Abstract
We examined for immediate and persistent changes in nAChRs in cerebral cortex, thalamus and striatum of male rats caused by prenatal exposure to nicotine from gestational day 3 to postnatal day 10 (PN10), and how such exposure affected the responses of adolescents to subsequent nicotine challenge. Receptor numbers were assessed by [3H]epibatidine binding and receptor function was measured by acetylcholine-stimulated 86Rb efflux (cerebral cortex and thalamus) and nicotine-stimulated dopamine release (striatum). Immediate effects of prenatal nicotine, assessed in PN10 animals, were not detected for any parameter. A subsequent 14 day nicotine exposure in adolescence revealed persistent changes caused by prenatal nicotine exposure. Nicotine exposure in adolescents caused up-regulation of binding in all three regions; however, this up-regulation was lost in thalamus from animals prenatally exposed to nicotine. Nicotine exposure in adolescents caused decreased nicotine-stimulated dopamine release in striatum; this effect was also lost in animals prenatally exposed to nicotine. Comparison of parameters in PN10 and PN42 rats revealed developmental changes in the CNS cholinergic system. In thalamus, binding increased with age, as did the proportion of 86Rb efflux with high sensitivity to acetylcholine. In cortex, binding also increased with age, but there was no change in total 86Rb efflux, and the proportion of high to low sensitivity efflux declined with age. Nicotine-stimulated striatal dopamine release (both total and α-conotoxin MII-resistant release) increased with age in naïve animals, but not in those prenatally exposed to nicotine. These findings demonstrate that prenatal exposure to nicotine causes alterations in the regulation of nAChRs by nicotine that persist into adolescence. These changes may play a role in the increased risk for nicotine addiction observed in adolescent offspring of smoking mothers.
Keywords: Nicotine, pregnancy, prenatal exposure, adolescence, nicotinic receptor subtypes
INTRODUCTION
Approximately 25% of all pregnant women in the United States smoke (Slotkin 1998). Maternal smoking during pregnancy is associated with a number of adverse behavioral and cognitive outcomes in the offspring (Lichtensteiger et al. 1988). The underlying neurobiological mechanism responsible for these deficits is still unknown, but nicotine-induced alterations in development of central neurotransmitter systems have been implicated (Lichtensteiger et al. 1988; Pliszka et al. 1996; Castellanos 1997). The effects of nicotine, the principal psychoactive agent in tobacco, are mediated through various subtypes of neuronal nicotinic acetylcholine receptors (nAChRs), but little is currently known about the properties of nAChRs during early development (Azam et al. 2007).
In the developing brain, neurotransmitter activation of receptors, including activation of nAChRs by acetylcholine (ACh), plays a unique role in establishing proper synaptic connectivity. Fetal nicotine exposure causes activation of nAChRs at inappropriate times and at levels beyond those experienced in the normal course of development by endogenous ACh; such non-physiological activation is likely to cause severe alterations in development (Hohmann et al. 1988; Navarro et al. 1989; Slikker, Jr. et al. 2005). Evidence for altered CNS cholinergic neurotransmission by prenatal nicotine exposure comes from experiments measuring the ratio of choline uptake to choline acetyltransferase activity, a biochemical marker of neuronal impulse activity. This ratio normally peaks by postnatal day 10; however, rats exposed to prenatal nicotine showed blunted activity before and during that developmental spike (Navarro et al. 1988). Prenatal nicotine exposure also disrupts the programming of cholinergic function in adolescence and adulthood (Abreu-Villaca et al. 2004; Slikker, Jr. et al. 2005; Navarro et al. 1989), and the vulnerability of cholinergic systems to persistent disruption by nicotine continues into adolescence (Abreu-Villaca et al. 2003; Trauth et al. 1999; Trauth et al. 2000).
Adolescence is known to be a vulnerable period for development of drug addiction, including nicotine addiction. One contributing factor that predisposes adolescents to nicotine addiction is maternal smoking during pregnancy. A recent study reported that offspring of mothers who smoked during pregnancy were more likely to smoke regularly than adolescents whose mothers did not smoke at all or who had smoked only before and/or after pregnancy (Al Mamun et al. 2006). A deficient response to nicotinic stimulation in adolescents whose mothers smoked during pregnancy may serve to drive higher cigarette consumption, which could in turn increase the likelihood of eventual addiction (Chassin et al. 1996; Chen and Millar 1998). Evidence for this comes from a study of prenatal exposure in rats which was found to impair nAChR up-regulation in response to adolescent nicotine treatment (Abreu-Villaca et al. 2004). We have previously reported that chronic nicotine alters the expression and function of specific subtypes of nAChRs in adult rats, and furthermore that nicotine’s effects differ in adolescent rats exposed to chronic nicotine compared to those in adults (Perry et al. 2007; Doura et al. 2008). Prenatal nicotine exposure may lead to changes that subsequently affect the expression and function of nAChRs in the adolescent brain that have been re-exposed to nicotine, contributing to an altered biological response and an increased susceptibility for addiction.
In this study, we used an established rat model of nicotine administration that maintains steady-state plasma levels at those found in moderate to heavy smokers (Murrin et al. 1987; Hukkanen et al. 2005; Slotkin 1998; Matta et al. 2006). Nicotine is administered from gestational day 3 through postnatal day 10. Thus, animals sacrificed at PN10 allowed determination of the immediate effects of prenatal nicotine exposure. In some offspring, a subsequent 14 day course of nicotine was administered during adolescence. Animals sacrificed at PN42 allowed determination of persistent effects of prenatal nicotine exposure. Persistent effects include those that were directly observable, in animals that did not receive nicotine during adolescence, and indirect effects, determined by changes in the response to nicotine given during adolescence. We tested for the effects of exposure patterns on nAChR number and function in male rats using [3H]epibatidine binding, ACh-stimulated 86Rb efflux and nicotine-stimulated [3H]dopamine release.
