * = p<0.05 compared to gland at the same time point.
CD8+ cells expressing Granzyme B were evenly distributed between stroma and gland after PC61 administration suggesting that removal of Tregs facilitated tumor infiltration by effector cells (Figure 5B). However, compensatory movement of regulatory cells to the same compartment may keep effector function in check and prevent tumor rejection.
In situ vaccination with LIGHT-expressing tumor cells decreases tumor burden
Experiments in transplanted tumor models have demonstrated that introduction of the TNF receptor superfamily member LIGHT at the tumor site can recruit and activate naïve T cells, causing rejection of antigenically unrelated tumors. LIGHT is a ligand for herpes viral entry mediator (HVEM) expressed on T cells, and lymphotoxin-β receptor expressed on stromal cells (28). Ligation of these two receptors increased localization of effector T cells within the tumor (30) and may enhance extravasation of tumor stroma. We tested the hypothesis that intraprostatic injection of LIGHT-expressing tumor cells would increase infiltration of the cancerous glands by functional CD8+ T cells. The highly immunogenic C57BL/6-derived sarcoma tumor cell line UV-8101-RE (32) was transduced with a replication-incompetent retrovirus expressing mutant LIGHT (mLIGHT) (Supplementary Figure S3). Two days after injection of PC61 antibody, Pten knockout mice were castrated, and simultaneously injected with 1×106 UV-8101-RE-mLIGHT or UV-8101-RE cells alone into both anterior and both dorso-lateral prostate lobes. Prostates and spleens were harvested and analyzed 2.5 weeks later. On the day of the surgery, prostate lobes and seminal vesicles were enlarged and clearly visible. At the time of harvest, seminal vesicles were shrunken and prostate lobes were reduced in size (data not shown).
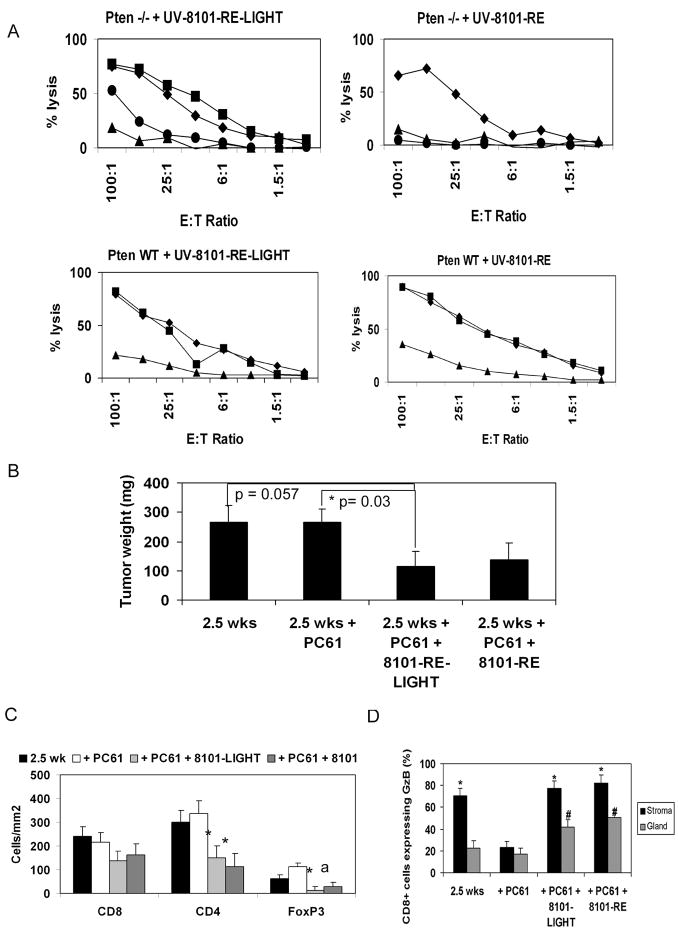
Mixed Lymphocyte Tumor cell Cultures (MLTCs) of spleens from immunized mice showed that both UV-8101-RE and UV-8101-RE-mLIGHT vaccinated mice elicited a strong and specific lytic response by 2.5 weeks post-vaccination, that was indistinguishable from that of wild-type non-tumor bearing mice (Figure 6A). Tumor wet weights from animals injected with UV-8101-mLIGHT were significantly smaller compared to weights of tumors from animals that were castrated and treated with PC61 antibody (Figure 6B), and approaching significance compared to animals 2.5 weeks after castration alone. In contrast, although Pten knockout mice injected with UV-8101-RE cells also mounted a specific CTL response to the vaccine, there was no significant reduction in prostate tumor weight.
