Abstract
Objectives
We measured efflux from macrophages to apoB–depleted serum from 263 specimens and found instances where serum having similar HDL-C differed in their efflux capacity. Thus we wanted to elucidate why efflux capacity could be independent of total HDL-C or apoA-I.
Methods and Results
To understand why sera with similar HDL-C or apoA-I could differ in total efflux capacity we assessed their ability to promote efflux via the pathways expressed in cAMP treated J774 macrophages. Briefly, macrophages were pre-incubated with Probucol to block ABCA1, BLT-1 to block SR-BI and both inhibitors to measure residual efflux. ABCG1 efflux was measured with transfected BHK-1 cells. We used apoB-depleted serum from specimens with similar HDL-C values at the 25th and 75Th percentile. Specimens in each group were classified as having high or low efflux based on total efflux being above or below the group average. We found that, independently of HDL-C, sera with higher efflux capacity had a significant increase in ABCA1 mediated efflux which was significantly correlated to the concentration of preβ-1 HDL. The same result was obtained when these sera were similarly analyzed based on similar apoA-I.
Conclusion
Sera with similar HDL-C or apoA-I differ in their ability to promote macrophage efflux due to differences in the concentration of preβ-1 HDL.
Keywords: macrophages, cholesterol efflux, ABCA1, HDL-C, preβ-1 HDL, apoA-I
Epidemiological and interventional 1-4 studies demonstrate an inverse relationship between HDL cholesterol (HDL-C) levels and coronary heart disease (CHD), an observation also supported by animal studies5; thus high HDL-C levels are thought to independently reduce CHD risk. Although HDL has been shown to have both anti-oxidant and anti-inflammatory properties 6, its beneficial anti-atherogenic effect is likely due to its central role in reverse cholesterol transport (RCT), i.e. the transport of cholesterol from peripheral tissues to the liver for excretion to reduce its accumulation in tissue cells such as vessel wall macrophages7. Since both HDL metabolism and cholesterol transport are complex processes, it has been difficult to obtain in vivo evidence that modulating HDL levels can affect removal of cholesterol from macrophage foam cells in the vessel wall and reduce atherosclerotic lesions. Overexpression of apolipoprotein A-I (apoA-I) in mice can reduce progression of atherosclerotic lesions 8 and infusion of apoA-I/phospholipid complexes in humans promotes lesion regression and increases fecal excretion of bile acids 9. On the other hand, results of studies in subjects with monogenic disorders of HDL metabolism10,11 and post hoc analysis of epidemiological studies raise questions regarding the mechanism underlying the association between HDL-C levels and CHD12.
We recently demonstrated that, in healthy individuals having a wide range of HDL-C and apoA-I levels, the capacity of serum HDL to promote cholesterol efflux from macrophages in vitro is negatively correlated with measures of carotid intima thickness (CIMT) independently of HDL-C and apoA-I levels, suggesting that measures of HDL function may be additional predictors of cardiovascular risk13. HDL exists as a heterogeneous population of particles differing in size and composition6. In addition, efflux of cellular cholesterol is mediated by a number of pathways including aqueous diffusion, ABCA1, ABCG1 and SR-BI with different HDL particles best suited to promote efflux via each of these pathways14. Thus the efficiency of an individual serum to accept cellular cholesterol depends both on the distribution of HDL particles and the cholesterol transporters expressed in the cell being used as a cholesterol donor. Since HDL subfractions differ in their ability to remove cholesterol from macrophages, the fact that individuals with similar HDL-C may have different distribution of HDL particles provides a rationale for the increased predictive value of measures of HDL function we observed.
In this study, we took advantage of the efflux data previously generated and identified subjects having similar HDL-C but significantly different total macrophage efflux. We assumed that differences in total efflux resulted from differences in the levels of functional HDL particles. We then used a published inhibitor-based assay 15 to measure the relative contribution of different pathways to the total efflux capacity of a given serum as an indication of the relative concentration of HDL particles present. Our results show that subjects with similar HDL-C but higher total macrophage efflux capacity have significantly higher ABCA1 mediated efflux and this efflux is associated with the level of preβ-1 HDL in serum. The same results were obtained with a subset of the same sera chosen to have similar apoA-I.
Methods
Study Subjects
As previously13, efflux was measured using serum aliquots, specifically collected for this purpose, obtained from well-characterized subjects participating in a prospective, observational study to investigate the effect of HDL-C on markers of oxidative stress and inflammation and their relationship to subclinical atherosclerosis. By design, the subjects enrolled were healthy non-smokers, with no clinically evident CHD, a broad range of HDL-C levels and no drugs known to significantly affect HDL levels. The study was approved by the University of Pennsylvania Institutional Review Board and all subjects gave their informed consent for participation. About equal numbers of males and females were recruited but the experiments reported here were done with serum from female donors because more serum aliquots were available. These were selected to have similar HDL-C by choosing sera with HDL-C within a 6% range (HDL-C ± 6%) which is below the 7% biological variation for HDL-C and apoA-I 16. In addition, to confirm our results, a few critical experiments were repeated using serum from male donors also chosen to have similar HDL-C.