RESULTS
Biphasic response for ACh-stimulated 86Rb efflux
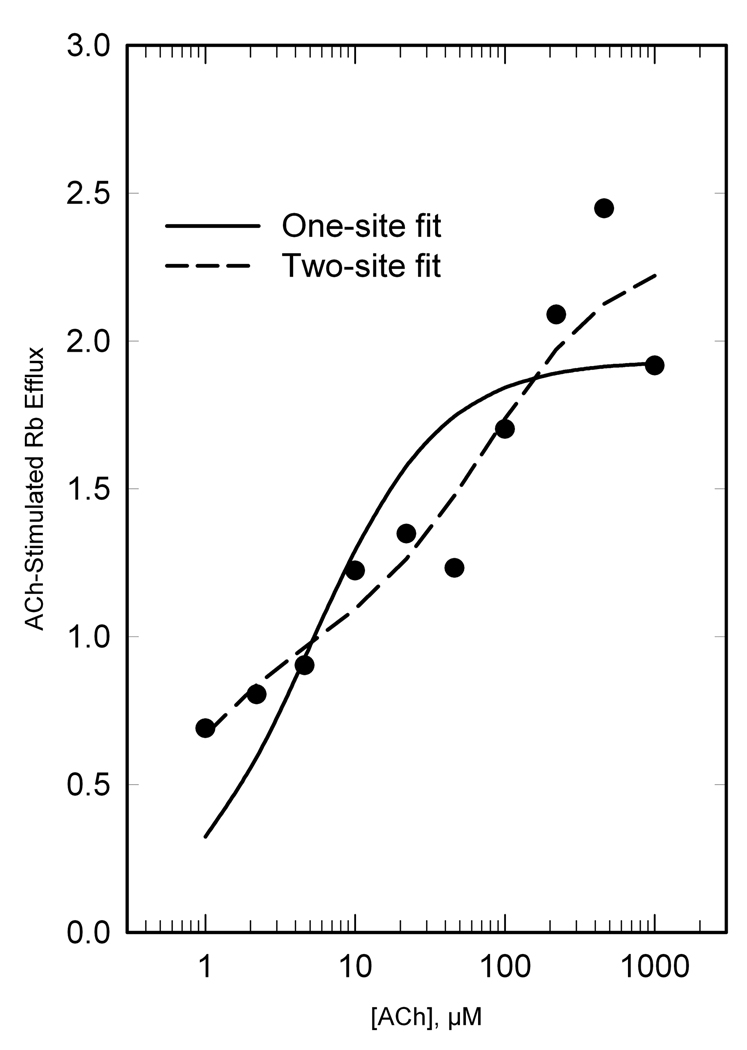
Initial evaluation of concentration-response curves for ACh-stimulated 86Rb efflux indicated the possibility of a biphasic response. Marks and colleagues previously demonstrated a biphasic ACh concentration-response curve for 86Rb efflux in mouse synaptosomes (Marks et al. 1999) (Gotti et al. 2008). When fitted to the Hill equation, data from all six treatment groups and both regions showed Hill coefficients less than unity (Table 1), supporting a biphasic response. Accordingly, we analyzed 86Rb efflux data from all treatment groups for a two-site fit (Marks et al. 1999) (Gotti et al. 2008). Results of the two-site fits from thalamus and cortex of rats for each of the six treatment groups provided twelve separate determinations for the high sensitivity potency (Khs) and low sensitivity potency (Kls). No significant differences in either Khs (average 1.92 µM) or Kls (average 143 µM) were observed between regions or among treatment groups. Subsequently, results for each experiment were re-fit to the two-component model, with the values for Khs and Kls fixed at 1.92 and 143 µM respectively, to obtain estimates of maximal responses for the high sensitivity component (Ehs) and low sensitivity component (Els) for every individual rat. An example showing the one-site and two-site fit is shown for one treatment group in Fig. 1. In this example, the proportion of low and high sensitivity components is approximately equal; the proportion varied considerably among treatment groups (Table 1).
TABLE 1.
Parameters of ACh-stimulated 86Rb efflux in thalamus and cerebral cortex: Hill coefficient (nH), total efflux, and percent of total efflux with high sensitivity to ACh (HS).
| Treatment group |
Thalamus | Cortex | ||||
|---|---|---|---|---|---|---|
| nH | Total efflux |
% HS | nH | Total Efflux |
% HS | |
| PreSal | 0.43 | 1.43 | 41% | 0.22 | 1.32 | 54% |
| PreNic | 0.44 | 1.76 | 28% | 0.30 | 1.65 | 51% |
| PreSal/AdolSal | 0.37 | 2.31 | 51% | 0.58 | 1.80 | 49% |
| PreSal/AdolNic | 0.51 | 1.97 | 65% | 0.37 | 1.94 | 43% |
| PreNic/AdolSal | 0.62 | 2.18 | 50% | 0.84 | 1.78 | 33% |
| PreNic/AdolNic | 0.83 | 1.53 | 71% | 0.46 | 1.71 | 40% |
Figure 1. ACh stimulation of 86Rb efflux from thalamic synaptosomes of PreSal/AdolSal (adolescent) rats, illustrating two components.
Data were fit to both a one-site model (solid line) and a two-site model (dashed line) as described in Methods. For the two-site fit, we determined the potency (K) and capacity (Emax) for the high sensitivity component (Khs and Ehs) and low sensitivity component (Kls and Els). Subsequently, results for each experiment were fit to a two-component model, with values for Khs and Kls fixed at 1.92 and 143 µM respectively, to obtain estimates of Ehs and Els for every individual rat. In this example, the proportion of high sensitivity to low sensitivity component was 51%/49%.
Effects of age on nAChR expression and function
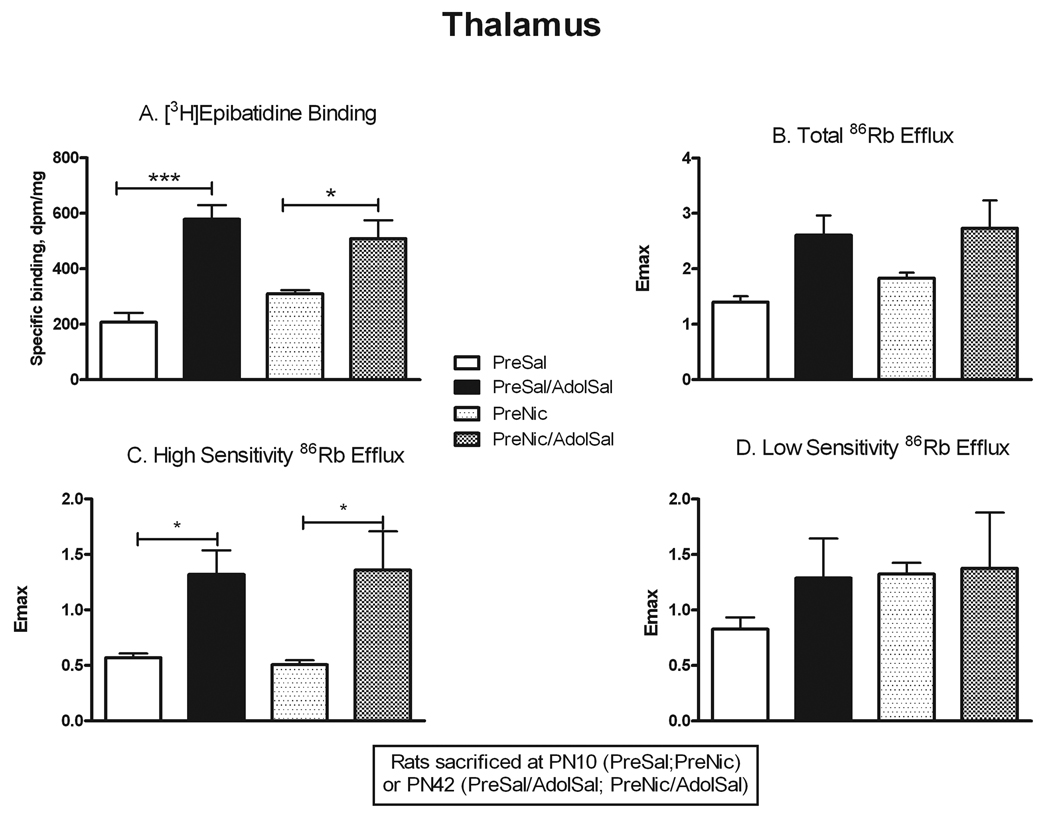
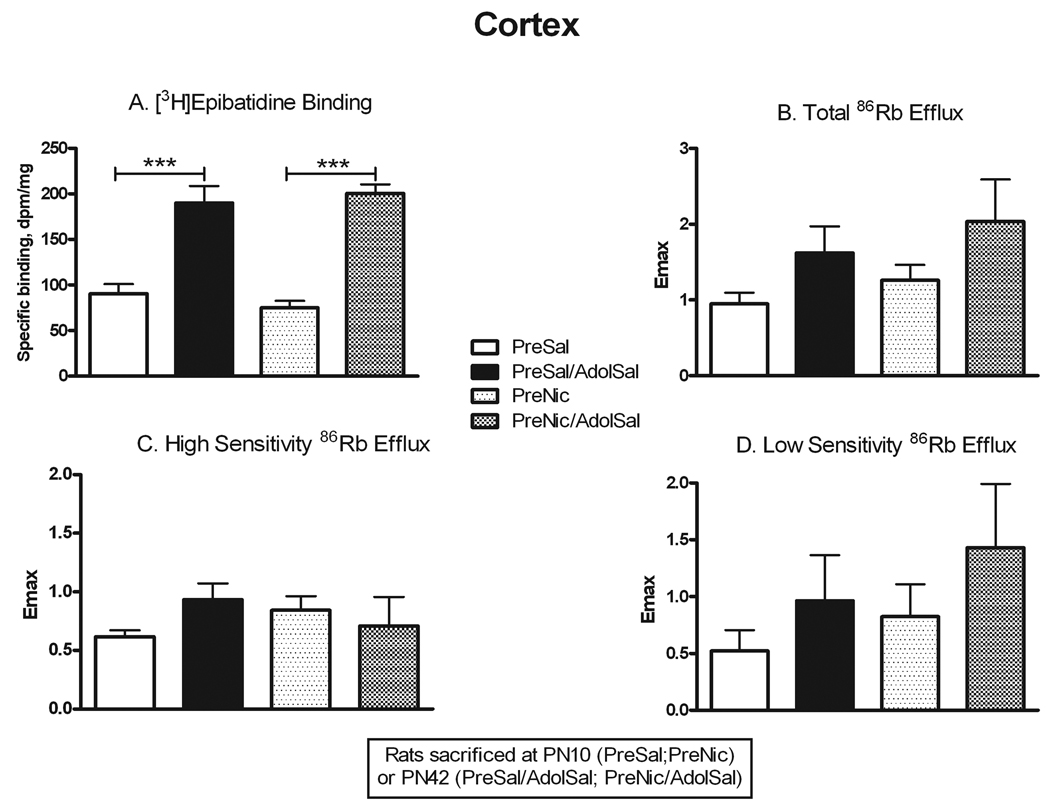