FIGURE 6. Vaccination with LIGHT-expressing tumor cells together with castration and PC61 administration reduced prostate tumor weight.
(A) Splenocytes from vaccinated mice were stimulated in vitro with UV-8101-LIGHT or UV-8101-RE. ■ UV-8101-RE-LIGHT; ◆ UV-8101-RE; ●Pten CaP8; ▲ TRAMP C-1. (B) Prostate wet weight of mice was determined. p = 0.057 between 2.5 weeks and 2.5 weeks + PC61 + UV-8101-LIGHT. p = 0.03 between 2.5 weeks + PC61 and 2.5 weeks + PC61 + UV-8101-RE-LIGHT; (C) FoxP3+ cells are significantly decreased in the tumor after vaccination with immunogenic sarcoma cells. ■ 2.5 weeks; □ 2.5 weeks + PC61 treatment;  2.5 weeks + PC61 + UV-8101-RE-LIGHT;
2.5 weeks + PC61 + UV-8101-RE-LIGHT;  2.5 weeks + PC61 + UV-8101-RE. * = p<0.05 compared to 2.5 weeks and 2.5 weeks + PC61; a = p<0.05 compared to castration and PC61 treatment alone; (D) Proportion of Granzyme B+ cells within the prostate glands is significantly increased after intra-prostatic vaccination. ■ stroma,
2.5 weeks + PC61 + UV-8101-RE. * = p<0.05 compared to 2.5 weeks and 2.5 weeks + PC61; a = p<0.05 compared to castration and PC61 treatment alone; (D) Proportion of Granzyme B+ cells within the prostate glands is significantly increased after intra-prostatic vaccination. ■ stroma,  gland. * = p < 0.05 compared to gland at the same time point; # = p<0.05 compared to gland at 2.5 weeks and 2.5 weeks + PC61 treatment. Data for 2.5 weeks and 2.5 weeks + PC61 are the same as in Figures 1 and 4.
gland. * = p < 0.05 compared to gland at the same time point; # = p<0.05 compared to gland at 2.5 weeks and 2.5 weeks + PC61 treatment. Data for 2.5 weeks and 2.5 weeks + PC61 are the same as in Figures 1 and 4.
Although CD8+ T cell numbers in the tumors of vaccinated mice were unchanged (Figure 6C), the number of FoxP3+ T cells significantly decreased within tumors vaccinated with UV-8101-RE-mLIGHT, or UV-8101-RE alone (Figure 6C). Thus, generation of a strong and productive CTL response within the tumor may augment Treg depletion initiated by PC61 treatment.
The proportion of CD8+ cells expressing Granzyme B was significantly increased in the prostate glands of mice vaccinated with UV-8101-RE cells, with or without the addition of LIGHT (Figure 6D), likely due to the generation of a strong CTL response against the UV-8101 tumor-specific antigen. Nevertheless, only vaccination with LIGHT-expressing tumor cells reduced tumor burden, suggesting that introduction of LIGHT in an immunogenic context may enhance bystander CTL responses to prostate tumor antigens. Two of five mice vaccinated with UV-8101-RE-mLIGHT also lysed Pten CaP8 cells, a tumor cell line derived from a Pten knockout mouse prostate tumor (one example shown in Figure 6A, top left panel), while mice vaccinated with UV-8101-RE cells did not lyse Pten CaP8 cells (Figure 6A, top right panel).
DISCUSSION
We evaluated the short-term and long-term effect of androgen ablation on the localization of T cell subsets to prostate tumors in a prostate-specific Pten knockout mouse model. Pten deletion is frequently observed in human prostate cancers (44). Therefore, evaluation of the effects of immune modulations in a model that has parallels to human disease is a particular strength of our studies.
Previous studies in human and mouse models have reported that androgen ablation, the preferred first-line therapy for localized prostate cancer, increases the number and functional status of T cells at the tumor site. However, this effect must be transient, since the tumor continues to grow. The increase in functional cell density we observed was not a result of smaller prostate volume, since tumor weights early and late after castration were similar. Androgen ablation alone was insufficient to prevent tumor recurrence, perhaps because the effector cells remained sequestered in the stroma.