Serum Lipid Parameters
Blood was drawn after a 12 hour fast and several plasma and serum aliquots were prepared and frozen (-70°C) for future studies. EDTA plasma aliquots were used for lipid and lipoprotein analyses performed in a CDC-standardized lipid laboratory as previously described17. The distribution of apoA-I containing HDL particles in apoB-depleted serum was measured using immunoblotting and image analysis after separation of the various particles with non-denaturing, 2-D gel electrophoresis as described18. To obtain particle mass the percent distribution of HDL particles was applied to the total apoA-I concentration. Levels of preβ-I HDL were also assayed using a commercial enzyme–linked immunoassay (pre-Beta 1-HDL ELISA by Daiichi, Polymedco, Inc. NY). We obtained a significant correlation between 2-D gel and Elisa preβ-I HDL values for all available values (r2=0.357, p=0.0069, n=19).
Assay of Cellular Cholesterol Efflux
J774 cells, maintained in RPMI plus 10% FBS and antibiotics in 5% CO2, were plated in 24-multi well plates (70,000 cells/well) and labeled for 24h in the presence of an ACAT inhibitor (2μg/ml CP113,818, a gift from Pfizer) using 0.5ml/well of 2μCi/ml [1,2-3H] cholesterol (Perkin Elmer) in RPMI plus 1% FBS. To up-regulate ABCA1 in J774 cells we incubated an additional 16h with 0.5ml/well medium containing 0.3mM Cpt-cAMP (Sigma) and 0.2% BSA in RPMI. The relative contribution of various efflux pathways was measured by 2h pretreatment of replicate wells of cAMP treated J774 cells with RPMI-0.2% BSA alone or this medium plus 20μM Probucol, 1μM BLT-1 or both to specifically inhibit ABCA1 and SR-BI15. The contribution of ABCG1 to total efflux capacity was directly measured by 4h incubation of ABCG1 transfected BHK-1 cells with the same specimens used with J774 cells. In BHK-I cells transfected with ABCG1, receptor expression is regulated by mifepristone; the difference between cells treated overnight with mifepristone (10nM) and untreated cells represents ABCG1 efflux19. Sera for these studies were aliquots stored at -70°C, used after a single thaw, and chosen to have similar HDL-C (HDL-C± 6%) at either the 25th (low HDL=45, n=22) or 75th (high HDL=73, n=18) percentile of the HDL-C distribution. Efflux was the fraction of total cellular cholesterol released in 4h to apoB-depleted serum, obtained after removal of apoB lipoproteins with Polyethylene Glycol (PEG, MW 8000, Sigma)18, and diluted to 2.8% (equivalent to 2% serum) in MEM-HEPES (0.5ml/well). We have routinely used this dilution to promote release of radioactive cholesterol to the medium in 4h that is well above background. Supplementary figure I shows the dependence of the various receptor-mediated efflux pathways expressed in J774 cells on the dose of apoB-depleted serum. In this figure, ABCA1 efflux is the difference between cAMP treated and control J774 cells, SR-BI is the efflux measured from Fu5AH cells where SR-BI is the major efflux pathway and ABCG1 efflux is the difference between mifepristone treated and untreated transfected BHK-1 cells. SR-BI and ABCG1 efflux show linear dose dependence; the dose-dependence for ABCA1 mediated efflux fits a non-linear regression that tends to plateau but a concentration of 2.8% apoB-depleted serum is below this point (GraphPad Prism 5, GraphPad Software Inc., San Diego, CA). To further validate our macrophage model, we measured ABCA1 efflux using BHK-1 cells transfected with ABCA1 where receptor expression is regulated with mifepristone and found that the dose dependence of ABCA1 efflux measured with BHK-1 cells was identical to that measured with J774 cells (Supplementary Fig. I). In every experiment, we monitored up-regulation of ABCA1 in J774 cells as increased efflux to apoA-I (20μg/ml) from cells treated with cAMP compared to untreated cells (11±4 fold stimulation, n=10 experiments) and used an aliquot of a standard serum pool at 2% to monitor inter-assay variability in total efflux from cAMP J774 cells and ABCG1 efflux from BHK-1 cells. Transfected BHK-1 cells were a kind gift from Dr. J. Oram. All cellular incubations were done at 37°C and expression of cholesterol transporters was confirmed by western blot in initial experiments. Sera from subjects with similar high or low HDL-C were defined as having low or high efflux capacity based on total J774 efflux below or above the average efflux for each group. The same analysis was done using a subset of these specimens chosen to have similar apoA-I at either the 25th (low apoA-I 127mg/dl, n=11) or 75th (high apoA-I=163mg/dl, n=10) percentile of the apoA-I distribution.