Comparison of neonatal rats (PN10) to adolescent rats (PN42) allowed determination of age-related changes in nAChR number and function independent of prenatal nicotine exposure (PreSal vs. PreSal/AdolSal), as well as in those animals exposed to nicotine prenatally only (PreNic vs. PreNic/AdolSal). Binding to α4β2* receptors in thalamus (Fig. 2) was significantly greater in adolescents compared to PN10 rats, both in saline-pretreated rats (+178%, p<0.001) and nicotine-pretreated rats (+64%, p<0.05). Total ACh-stimulated 86Rb efflux, while demonstrating a similar trend, showed no significant overall change (Fig. 2; Table 1). However, the high sensitivity component of the 86Rb efflux in thalamus did significantly increase with age in both treatment groups (+132% in saline-pretreated, +166% in nicotine-pretreated, both p<0.05). Because there was no concomitant change in the low sensitivity component, the ratio of high to low sensitivity efflux in thalamus also increased with age from PN10 to PN42 (Table 1). Thus, the up-regulation in α4β2* binding in thalamus with age appeared to correlate with a similar up-regulation in high sensitivity 86Rb efflux. Binding to α4β2* nAChRs in cortex (Fig. 3) also increased from PN10 to PN42 (+110% in saline-pretreated, +168% in nicotine-pretreated, both p<0.001). However, unlike in thalamus, no significant differences were seen in ACh-stimulated 86Rb efflux (Fig. 3), despite a trend towards increase in total efflux. The proportion of efflux in cortex with high sensitivity to ACh was relatively higher in neonates, declining somewhat in adolescents, again in contrast to results in thalamus (Table 1).
Figure 2. Changes in nAChR binding and ACh-stimulated 86Rb efflux from PN10 to PN42 in thalamus.
Rats were sacrificed either at PN10 (PreSal; PreNic) or at PN42 (PreSal/AdolSal; PreNic/AdolSal); the effect of age is shown for animals never receiving nicotine (PreSal v. PreSalAdolSal), and for those receiving prenatal nicotine (PreNic v. PreNicAdolSal). Means were analyzed by two-way ANOVA for age and treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=7–10); *p<0.05; ***p<0.001. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; age effect F(1,27) = 37.08; p<0.0001. B. Emax values for total ACh-stimulated 86Rb efflux from thalamic synaptosomes. C. Emax values for the high sensitivity component of ACh-stimulated 86Rb efflux; age effect F(1,24) = 18.22; p<0.0001. D. Emax values for the low sensitivity component of ACh-stimulated 86Rb efflux.
Figure 3. Changes in nAChR binding and ACh-stimulated 86Rb efflux from PN10 to PN42 in cerebral cortex.
Rats were sacrificed either at PN10 (PreSal; PreNic) or at PN42 (PreSal/AdolSal; PreNic/AdolSal); the effect of age is shown for animals never receiving nicotine (PreSal v. PreSalAdolSal), and for those receiving prenatal nicotine (PreNic v. PreNicAdolSal). Means were analyzed by two-way ANOVA for age and treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=6–10); ***p<0.001. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; age effect F(1,26) = 77.15; p<0.0001. B. Emax values for total ACh-stimulated 86Rb efflux from cortical synaptosomes; age effect F(1,16) = 5.54; p<0.05. C. Emax values for the high sensitivity component of ACh-stimulated 86Rb efflux. D. Emax values for the low sensitivity component of ACh-stimulated 86Rb efflux.
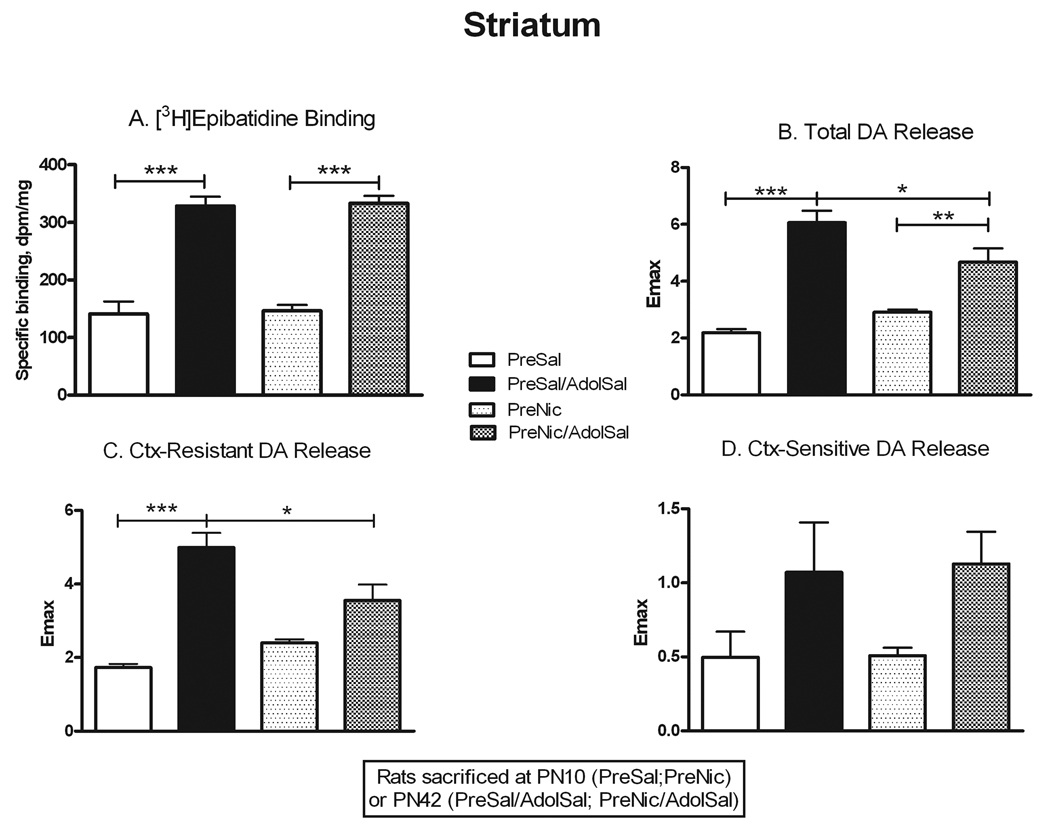
As in the other two regions, binding in striatum (Fig. 4) also increased significantly with age in both saline-pretreated rats (+133%, p<0.001) and nicotine-pretreated rats (+127%, p<0.001). To assess receptor functionality in this region, we measured nicotine-stimulated dopamine release. Total release followed a similar pattern of age-related increases as binding (+177%, p<0.001 in saline-pretreated, +61%, p<0.01 in nicotine-pretreated; Fig. 4). αCtxMII-resistant release (α4β2*-mediated) also increased in saline-pretreated animals (+188%, p<0.001), but this age effect was absent in nicotine-pretreated animals (Fig. 4). No significant changes occurred with age for the αCtxMII-sensitive component of release (α6*-mediated).