The timing and dosage of anti-CD25 treatment is critical, since CD25+ effector cells may also be depleted. In a previous study, only prophylactic anti-CD25 administration augmented effector cell function (42). We reasoned that anti-CD25 administration along with castration would be a prophylactic treatment for residual castration-resistant disease. Since regulatory T cells in the prostate tumor were not significantly depleted despite near complete systemic Treg depletion, intra-tumoral injection of PC61 may be necessary to deplete Tregs within the tumor.
PC61 administration increased Granzyme B+ CD8+ T cell access to the cancerous gland. However, CD8+ T cell numbers were slightly (but not significantly) reduced at the tumor site, indicating that CTL proliferation did not increase within the tumor. In addition, the percentage of Granzyme B+ cells within the tumor was significantly decreased, either due to depletion of effector cells or incomplete acquisition of effector function by CD8+ T cells. Thus, systemic administration of anti-CD25 antibody may be detrimental to the anti-tumor response, even when delivered in the setting of minimal residual disease. A study using a transplantable model of glioblastoma showed that Treg depletion by systemic PC61 treatment reduced tumor burden when the tumors were small. Later administration of PC61 did not affect tumor burden and also depleted effector cells (45).
One potential explanation for the inability of CD8+ T cells at the tumor site to mount a rejection response is the lack of co-stimulation that is necessary for acquisition of full effector function. Transduction of tumor cells with molecules such as B7-1 (46) and 4-1-BB ligand (47) provides co-stimulation in situ and augments effector function. Subcutaneous challenge of mice with tumor cells expressing the costimulatory molecule LIGHT increased the immunogenicity of the transduced tumor, and activated T cells specific for an antigenically unrelated tumor present at a distant site within the same animal (30). Furthermore, introduction of LIGHT in the tumor microenvironment increased effector T cell infiltration of the tumor.
We chose a highly immunogenic cell line as the vaccine to deliver LIGHT in order to elicit a strong immune response within the prostate tumor. Reduction in prostate tumor weight was only observed when LIGHT-expressing tumor cells were used as vaccine, indicating that introduction of a potent immunogen alone is insufficient to elicit a rejection response against undefined antigens expressed by the endogenous prostate tumor. As reported previously (30), we also observed that LIGHT can enhance function of bystander T cells within antigenically and histologically unrelated tumor types. Approximately half of the injected cells expressed LIGHT, and a further increase in retroviral transduction of the vaccine may increase the bystander effect. To our knowledge, our study is the first to show that LIGHT immunization has potential as a therapeutic manipulation for endogenous tumors.
Elicitation of a strong CTL response against the vaccine may have resulted in movement of Tregs out of the organ, or alternately may have enhanced Treg depletion initiated by anti-CD25 antibody treatment. We did not test whether LIGHT vaccination alone may be sufficient to deplete Tregs.
Additional experiments in this tumor model will address whether repeated vaccinations with LIGHT-expressing tumor cells can sustain Treg depletion and increased effector function within the tumor, further reducing tumor burden.
In conclusion, our results imply that combination immunotherapy which leads to an increased proportion of functional T cells that can efficiently access the cancerous glands may be most effective at eliminating or preventing the development of castration-resistant prostate cancer.