Statistical Analysis
Results are reported as mean ± SEM. Linear regression was used to establish associations between parameters. Differences between means were established with unpaired two-tailed t test. These analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) and statistical significance was assumed for p equal to or <0.05.
Results
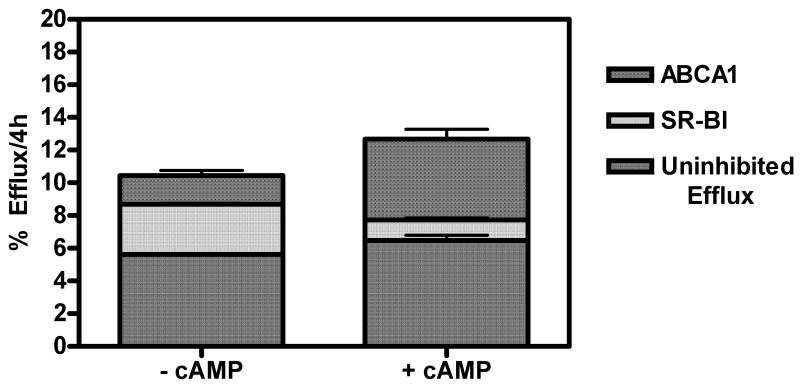
Figure 1 summarizes efflux from J774 macrophages to apoB-depleted serum isolated from a human serum pool. Although the total cholesterol released was only slightly increased by cAMP, this treatment significantly changed the relative contribution of different pathways. Compared to control J774 cells, cAMP increased ABCA1 mediated efflux (-cAMP=1.76±0.524, n=3 vs. +cAMP= 4.96±1.03, n=3; p=0.0086) but the contribution of SR-BI was decreased (-cAMP=3.07±0.111, n=3 vs. +cAMP= 1.25±0.242, n=3; p=0.0003). cAMP treatment also slightly increased in the residual efflux measured in cells treated with both Probucol and BLT-1. The major contribution to this efflux is aqueous diffusion which represents about 50% of the total efflux in control J774 cells; however after cAMP treatment this residual efflux may also include other inhibitor-resistant pathways such as ABCG1, which can be detected by western blot (data not shown). Figure 1 also shows a low level of ABCA1 efflux in control J774 cells. In agreement with Favari et al. 20, this efflux is Probucol sensitive as confirmed by >90% decrease in efflux to 20μg/ml apoA-I from both control and cAMP treated J774 cells (Supplementary Fig. II). Since we cannot specifically measure ABCG1 efflux in cAMP J774 cells, we used transfected BHK-1 cells. In this model, ABCG1 expression is regulated by mifepristone and as previously shown19, 21, mifepristone significantly stimulated cholesterol efflux to both HDL3 and apoB-depleted serum (data not shown).
Figure 1. Contribution of Efflux Pathways to Total Cholesterol Efflux.
Efflux from control and cAMP treated J774 macrophages was measured after 2h pretreatment with inhibitors to block specific cholesterol transporters. All cells were incubated for 4h with apoB-depleted serum isolated from aliquots of a human serum pool at 2.8% (equivalent to 2% serum). All procedures as in methods. Results are the average of 3 independent experiments.
Although we previously measured the efflux capacity of apoB-depleted serum from specimens obtained from a population of healthy male and female subjects 13, in the current study the majority of the experiments were done using serum from the females. There were no differences between the demographic characteristics of the entire population of female subjects (n=130) and those of subjects with similar low (n=22) or similar high (n=18) HDL-C whose serum was chosen for this study (Supplementary Table I).
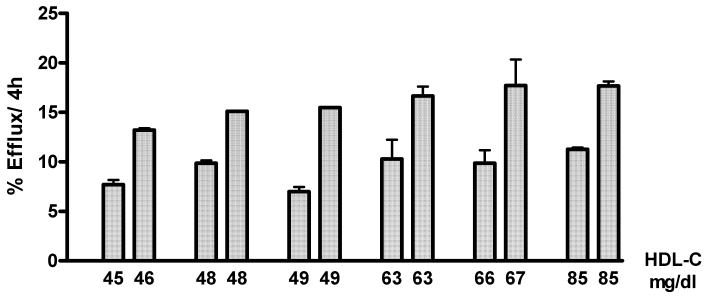
As expected, efflux was significantly associated to both the serum HDL-C and apoA-I levels (Supplementary Fig. IIIA, IIIB; n=130). However these graphs suggest that the apoB-depleted serum isolated from individuals having the same or similar HDL-C or apoA-I can promote significantly different efflux from J774 macrophages. This is better shown by the representative examples in figures 2 (HDL-C) and 3 (apoA-I).