Figure 4. Changes in nAChR binding and nicotine-stimulated dopamine release from PN10 to PN42 in striatum.
Rats were sacrificed either at PN10 (PreSal; PreNic) or at PN42 (PreSal/AdolSal; PreNic/AdolSal); the effect of age is shown for animals never receiving nicotine (PreSal v. PreSalAdolSal), and for those receiving prenatal nicotine (PreNic v. PreNicAdolSal). Means were analyzed by two-way ANOVA for age and treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=9–11); *p<0.05; **p<0.01; ***p<0.001. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; age effect F(1,27) = 141.45; p<0.0001. B. Emax values for total nicotine-stimulated dopamine release from striatal synaptosomes; age effect F(1,29) = 32.24; p<0.0001. C. Emax values for the conotoxin-resistant release (in the presence of 50 nM αCtxMII, leaving α4β2*-mediated release); age effect F(1,29) = 20.92; p<0.0001. D. Emax values for the conotoxin-sensitive release (conotoxin-resistant release subtracted from total release; mediated by α6*-nAChRs); age effect F(1,29) = 9.57; p<0.005.
Immediate effects of prenatal nicotine exposure
Continuous nicotine exposure in adult rats has been shown to dramatically alter both numbers and function of nAChRs (Perry et al. 2007). While chronic nicotine also up-regulates nAChRs in adolescent rats, this effect is significantly reduced compared to that in adults (Doura et al. 2008). To determine the immediate effects of prenatal nicotine exposure, we compared PN10 animals exposed to prenatal saline (PreSal) to PN10 animals exposed to prenatal nicotine (PreNic) immediately after cessation of exposure. Although there was a trend towards increased binding in thalamus, this did not reach statistical significance (Fig. 2). No immediate effects of prenatal nicotine were seen in nAChR binding in cortex or striatum (Figs. 3 and 4). Likewise, the assays for receptor functionality did not show any significant immediate effects of prenatal nicotine exposure (Figs. 2–4).
Persistent effects of prenatal nicotine exposure
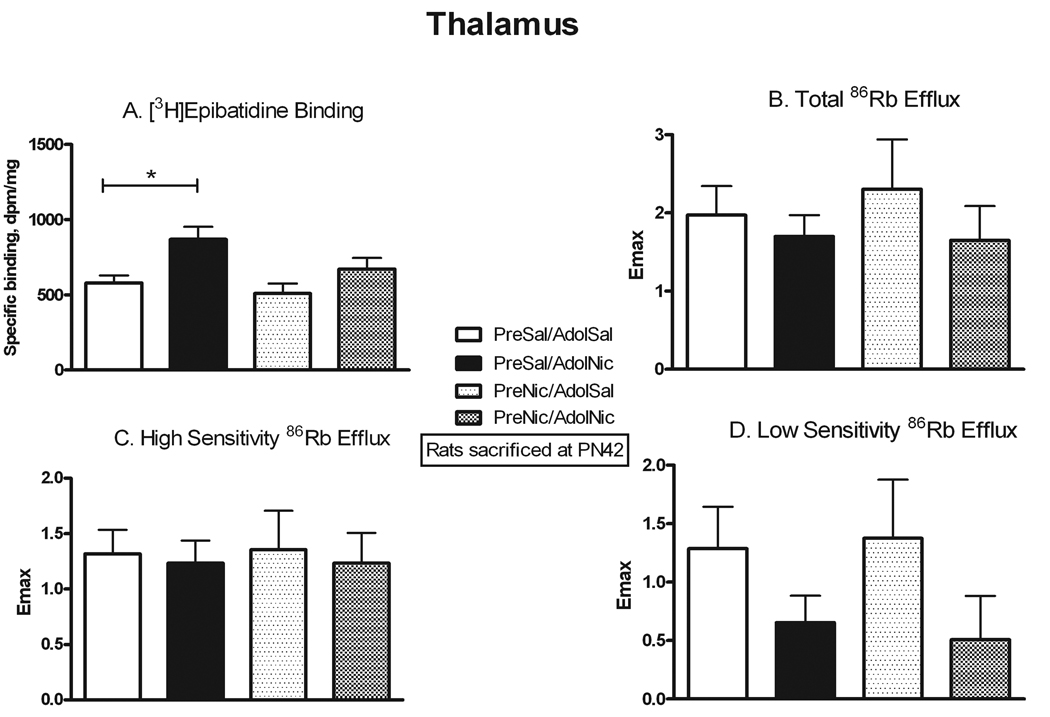
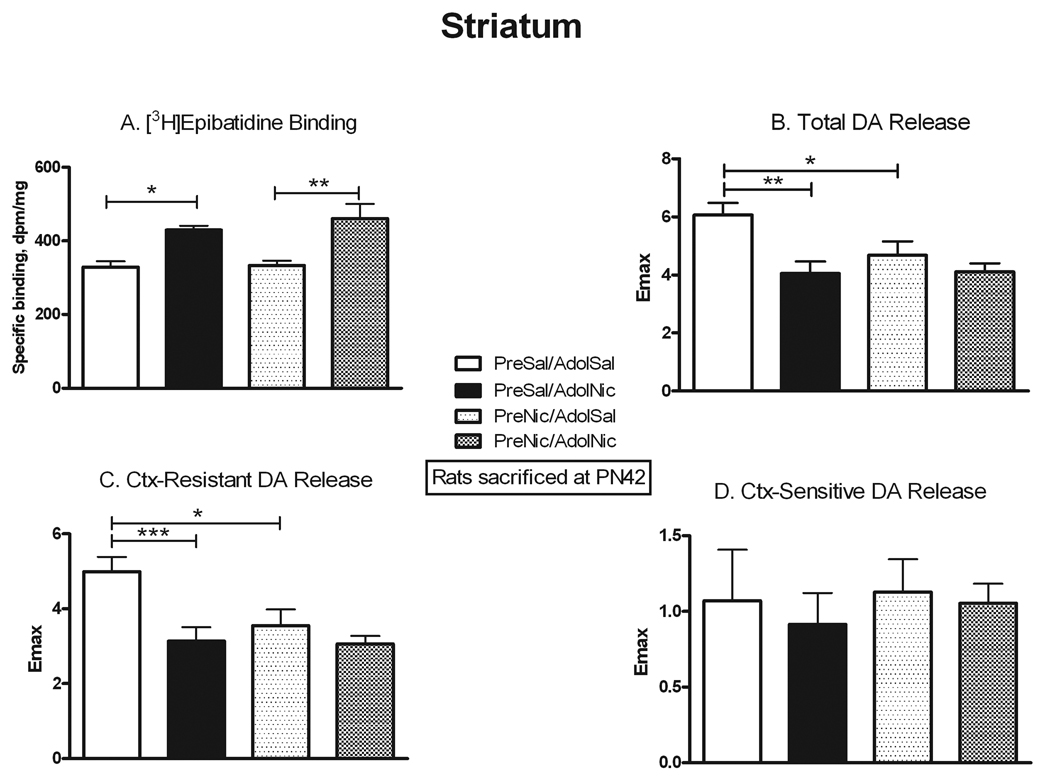
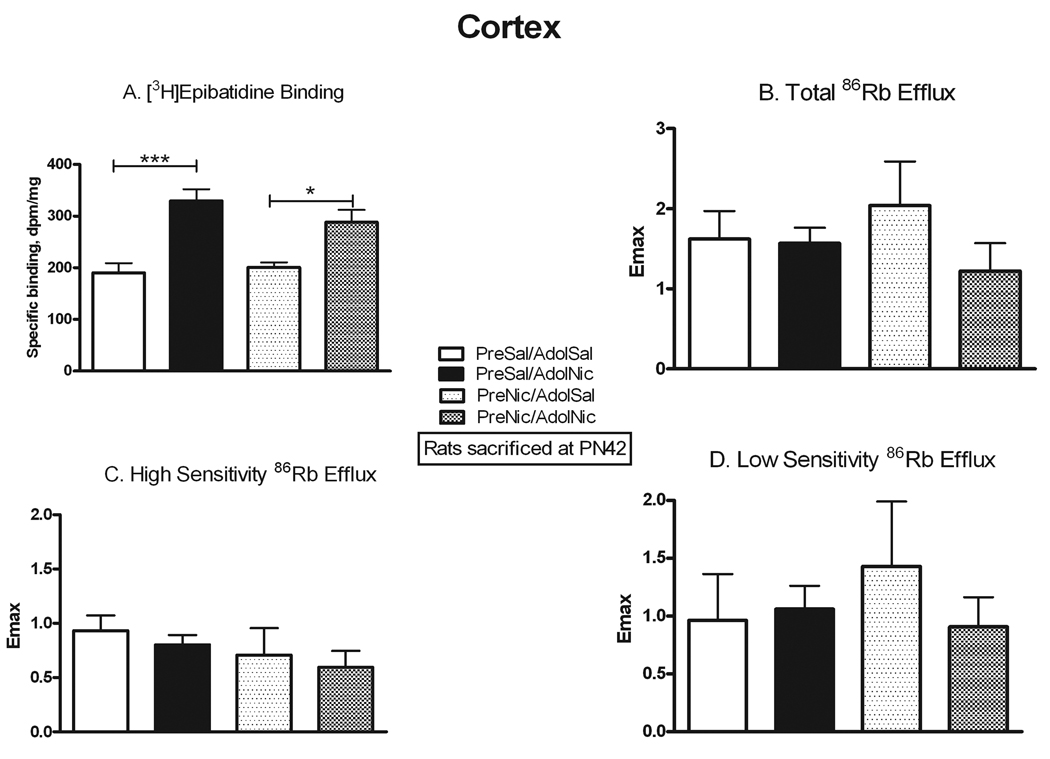
Previous studies have reported that prenatal nicotine exposure can cause behavioral changes that persist into adolescence. We used several approaches to assess whether similarly persistent changes occurred in nAChR number and function after prenatal nicotine. We allowed some animals that had been prenatally exposed to nicotine or saline to grow to adolescence, and then they were further challenged with either chronic nicotine or saline exposure. The comparison of nAChR binding and function in the four adolescent treatment groups is presented in Figures 5–7. To assess for direct persistent effects of prenatal exposure we compared binding and function in naïve animals (PreSal/AdolSal) to those in animals receiving only prenatal nicotine (PreNic/AdolSal). Such exposure did not alter binding in any region, nor were there any significant differences in the ACh-stimulated 86Rb efflux in thalamus or cortex (although the effect of adolescent nicotine on low sensitivity efflux approached significance, p=0.052). Likewise, comparison of results in those animals challenged with nicotine as adolescents (i.e. PreSal/AdolNic vs. PreNic/AdolNic) revealed no significant differences in any measured parameter. However, nicotine-stimulated dopamine release in rats previously exposed prenatally to nicotine (PreNic/AdolSal) was significantly lower than in those animals not prenatally exposed (PreSal/AdolSal). This decrease was seen both for total release (23% lower, p<0.05) and for αCtxMII-resistant (α4β2*-mediated) release (29% lower, p<0.05) (Fig 7).