Supplementary Material
Acknowledgments
We acknowledge the gift of a PtenloxP/loxP breeding pair by Dr. Yong Chen (WFUHS), the pcDNA3.1-mLIGHT plasmid by Dr. Yang-Xin Fu (University of Chicago), UV-8101-RE cells from Mrs. Karin Schreiber and Dr. Hans Schreiber (University of Chicago), Pten CaP8 cells by Dr. Hong Wu and TRAMP C-1 cells by Dr. Owen Witte (UCLA). We appreciate the assistance of Wei Du, Marie Plyler, and the Molecular Diagnostics lab of the WFUSM Department of Pathology. We thank Dr. Weiwei Huang (WFUHS) for teaching us intra-prostatic injections. We are grateful to Dr. Hong Wu for helpful suggestions on the manuscript and Drs. Hans Schreiber and Yang-Xin Fu for helpful suggestions on the LIGHT experiments. This work was supported by an American Cancer Society Research Scholar Award (RSG-07-196-01) and NIH R21CA124457 (both to P.D). E.J.A was supported by NIH T32GM063485.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Huggins C. Endocrine-induced regression of cancers. Science. 1967;156(3778):1050–4. doi: 10.1126/science.156.3778.1050. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED. Challenges in the management of prostate cancer. Br J Urol. 1992;70(Suppl 1):33–8. doi: 10.1111/j.1464-410x.1992.tb15865.x. [DOI] [PubMed] [Google Scholar]
- 3.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–71. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 4.Hess PR, Boczkowski D, Nair SK, Snyder D, Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55(6):672–83. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page ST, Plymate SR, Bremner WJ, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290(5):E856–E63. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 6.Roden AC, Moser MT, Tri SD, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098–108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 7.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArdle PA, Canna K, McMillan DC, McNicol AM, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91(3):541–3. doi: 10.1038/sj.bjc.6601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A(12):1797–803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty NG, Stevens RL, Mehrotra S, et al. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol Immunother. 2003;52(8):497–505. doi: 10.1007/s00262-003-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkord E, Rowbottom AW, Kynaston H, Williams PE. Correlation between CD8+ T cells specific for prostate-specific antigen and level of disease in patients with prostate cancer. Clin Immunol. 2006;120(1):91–8. doi: 10.1016/j.clim.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Differential CTLs specific for prostate-specific antigen in healthy donors and patients with prostate cancer. Int Immunol. 2005;17(10):1315–25. doi: 10.1093/intimm/dxh309. [DOI] [PubMed] [Google Scholar]
- 13.McNeel DG, Nguyen LD, Ellis WJ, Higano CS, Lange PH, Disis ML. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate. 2001;47(3):222–9. doi: 10.1002/pros.1066. [DOI] [PubMed] [Google Scholar]
- 14.Perambakam S, Xue BH, Sosman JA, Peace DJ. Induction of Tc2 cells with specificity for prostate-specific antigen from patients with hormone-refractory prostate cancer. Cancer Immunol Immunother. 2002;51(5):263–70. doi: 10.1007/s00262-002-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177(10):7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 17.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 19.Monnier-Benoit S, Mauny F, Riethmuller D, et al. Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol Oncol. 2006;102(1):22–31. doi: 10.1016/j.ygyno.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciavarra RP, Holterman DA, Brown RR, et al. Prostate tumor microenvironment alters immune cells and prevents long-term survival in an orthotopic mouse model following flt3-ligand/CD40-ligand immunotherapy. J Immunother. 2004;27(1):13–26. doi: 10.1097/00002371-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Degl’Innocenti E, Grioni M, Boni A, et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35(1):66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 23.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67(5):536–46. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng-Rogenski SS, Arredouani MS, Neeley YC, Lu B, Chinnaiyan AM, Sanda MG. Fas-mediated T cell deletion potentiates tumor antigen-specific tolerance in a mouse model of prostate cancer. Cancer Immunol Immunother. 2008;57(9):1357–65. doi: 10.1007/s00262-008-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Gao JX, Zhang H, Geiger TL, Liu Y, Zheng P. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J Immunol. 2002;169(9):4761–9. doi: 10.4049/jimmunol.169.9.4761. [DOI] [PubMed] [Google Scholar]
- 26.Degl’Innocenti E, Grioni M, Capuano G, et al. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68(1):292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 27.Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer Res. 2005;65(7):2947–55. doi: 10.1158/0008-5472.CAN-04-3271. [DOI] [PubMed] [Google Scholar]
- 28.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86(3):231–45. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 30.Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–9. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101(1-2):61–9. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 32.Dubey P, Hendrickson RC, Meredith SC, et al. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J Exp Med. 1997;185(4):695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67(13):6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 34.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16):3325–30. [PubMed] [Google Scholar]
- 35.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Ladoire S, Arnould L, Apetoh L, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14(8):2413–20. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 37.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 39.Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 40.Harrison JC, Dean PJ, el-Zeky F, Vander Zwaag R. Impact of the Crohn’s-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis. Hum Pathol. 1995;26(1):31–8. doi: 10.1016/0046-8177(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 41.Mrass P, Takano H, Ng LG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203(12):2749–61. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–38. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 44.Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28(2):145–53. [PubMed] [Google Scholar]
- 45.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 47.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–44. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.