Figure 2. Efflux from cAMP Treated J774 Cells to Serum Pairs with Similar HDL-C.
Total cholesterol efflux from cAMP treated J774 macrophages was measured after 4h incubation with 2.8% apoB-depleted serum as in methods. There were significant differences in efflux (p<0.05) between all pairs with the same or similar HDL-C as determined by unpaired, 2-tailed t tests.
To investigate why serum with similar HDL-C showed high and low efflux efficiency we measured the relative contributions of different pathways to total efflux in two groups of specimens with HDL-C ± 6%: a low HDL group (n=22) with average HDL-C=48mg/dl (range:45-51mg/dl) and a high HDL group (n=18) with average HDL-C=73 mg/dl (range:69-77mg/dl). We used the apoB-depleted serum from each group to measure cholesterol efflux from cAMP treated J774 with no pretreatment or pretreated 2h with Probucol to block ABCA1, BLT-1 to block SR-BI and with both inhibitors to measure the residual efflux. In parallel assays, each apoB-depleted serum was used to assess ABCG1 directly, by measuring efflux from transfected BHK-1 cells, untreated and treated with mifepristone to induce receptor expression. We defined low and high efflux capacity based on total J774 efflux below or above the average of all sera with similar HDL-C and asked if these specimens differed in their ability to promote efflux via individual pathways.
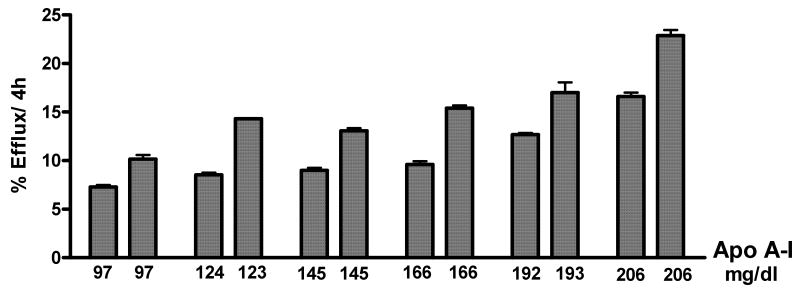
The results of this analysis are shown in Table 1 (low HDL-C sera) and Table 2 (high HDL-C sera). As can be seen, the most dramatic difference we found was that specimens with high efflux capacity had significantly increased ability to promote efflux via ABCA1. We also found a significant but less pronounced increase in the ability of high efflux capacity serum to promote residual or inhibitor-resistant efflux. There were no significant differences in either SR-BI mediated efflux from J774 cells or in ABCG1 mediated efflux from BHK-1 cells. Sera with similar apoA-I (Fig. 3) can also have significantly different macrophage efflux. Thus we selected a subset of the same specimens based on similar apoA-I (apoA-I±6%) and analyzed the efflux values we had obtained. Two groups were formed, one with low apoA-I (average =127mg/dl, =25th percentile, range 119-135, n=11) and one with high apoA-I (average =163mg/dl, =75th percentile, range=153-173, n=10). Sera with similar low levels of apoA-I (127mg/dl) and total macrophage efflux above the average for the group had higher ABCA1 efflux when compared to sera with total efflux below the mean for the group (9.98±0.320 %/4h, n=5 vs. 7.72±0.533 %/4h, n=6; p=0.0073). Likewise, sera with high efflux capacity and similar high apoA-I levels (163mg/dl) also had higher ABCA1 efflux (10.74±0.855 %/4h, n=4 vs. 7.25±0.581 %/4h, n=6; p=0.0004). There were no significant differences in the efflux mediated by any other pathway. Thus, independently of HDL-C or apoA-I level, the increased efficiency of a given apoB-depleted serum to remove cholesterol from macrophages is due to the fact that it can more efficiently promote cholesterol efflux via ABCA1.
Table 1. Average Efflux (% per 4h) For Serum Specimens From Females With HDL-C =48± 6% (HDL-C= 45-51).
| Mean ± SEM | ||||
|---|---|---|---|---|
| Efflux Pathway | Cell Model | Efflux Below Mean (n=11) | Efflux Above Mean (n=11) | P Value |
| Total J774 | J774+ cAMP | 12.7±0.3 | 16.6±0.3 | <0.0001 |
| Uninhibited Efflux | J774+ cAMP | 4.8±0.2 | 5.4±0.2 | p=0.0111 |
| SR-BI | J774+ cAMP | 0.80±0.3 | 1.2±0.3 | NS |
| ABCA1 | J774+ cAMP | 7.6±0.3 | 10.7±0.4 | <0.0001 |
| ABCG-1 | G1-BHK | 2.9±0.5 | 3.4±0.2 | NS |
Efflux values to the serum apoB-depleted serum are given as Mean ± SEM. Mean total J774 efflux for all sera analyzed (n=22) =14.7% per 4h (11.0-18.7). Average total HDL-C in each group was 48 which represents the 25th percentile for the range of HDL-C values in this set of serum specimens from all female donors (n=130).