Figure 5. Persistent effects of prenatal nicotine exposure on nAChR numbers and ACh-stimulated 86Rb efflux in thalamus.
All animals were sacrificed at PN42; the effect of nicotine administered in adolescents is shown in animals never before exposed (PreSal/AdolSal v. PreSal/AdolNic), and in animals prenatally exposed to nicotine (PreNic/AdolSal v. PreNic/AdolNic). Means were analyzed by two-way ANOVA for prenatal treatment and adolescent treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=7–10); *p<0.05. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; adolescent nicotine effect, F(1,28) = 10.58; p<0.005. B. Emax values for total ACh-stimulated 86Rb efflux from thalamic synaptosomes. C. Emax values for the high sensitivity component of ACh-stimulated 86Rb efflux. D. Emax values for the low sensitivity component of ACh-stimulated 86Rb efflux. There was a suggestion of an adolescent nicotine effect: F(1,23) = 4.196, p=0.052.
Figure 7. Persistent effects of prenatal nicotine exposure on nAChR numbers and nicotine-stimulated dopamine release in striatum.
All animals were sacrificed at PN42; the effect of nicotine administered in adolescents is shown in animals never before exposed (PreSal/AdolSal v. PreSal/AdolNic), and in animals prenatally exposed to nicotine (PreNic/AdolSal v. PreNic/AdolNic). Means were analyzed by two-way ANOVA for prenatal treatment and adolescent treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=7–10); *p<0.05; **p<0.01; ***p<0.001. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; adolescent nicotine effect F(1,28) = 23.46, p<0.0001. B. Emax values for total nicotine-stimulated dopamine release from striatal synaptosomes. Interaction between adolescent and prenatal nicotine treatment, F(1,34) = 121.56; p<0.0001; adolescent nicotine effect F(1,34) = 14.12, p<0.001; prenatal nicotine effect F(1,34) = 90.44, p<0.0001. C. Emax values for the conotoxin-resistant release (in the presence of 50 nM αCtxMII, leaving α4β2*-mediated release). Interaction between adolescent and prenatal nicotine treatment, F(1,31) = 74.33; p<0.0001; adolescent nicotine effect F(1,31) = 11.17, p<0.005; prenatal nicotine effect F(1,31) = 117.28, p<0.0001. D. Emax values for the conotoxin-sensitive release (conotoxin-resistant release subtracted from total release; mediated by α6*-nAChRs).
Effects of prenatal nicotine exposure on adolescent response to nicotine challenge
Another way to examine for persistent effects of prenatal nicotine exposure is to test whether the effects of adolescent nicotine exposure on receptor number and function differ in animals with prior prenatal nicotine exposure. In agreement with our previous autoradiographic study in adolescents (Doura et al. 2008), α4β2* binding was significantly up-regulated by chronic nicotine exposure in thalamus (+50%, p<0.05) and cortex (+74%, p<0.001) in naïve adolescent rats (i.e. PreSal/AdolSal vs. PreSal/AdolNic; Figs. 5 and 6). However, while up-regulation was also seen in cortex (+43%, p<0.05; Fig 6) from adolescent rats that had been prenatally exposed to nicotine (PreNic/AdolSal vs. PreNic/AdolNic), up-regulation did not occur in thalamus from prenatally exposed rats (Fig. 5). Despite binding increases with adolescent nicotine, no changes were seen in either component of ACh-stimulated 86Rb efflux, regardless of prenatal treatment (Figs. 5 and 6), although there was a non-significant trend towards an increase in ratio of high to low sensitivity in thalamus.
Figure 6. Persistent effects of prenatal nicotine exposure on nAChR numbers and ACh-stimulated 86Rb efflux in cerebral cortex.