Table 2. Average Efflux (% per 4h) For Serum Specimens From Females With HDL-C= 73 ± 6% (HDL-C=69-77).
| Mean ± SEM | ||||
|---|---|---|---|---|
| Efflux Pathway | Cell Model | Efflux Below Mean (n=9) | Efflux Above Mean (n=9) | P Value |
| Total J774 | J774+ cAMP | 11.7±0.4 | 15.0±0.5 | <0.0001 |
| Uninhibited Efflux | J774+ cAMP | 4.6±0.2 | 5.7±0.3 | 0.0102 |
| SR-BI | J774+ cAMP | 1.4±0.2 | 1.4±0.3 | NS |
| ABCA1 | J774+ cAMP | 6.2±0.5 | 8.5±0.5 | 0.0072 |
| ABCG-1 | G1-BHK | 2.7±0.3 | 2.6±0.3 | NS |
Efflux values to the serum apoB-depleted serum are given as Mean ± SEM. Mean total J774 efflux for all sera analyzed (n=18) =13.4% per 4h (10.2-17.6). Average total HDL-C in each group was 73 which represents the 75th percentile for the range of HDL-C values in this set of serum specimens from all female donors (n=130).
Figure 3. Efflux from cAMP Treated J774 Cells to Serum Pairs with Similar ApoA-I.
Total cholesterol efflux from cAMP treated J774 macrophages was measured as in figure 2. There were significant differences in efflux (p<0.05) between all pairs with the same or similar apoA-I as determined by unpaired, 2-tailed t tests.
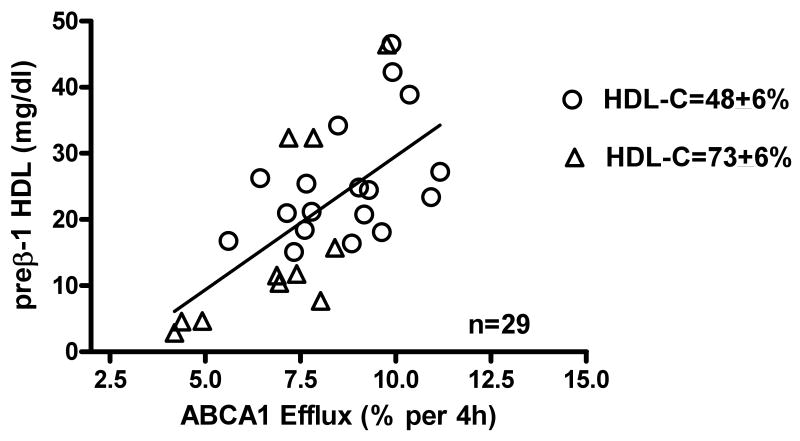
Since the differences in efflux shown in Tables 1 and 2 were not related to serum HDL-C we used 2D gel electrophoresis to measure preβ-1 HDL levels 18. Figure 4 shows that there is a significant correlation between ABCA1 efflux and the level of preβ-1 HDL in all the specimens analyzed using 2-D gels (n=29). The r2 value (r2=0.425) obtained suggests that 43% of the variability in ABCA1 efflux can be explained by the level of preβ-1 HDL in these sera. There were also significant associations between ABCA1 efflux and the preβ-1 HDL levels in sera having similar low or high HDL-C (low HDL-C: r2=0.220, p=0.05, n=18; high HDL-C: r2=0.535, p=0.011, n=11). Although we did not have preβ-1 HDL values for all the sera chosen on the basis of similar apoA-I, the ABCA1 efflux mediated by all the specimens in this smaller subset was also significantly associated with the serum preβ-1 HDL levels measured (r2=0.357, p=0.040, n=12).
Figure 4. Correlation of ABCA1 Efflux to the Concentration of preβ-1 HDL in Serum with Similar High or Low HDL-C.
ABCA1 efflux from cAMP J774 cells was the Probucol sensitive efflux measured after 4h incubation with 2.8% apoB-depleted serum from specimens with similar HDL-C (HDL-C±6%) at either the 25th percentile (open circles: HDL-C=48, range=45-51, n=22) or the 75th percentile (open triangles: HDL-C=73, range 69-77, n=18). The specific contribution of ABCA1 to cholesterol efflux from cAMP treated J774 macrophages was significantly associated to the serum level of preβ-1 HDL (r2=0.425, p=0.0002, n=29) measured by 2D gels. All procedures as in methods.
Although we had focused these studies on the apoB–depleted serum from female donors, confirmatory experiments were done with apoB-depleted serum from healthy males who participated in the same clinical study and have a similar demographic profile (data not shown). We found that whether the specimens from male donors had similar low HDL-C (HDL-C= 38mg/dl±6%, n=14) or similar high HDL-C (HDL-C=63mg/dl±6%, n=16), sera with higher capacity to promote efflux from J774 macrophages had significantly higher ABCA1 efflux (supplementary tables II and III).