All animals were sacrificed at PN42; the effect of nicotine administered in adolescents is shown in animals never before exposed (PreSal/AdolSal v. PreSal/AdolNic), and in animals prenatally exposed to nicotine (PreNic/AdolSal v. PreNic/AdolNic). Means were analyzed by two-way ANOVA for prenatal treatment and adolescent treatment; F ratios and significance shown for those comparisons with a significant main effect. Unless noted, there were no significant interactions between parameters. For those with a significant main effect, we proceeded to analyze the four treatment means by one-way ANOVA and Tukey’s post-test. Means +/− S.E.M. are shown (N=6–10); *p<0.05; ***p<0.001. A. Binding of 0.5 nM [3H]epibatidine, in the presence of 50 nM αCtxMII to inhibit binding to α6* nAChRs; adolescent nicotine effect F(1,27) = 34.16; p<0.0001. B. Emax values for total ACh-stimulated 86Rb efflux from cortical synaptosomes. C. Emax values for the high sensitivity component of ACh-stimulated 86Rb efflux. D. Emax values for the low sensitivity component of ACh-stimulated 86Rb efflux.
Binding to striatal nAChRs increased in adolescents exposed to nicotine, both in naïve animals (+30%, p<0.05) and those prenatally exposed to nicotine (+38%, p<0.01); Fig. 7. Nicotine-stimulated dopamine release, however, actually decreased after chronic adolescent nicotine in naïve rats, both total release (−33%, p<0.01) and conotoxin-resistant release (α4β2*-mediated; −37%, p<0.001) (Fig. 7). In rats prenatally exposed to nicotine, however, there was no longer any significant change in dopamine release caused by adolescent nicotine exposure. This effect was due to the previously noted decrease in the baseline release in the PreNic/AdolSal group.
Regional differences
Binding to α4β2* nAChRs differed significantly among the three regions at each of the two ages, and regardless of nicotine treatment. Comparison of ACh-stimulated 86Rb efflux in cortex and thalamus revealed that total efflux was in general lower in cortex than thalamus. The proportion of high sensitivity release was greater in cortex than thalamus in neonates, whereas the opposite was true in adolescents.
DISCUSSION
The present results demonstrate that the effects of nicotine exposure in adolescent rats are altered by previous prenatal nicotine exposure. Prenatal exposure to tobacco smoke is associated with a wide variety of negative developmental outcomes. Because nAChRs are known to play a role in CNS development during gestation, activation of these receptors by nicotine at developmentally inappropriate times and at stimulation levels generally much higher than normal is likely to contribute to long-term changes in neurochemistry and behavior in the offspring (Slotkin 1998). Chronic exposure to nicotine has long been known to alter both function and numbers of nAChRs in adult animals, which is likely to play a role in the addictive properties of this drug. The alterations in nicotine’s regulatory activity in adolescent rats caused by prenatal exposure may thus be relevant to the propensity for nicotine addiction in adolescents, and in particular the enhanced vulnerability to addiction in those whose mothers smoked during pregnancy (Al Mamun et al. 2006).
In examining how prenatal nicotine might affect the developing CNS, we distinguished between immediate effects (those measurable immediately following end of exposure), and persistent effects (in this case, measured in late adolescence). Somewhat surprisingly, we detected no immediate effects on binding to α4β2* nAChRs in thalamus, cortex or striatum. This subtype has been shown to be readily up-regulated by chronic nicotine in adult rats (Flores et al. 1992; Marks et al. 2004; Doura et al. 2008). We have recently determined that up-regulation in adolescents is markedly less than seen in adults with the same exposure (Doura et al. 2008). The present results suggest that sensitivity to nicotine-induced up-regulation may increase over time from neonates to adolescents to adults.
Given the lack of immediate effects of prenatal nicotine exposure on nAChRs, it was not surprising that we also did not detect direct effects of prenatal nicotine in adolescent animals one month after the end of prenatal exposure. However, this did not mean that prenatal exposure was without effect on the CNS nicotinic system. One type of effect was seen in the ability of prenatal exposure to blunt the normal age-related increase in αCtxMII-resistant dopamine release; while this α4β2*-mediated release increased in parallel to the binding increase from PN10 to PN42 in naïve animals, in animals prenatally exposed to nicotine there was no longer a significant change with age. Further evidence for a persistent alteration derived from analysis of the effects of subsequent adolescent nicotine exposure. While such exposure caused predictable up-regulation of binding in all three regions in naïve animals, up-regulation was lost in thalamus from animals previously exposed to nicotine in utero. Likewise, the decrease in nicotine-stimulated dopamine release in striatum (both total and αCtxMII-resistant) elicited by adolescent nicotine treatment was lost in previously exposed animals, consistent with the loss of the age-related increase just discussed. Thus, the ability of nicotine to alter the functional response of α4β2* nAChRs in thalamus and striatum was blunted in animals that were prenatally exposed.
Besides demonstrating the differential effects of nicotine exposure, the present results yield insight into the age-related changes of the CNS nicotinic cholinergic system. Comparison of PN10 and adolescent animals revealed large increases in binding to α4β2* nAChRs in cortex, thalamus and striatum during this period of development. Expression of this subtype appears to peak during adolescence, as our previous autoradiographic studies revealed that binding to α4β2* (and α7) nAChRs is higher in adolescents compared to adult rats in almost all region surveyed (Doura et al. 2008). In parallel to the increased receptor number during the period from PN10 to PN42, we also found large increases in function in ACh-stimulated 86Rb efflux in thalamus, and nicotine-stimulated dopamine release in striatum, but no functional changes in cortex.
The results for the two components of ACh-stimulated 86Rb efflux revealed several interesting trends, although the variance in these data sometimes precluded rigorous statistical conclusions. Comparison of results from PreSal vs. PreSal/AdolSal showed an increase in total release with age in thalamus and cortex from naïve animals; the bulk of the increase consisted of an increase in the high sensitivity component, which was statistically significant in thalamus. Comparison of results from PreNic vs. PreNic/AdolSal also showed an increase in total release with age; in thalamus, the increase was confined to the high sensitivity component, whereas in cortex the reverse was true. This finding suggests an effect of prenatal exposure in cortex: the increase in high sensitivity efflux occurring with age in naïve animals is suppressed in animals prenatally exposed to nicotine. Changes with age can also be inferred by comparing the effect of chronic nicotine at the two ages. In cortex, there was little apparent change at either age. In neonatal thalamus, the effect of chronic nicotine (i.e. PreSal vs. PreNic) was to cause a shift towards the low sensitivity component, whereas in adolescent thalamus, the effect of chronic nicotine (i.e. PreSal/AdolSal vs. PreSal/AdolNic) was to cause a shift towards the high sensitivity component.
Possible explanations for the two sensitivity components detected in the present study include the inclusion of an additional subunit in the fifth “structural” position with α4 and β2 (e.g. α5), or contribution from another nAChR subtype. For example, α3β4 nAChRs are present in several thalamic structures, and ACh potency for stimulation of 86Rb efflux in cells expressing α3β4 nAChRs is 110 µM (Meyer et al. 2001), which is close to the Kls of 143 µM determined here. A third possibility is alternate α4β2* stoichiometry. Marks and colleagues previously demonstrated two components of ACh-stimulated 86Rb efflux in mouse brain synaptosomes based on differential sensitivity to the competitive antagonist DHβE (Marks et al. 1999); subsequent reports found biphasic concentration-response curves for ACh-stimulated 86Rb efflux (Gotti et al. 2008). In these mouse studies, the two components of ACh-stimulated 86Rb efflux appeared to both be mediated by α4β2* nAChRs (Gotti et al. 2008). It has been suggested that the molecular basis for the differential sensitivity may be expression of α4β2* nAChRs with different α/β stoichiometry (i.e. α4[2]β2[3] v. α4[3]β2[2]) (Briggs et al. 2006) (Moroni et al. 2006). If this mechanism underlies the two components measured in the current studies, it would imply that rat brain α4β2* nAChRs (at least in thalamus) undergo a shift in stoichiometry during early development towards the high sensitivity α4[2]β2[3] form.
Of interest were the differences between nicotine’s effects on receptor number and those on receptor function. While increases in receptor number with age from PN10 to PN42 tracked well with increases in function mediated by these receptors in striatum (dopamine release) and thalamus (the high sensitivity component of 86Rb efflux), this was not true in cortex, where up-regulation was not accompanied by altered function. Similarly, up-regulation of binding induced by adolescent nicotine was not accompanied by increased function in thalamus or cortex, and in striatum the function actually decreased after nicotine exposure. Because the functional activities were measured in synaptosomal preparations and binding was done in standard homogenates, we tested whether binding experiments in synaptosomal preparations might yield different results when compared to standard homogenate preparations, but they did not (results not shown).
We previously reported that the extent of nicotine-induced up-regulation of [3H]epibatidine binding from adult rats correlated well with the extent of increased nicotine-stimulated 86Rb efflux in four different brain regions (Nguyen et al. 2004), and that nicotine-induced changes in α-CtxMII-sensitive and α-CtxMII-resistant dopamine release in adult striatal membranes also followed changes in their respective receptors (Perry et al. 2007). However, it is possible that not all up-regulated binding sites represent functional receptors, with proper intracellular localization and plasma membrane insertion. Marks and colleagues reported that chronic nicotine in mice had dose-related effects on up-regulation of α4β2* binding sites and functional activity, but that there was an overall decrease in function per receptor unit (Marks et al. 2004). The nature of saturation binding experiments is such that all properly assembled receptors will likely be included in the measurement, and the time of incubation is such that most or all binding sites are in a high-affinity, desensitized conformation, whereas the functional assays are much briefer and done under more physiological conditions. Furthermore, while chronic exposure to nicotine often causes receptor up-regulation, it also can lead to receptor desensitization and tolerance, which could be an explanation of the dissociation between results of binding and functional assays (Marks et al. 1993b).
The finding of receptor up-regulation and functional down-regulation in striatum was particularly striking. The binding analysis measured only α4β2* nAChRs, excluding α6* nAChRs by inclusion of α-CtxMII. The α6* subtype has been reported to be differentially regulated from α4β2*; the latter is typically up-regulated in most regions, while the former is either unaffected or in some cases down-regulated (Perry et al. 2007) (McCallum et al. 2006) (Mugnaini et al. 2006). We previously found that α-CtxMII-sensitive nicotine-stimulated dopamine release was selectively down-regulated by chronic nicotine in adult rats, whereas the α-CtxMII-resistant component (α4β2*-mediated) was unaffected (Perry et al. 2007). In neonates, there was no measurable effect of chronic nicotine on either component of dopamine release. In adolescents, the αCtxMII-resistant component showed a significant decrease, whereas the αCtxMII-sensitive component was unchanged. The fraction of total release that was αCtxMII-sensitive was 18–23% in neonates, and 18–26% in adolescents; these values are lower than previously reported in adult rats by us and others (38–49%) (Kulak et al. 1997) (Kaiser et al. 1998) (Cao et al. 2005) (Perry et al. 2007). Thus, the proportion of striatal dopamine release mediated by α6* nAChRs, and the regulation of this dopamine release by chronic nicotine, appear to depend upon the developmental stage of the animal.
In summary, our results demonstrate that exposure to prenatal nicotine causes persistent alterations in nAChRs, and importantly it changes the response to subsequent nicotine challenge in adolescent rats. The finding that prior prenatal nicotine exposure decreases the response to adolescent nicotine on striatal dopamine release is of particular interest. Dopamine release from the ventral striatum (nucleus accumbens) is associated with reward induced by nicotine and other drugs of abuse. If this effect is blunted by prior prenatal exposure, this suggests a scenario whereby adolescents previously exposed to nicotine in utero may require a higher rate of cigarette use to achieve equivalent stimulation, leading presumably to an increased likelihood and/or intensity of addiction (Abreu-Villaca et al. 2004; Chen and Millar 1998). It would be of interest to determine whether the acute affects of nicotine in this system are also affected by prenatal exposure, which could further our understanding of the susceptibility of adolescents to the addictive properties of nicotine and related drugs of abuse.
METHODS
Prenatal and adolescent nicotine exposure
All animal experiments were done in accordance with the guidelines and under the approval of the George Washington University Institutional Animal Use and Care Committee. Adult pregnant female Sprague-Dawley rats were weighed and anesthetized on the third day of gestation, and Alzet 28-day osmotic minipumps (model 2004, Durect Corporation, Cupertino, CA) that contained nicotine or saline were implanted subcutaneously. Osmotic minipumps were chosen to avoid spikes of high plasma nicotine concentrations and subsequent episodic fetal hypoxia/ischemia that can be produced by nicotine injections. Pumps were filled to deliver saline or 6 mg/kg/day nicotine, expressed as free base nicotine (37 µmol/kg); this dose has been shown to yield nicotine blood levels approximately equivalent to those that occur in moderate to heavy smokers (Murrin et al. 1987; Hukkanen et al. 2005; Slotkin 1998; Matta et al. 2006). Following light anesthesia with isoflurane, a patch between shoulder blades was shaved and wiped with betadine, and a small incision was made. A hemostat was used to make a subcutaneous pocket and a minipump containing sterile saline or nicotine was inserted. The wound was closed with three wound clips, topical antibiotics were applied, and the animal was allowed to recover from anesthetic before returning to the animal facility. After surgery, rats were housed separately and inspected daily for general health and infection throughout the treatment. After birth, the litter was culled and male pups continued to be exposed to nicotine through maternal milk until day PN10 (Huang et al. 2005). At postnatal day 10, animals were sacrificed by decapitation and brains were removed for dissection of thalamus, cortex, and striatum. Fresh tissue was used immediately for ACh-stimulated 86Rb efflux or nicotine-stimulated [3H]dopamine release, or frozen and kept at −80°C for [3H]epibatidine binding. These two treatment groups (PN10 animals exposed to prenatal saline or nicotine) are abbreviated as PreSal and PreNic. Ten animals were used for each treatment group; each animal used for binding studies or for functional assays was obtained from a separate litter.
Additional pups from each litter were chosen to be cross-fostered at PN10 to prevent further exposure to nicotine. At PN28, these animals were then implanted with Alzet 14-day osmotic minipumps (model 2002, Durect Corporation, Cupertino, CA) to deliver saline or 6 mg/kg/day nicotine using the same procedures as described above for the adult females. At PN42, rats were sacrificed by decapitation and brains were removed for dissection of thalamus, cortex, and striatum. Fresh dissected brain regions from other animals were used immediately for ACh-stimulated 86Rb efflux or nicotine-stimulated [3H]dopamine release, or frozen and kept at −80°C for [3H]epibatidine binding. The four adolescent treatment groups are abbreviated based on prenatal and adolescent treatment regimen: PreSal/AdolSal; PreSal/AdolNic; PreNic/AdolSal; PreNic/AdolNic. Ten animals were used for each treatment group; each animal used for binding studies or for functional assays was obtained from a separate litter.
[3H]Epibatidine binding
For receptor binding, thalamic, cortical, and striatal tissue was dissected and weighed. Tissue was homogenized by Polytron (20 s, setting 6) in 50 mM TrisHCl, pH 7.4. The homogenate was then centrifuged (35,000 × g, 10 min), and the pellet was resuspended in Tris by homogenization and re-centrifuged. The resulting pellet was resuspended at concentrations of 100 mg/ml (cortex) or 50 mg/ml (thalamus, striatum) and kept on ice. Binding to 100 µl aliquots was performed by addition of 0.5 nM [3H]epibatidine (55.5 Ci/mmol, Perkin-Elmer, Boston, MA, USA) in the presence of 50 nM α-Ctx MII (to block α6* nAChR binding); parallel samples also contained 100 µM nicotine (to define nonspecific binding). Triplicate determinations were used for all conditions. Final volume was 0.5 mL; samples were incubated for 2 h at room temperature in the dark, then filtered over GF/B filters pre-treated with 0.5% poly(ethyleneimine) (PEI), using a Brandel cell harvester (Gaithersburg, MD, USA), and radioactivity on filters measured by liquid scintillation counting. Under these conditions, the predominant nAChR subtype measured was α4β2*. The concentration of [3H]epibatidine employed should yield >90% occupancy of this receptor.