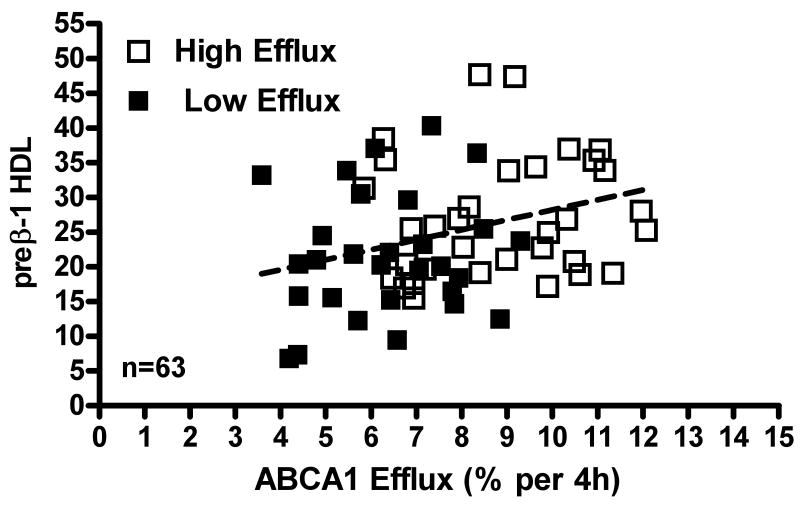
To better link high efflux capacity to increased ABCA1 and preβ-1 HDL, we calculated the average preβ-1 HDL concentration for all sera with high vs. low efflux capacity. As expected, high efflux sera had higher preβ-1 HDL. However, the average values were not significantly different (high = 25+3.5mg/dl, n=15 vs. low= 19+2.6mg/dl, n=14), possibly due to the low number of sera in each group. To increase the statistical power of this analysis, we obtained a commercially available ELISA to establish preβ-1 HDL in all the specimens from female and male donors for which we had ABCA1 efflux (Fig. 5, n=63). Increasing the number of data points allowed us to establish a significant difference in the preβ-1 HDL values measured in specimens with high versus low ABCA1 efflux (high = 27+1.ng/ml, n=33 vs. low=22+1.7ng/ml n=29; p=0.017).
Figure 5. Correlation of ABCA1 efflux to preβ-1 HDL Levels in Serum with High or Low Efflux Capacity.
ABCA1 efflux was measured as in figure 4 using apoB-depleted serum form male and female donors. Levels of preβ-1 HDL in all these specimens (n=63) were measured using a commercial ELISA (see methods) and are reported as ng/ml/aliquot assayed. These values were significantly correlated to ABCA1-mediated efflux (r2=0.102, p=0.011, n=63). Open squares= specimens with high efflux. Closed squares=specimens with low efflux.
Discussion
Studies have shown that measures of HDL subclasses in different populations such as participants in the VA-HIT study 22, post menopausal women23, and Finnish families with low HDL 24 are better predictors of coronary artery disease than measures of HDL-related parameters in serum. We recently reported that the efflux capacity of the apoB-depleted serum isolated from over 200 healthy individuals is negatively associated to CIMT independently of serum HDL-C and apoA-I 13. Others have shown that enhanced ABCA1 efflux from J774 cells to serum from Type IV hypertriglyceridemic subjects was associated to the serum preβ-1 HDL25 and that, in patients with diabetic nephropathy, defects in ABCA1 efflux are related to decreased levels of preβ-1 HDL26. In addition, depletion of preβ-1 HDL by chymase treatment of HDL3 impaired ABCA1 but not SR-BI mediated efflux from J774 macrophages 27. Thus there is increasing evidence that measures of HDL subfractions, especially preβ-1 HDL, and measures of HDL function, such as in vitro assay of cholesterol efflux, may be useful adjuncts to the traditional assays of serum HDL-C and apoA-I when evaluating cardiovascular risk.
The experiments we now report agree with the concept that measures of HDL parameters in serum do not always reflect HDL function. We measured cholesterol efflux to the apoB-depleted serum, isolated from human specimens by removing apoB lipoproteins18, and used cAMP treated J774 macrophages as cholesterol donors. Although aqueous diffusion and ABCA1 are the major pathways contributing to efflux in these cells, other efflux receptors are expressed and likely functional. As expected we found significant associations between efflux and both the serum HDL-C and apoA-I levels when we considered efflux results obtained with all specimens from female donors (supplementary Fig III A, B; n=130). These associations validate our assay and, in agreement with figure 1, suggest that efflux from the J774 macrophages is mediated by numerous pathways interacting with both lipid–rich and lipid-poor HDL particles. However, many individuals with the same serum HDL-C or apoA-I have different capacities to promote cellular cholesterol efflux. This is clearly seen in figures 2 and 3 where we show representative pairs of individual specimens with the same or similar HDL-C (Fig 2) or apoA-I (Fig. 3) but significantly different efflux efficiencies. Results in tables 1 and 2 and figure 4 indicate why neither measures of HDL-C nor apoA-I predict the capacity of individual sera to promote efflux from macrophages. Thus when we used a published method15 to establish the relative contribution of various efflux pathways with serum samples having either similar low HDL-C (Table 1) or high HDL-C (Table 2), serum having a higher capacity to promote efflux of cholesterol from J774 macrophages could more efficiently promote efflux via ABCA1 which in turn was significantly associated with the concentration of preβ-1 HDL (Fig. 4). Figure 5 shows that, when we measured preβ-1 HDL in all sera for which we had ABCA1 efflux (n=63), higher levels of preβ-1 HDL resulted in higher ABCA1 efflux.
Since total macrophage efflux may also be independent of apoA-I levels (fig. 3), we analyzed a subset of the same specimens based on similar high or low apoA-I. As for HDL-C, regardless of apoA-I levels, specimens with higher efflux showed a higher capacity to promote efflux via ABCA1 and this efflux was associated with the levels of serum preβ-1 HDL.
It is thought that the activity of phospholipid transfer protein (PLTP), hepatic triglyceride lipase (HTLP) and cholesteryl ester transfer protein (CETP) can increase serum preβ-1 HDL levels whereas lecithin-cholesterol acyltransferase (LCAT) activity has the opposite effect 24,26,28. Our data base includes only CETP mass and the level of CETP in sera we identified as having higher ABCA1 efflux and preβ-1 HDL was not different than that in low efflux sera. This could be either because the number of sera in each group is too small or because measures of CETP activity would be a better indicator.
It has been reported that modifications of HDL particles affect their functionality by either changing their specificity towards receptors 29,30 or their ability to promote efflux. Thus Francis et al. have shown that oxidative tyrosylation of HDL promotes formation of AI-AII heterodimers that can enhace efflux 31,32. In contrast, oxidation of apo A-I by myeloperoxidase can significantly impair ABCA1-mediated efflux. 33. Since we used a serum specimens from a healthy normolipedemic population we believe it is unlikely that our results were significantly affected by the presence of abnormal HDL.
The experiments reported in this paper were done with apoB-depleted serum from female donors; however comparable results were obtained in a small study using apoB-depleted serum from healthy male donors who participated in the same clinical study and have a similar demographic profile. Thus for specimens with similar low or high HDL-C, a higher capacity to promote efflux from J774 macrophages was due to significantly higher ABCA1 efflux (supplementary tables II and III).
In summary, our experiments show for the first time that the apoB-depleted serum from individuals with similar levels of HDL- C or apoA-I may have very different capacities to remove cholesterol from macrophages. In our cell model, specimens from subjects with either similar HDL-C or apo A-I that have higher efflux capacity can better promote efflux via ABCA1 and this is related, in large part, to the serum concentration of preβ-1 HDL. These novel results add to the increasing evidence that measures of HDL function, such as its efflux capacity, can be useful when assessing an individual's CHD risk.
Supplementary Material
Acknowledgments
This project was supported by HL-22633. The authors would like to acknowledge Vinh Nguyen, The Children's Hospital of Philadelphia, for excellent technical assistance. We thank Dr. Jack Oram (University of Washington, Seattle, WA) for the ABCG1and ABCA1 transfected BHK cells. The authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margarita de la Llera-Moya, Division of Gastroenterology and Nutrition, The Children's Hospital of Philadelphia.
Denise Drazul-Schrader, Division of Gastroenterology and Nutrition, The Children's Hospital of Philadelphia.
Bela F. Asztalos, Lipid Metabolism Laboratory, Tufts University
Marina Cuchel, The Institute of Translational Medicine and Therapeutics, The University of Pennsylvania School of Medicine.
Daniel J. Rader, The Institute of Translational Medicine and Therapeutics, The University of Pennsylvania School of Medicine
George H. Rothblat, Division of Gastroenterology and Nutrition, The Children's Hospital of Philadelphia
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Amer J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Genest JJ, Marcil M, Dennis M, Yu L. High density lipoproteins in health and disease. J Investig Medl. 1999;47:31–42. [PubMed] [Google Scholar]
- 4.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT a randomized control trial. J Am Med Assoc. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 5.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein:a new therapeutic target at the crossroads of dyslipidemia, inflammation and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 7.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 8.Breslow JL. Transgenic mouse models of lipoprotein metabolism and arterosclerosis. Proc Nat Acad Sci USA. 1993;90:8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis. A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 10.Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, Salvetti M, Monteduro C, Zulli R, Muiesian ML, Agabiti-Rosei E. Cardiovascular status of carriers of th eapo lipoprotein A-I (Milano) mutant: the Limone sui Garda study. Circulation. 2001;103:1949–1954. doi: 10.1161/01.cir.103.15.1949. [DOI] [PubMed] [Google Scholar]
- 11.Hirano K, Yamashita S, Nakajima N, Arai T, Maruyama T, Yoshida Y, Ishigami M, Sakai N, Kameda-Takemura K, Matsuzawa Y. Genetic cholesterol ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinimia caused by CETP gene mutation is not associated with longevity. Aterioscler Throm Vasc Biol. 1997;17:1053–1059. doi: 10.1161/01.atv.17.6.1053. [DOI] [PubMed] [Google Scholar]
- 12.Cuchel M, Rader DJ. Genetics of increased HDL cholesterol levels: insights into the relationship between HDL metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1710–1712. doi: 10.1161/01.ATV.0000092947.15939.93. [DOI] [PubMed] [Google Scholar]
- 13.Cuchel M, de la Llera-Moya M, Phillips JA, Wolfe ML, Rothblat GH, Rader DJ. Cholesterol efflux capacity of serum predicts carotid intimal-medial thickness independently of HDL-C and apo A-I levels. Circulation. 2008;118:S_ 371. Abstract 1695. [Google Scholar]
- 14.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metabolism. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The role of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Smith SJ, Cooper GR, Myers GL, Sampson EJ. Biological variability in concentrations of serum lipids: sources of variation among results from published studies and composite predicted values. Clin Chem. 1993;39:1012–1022. [PubMed] [Google Scholar]
- 17.McGillicuddy FC, de la Llera-Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apo lipoproteins. J Biol Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 20.Favari E, Zannotti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Atheroscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Value of high density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the veterans affairs HDL Intervention trial. Aterioscler Throm Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Herrington DM, Reboussin DM, Sherman M, Horvath KV, Cupples LA, White C, Demissie S, Schaefer EJ, Asztalos BF. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 2008;28:575–579. doi: 10.1161/ATVBAHA.107.157123. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe H, Soderlund S, Soro-Paavonen A, Hiukka A, Leinonen E, Alagona C, Salonen R, Tuomainen TP, Ehnoholm C, Jauhiainen M, Taskinen MR. Decreased high-density lipoprotein (HDL) particle size, prebeta-, and large HDL subspecies concentration in Finnish low-HDL families: relationship with intima-media thickness. Arterioscler Thromb Vasc Biol. 2006;26:897–902. doi: 10.1161/01.ATV.0000209577.04246.c0. [DOI] [PubMed] [Google Scholar]
- 25.Attia N, Ramaharo A, Paul JL, Cambillau M, Beaune P, Grynberg A, Simon A, Fournier N. Enhanced removal of cholesterol from macrophage foam cells to serum from type IV hypertriglyceridemic subjects. Atherosclerosis. 2008;198:49–56. doi: 10.1016/j.atherosclerosis.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Tan KCB, Shiu SWM, Wong Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab Res Rev. 2008;24:617–623. doi: 10.1002/dmrr.895. [DOI] [PubMed] [Google Scholar]
- 27.Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, Kovanen PT. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279:9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor PM, Zysow BR, Schoenhaus SA, Ishida BY, Kunitake ST, Naya-Vigne JM, Duchateau PN, Redberg RF, Spencer SJ, mark S, Mazur M, Heilbron DC, Jaffe RB, Malloy MJ, Kane JP. Prebeta-1 HDL in plasma of normolipedemic individuals: influences of plasma lipoproteins, age, and gender. J Lipid Res. 1998;39:670–678. [PubMed] [Google Scholar]
- 29.Kleinherenbrink-Stins MF, Schouten D, vanderBroom J, Brouwer A. Nitrosylated high density lipoprotein is recognized by a scavenger receptor in rat liver. J Lipid Res. 1989;30:511–520. [PubMed] [Google Scholar]
- 30.La Ville AE, Sola R, Balanya J, Turnner PR, Masana L. In vitro oxidised HDL is recognised by the scavenger receptor of macrophages:Implications for its protective role in vivo. Atherosclerosis. 1994;105:179–189. doi: 10.1016/0021-9150(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 31.Francis GA, Mendez AJ, Bierman EL, Heinecke JW. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc Natl Acad Sci USA. 1993;90:6631–6635. doi: 10.1073/pnas.90.14.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang WQ, Merriam DL, Moses AS, Francis GA. Enhanced cholesterol efflux by tyrosyl radical-oxidized high density lipoprotein is mediated by apolipoprotein AI-AII heterodimers. J Biol Chem. 1998;273:17391–17398. doi: 10.1074/jbc.273.28.17391. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.