Means of the binding values from all groups of animals for each region (N=8) were compared using analysis of variance (ANOVA). A three-way ANOVA [region (thalamus, cortex, and striatum), prenatal treatment (saline or nicotine) and adolescent treatment (saline or nicotine)] was used to analyze the effect of region on prenatal and adolescent treatment on binding. Subsequently, two-way ANOVAs (prenatal treatment v. adolescent treatment; nicotine treatment v. age) were used to analyze the effect of prenatal treatment on adolescent treatment and the effect of age (PreSal, PreSal/AdolSal) or age at nicotine treatment (PreNic, PreSal/AdolNic) on binding in thalamus, cortex, and striatum individually. The effects on each individual treatment group were evaluated with one-way ANOVA with Tukey’s post-hoc test to compare the effects of prenatal nicotine exposure in adolescence (PreSal/AdolSal, PreSal/AdolNic, PreNic/AdolSal, PreNic/AdolNic) or to compare the effects of age (PreSal, PreSal/AdolSal) and age at nicotine exposure (PreNic, PreSal/AdolNic). Differences were deemed significant at p<0.05.
In parallel experiment, synaptosomes were substituted for homogenized membranes to look for differences in binding using these two methods for tissue preparation; none were found (not shown).
Determination of ACh-stimulated 86Rb efflux
Immediately following decapitation, thalamic and cortical tissue was removed and homogenized by hand with a Teflon-glass tissue grinder in ice-cold 0.32 M sucrose buffered to pH 7.5 with 5 mM HEPES. Synaptosomes were prepared and the 86Rb efflux assay performed according to the methods of Marks (Marks et al. 1993a) (Marks et al. 1999). The homogenate was centrifuged at 12,000 × G for 20 min; 25 µl aliquots of synaptosomal suspension was incubated for 40 min in uptake buffer (140 mM NaCl; 1.5mM KCl; 2mM CaCl2; 1mM MgSO4; 25 mM HEPES hemisodium; 20mM glucose; pH 7.5) containing 4 µCi of carrier-free 86RbCl. 5 µl of 80 uM DFP was added and samples were incubated 5 min more to inhibit acetylcholinesterase activity. Uptake by the synaptosomes was terminated by filtration of the sample onto a 6 mm diameter glass fiber filter (Type AE; Gelman, Ann Arbor, MI) under gentle vacuum followed by two washes with 0.5 ml of uptake buffer. After filtration and wash, the glass fiber filter containing the loaded synaptosomes was transferred to a polypropylene platform. Perfusion buffer (135 mM NaCl; 5mM CsCl; 1.5 mM KCl; 2mM CaCl2; 1mM MgSO4; 25mM HEPES hemisodium; 20mM glucose; 50 nM tetrodotoxin; 0.1% bovine serum albumin; pH 7.5) was delivered to the filter at a rate of 2.5 ml/min. Tetrodotoxin was included in the buffer to prevent activation of sodium channels. Buffer was actively removed at a rate of 3 ml/min. Sample effluent was pumped through a 200 µl Cherenkov cell in a β-RAM Radioactivity HPLC Detector (IN/US Systems, Inc., Tampa, FL) to achieve continuous monitoring of 86Rb efflux from the sample. Stimulation of the synaptosomes was achieved by diverting perfusion buffer flow through a 1 ml loop containing the test solution by means of a 4-way rotary Teflon injection valve (Alltech Associates, Inc., Deerfield, IL). Stimulation time was 5 seconds. The 86Rb efflux was monitored for 4 minutes and timing adjusted so that any efflux resulting from stimulation was located in the middle of the sampling period. This timing permitted the definition of basal efflux rate measured before and after agonist application.
Primary data from ACh-concentration-effect curves (from 1–1000 µM ACh) were analyzed using the nonlinear least-squares algorithm in SigmaPlot 2001 (Systat Software, Inc., Point Richmond, CA). The magnitude of agonist stimulated 86Rb efflux was determined as the difference between the average of the peak values and the baseline (the average of the five data points before and after the peak). This value was then multiplied by the number of data points in the peak and divided by the baseline value to normalize the counts so that the signal size represented the percentage of 86Rb efflux above baseline that was stimulated by agonist exposure. ACh concentration curves were fit using the Michaelis-Menten equation: y = E × [ACh]/K + [ACh]; the Hill equation: y = E×[ACh]n/K + [ACh]; and a two-component model: E = (Ehs × [ACh])/(Khs + [ACh])+(Els × [ACh])/(Kls + [ACh]). E represents the total 86Rb efflux at each concentration of ACh, and Ehs and Els represent the 86Rb efflux for the components with higher sensitivity (Khs) and lower sensitivity (Kls) to stimulation by ACh.
Statistical analysis was done by analysis of variance (ANOVA) using SPSS (SPSS, Inc. Chicago, IL). A three-way ANOVA [region (thalamus or cortex), prenatal treatment (saline or nicotine), and adolescent treatment (saline or nicotine)] was used to analyze for the effect of region on prenatal and adolescent treatment on Etot, Ehs, and Els. Subsequently, two-way ANOVAs (prenatal treatment v. adolescent treatment, nicotine treatment v. age) were used to analyze the effect of prenatal treatment on adolescent treatment and the effect of age or nicotine treatment on Etot, Ehs, and Els in thalamus and cortex individually. The effects on each individual treatment group (N=6–10) were evaluated with one-way ANOVA followed by Tukey’s post-test to compare the effects of prenatal nicotine exposure in adolescence (PreSal/AdolSal, PreSal/AdolNic, PreNic/AdolSal, PreNic/AdolNic) or to compare the effects of age (PreSal, PreSal/AdolSal) and age at nicotine exposure (PreNic, PreSal/AdolNic).
Measurement of nicotine-stimulated [3H]dopamine release
Release was measured as previously described (Perry et al. 2007). Immediately following decapitation, striatal tissue was removed and placed into ice-cold 0.32 M sucrose buffered with 5 mM HEPES at pH 7.5. Tissue was homogenized with 10–20 strokes of a Teflon homogenizer, then centrifuged 20 min at 12,000 × G. Pellets were resuspended in 1.6 ml uptake buffer (128 mM NaCl, 2.4 mM KCl, 3.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, pH 7.5, 10 mM glucose, 1 mM ascorbic acid, 10 µM pargyline) to create the synaptosomal mixture. This mixture was then incubated for 10 min at 37°C before addition of 100 nM [3H]dopamine, followed by an additional 5 minute incubation at 37°C. All subsequent steps were done at room temperature.
Synaptosomes (80 µl aliquots) were applied onto glass fiber filters and perfused with perfusion buffer (uptake buffer containing 0.1% BSA and 10 µM nomifensine) at 1 ml/min for 10 min before beginning of fraction collection. For some samples, the second 5 min of this perfusion included 50 nM α-CtxMII to block α6* nAChRs (Salminen et al. 2004) (Grady et al. 2002). Fractions were collected every 18 sec for 4 min; nicotine-stimulated [3H]dopamine release was obtained by perfusing with different concentrations of nicotine in perfusion buffer for a total of 1 min during the collection period. The radioactivity of the fractions as well as that remaining on the filter after perfusion was measured by scintillation counting (Beckman 6500).
Radioactivity in fractions immediately before and after the stimulated peak was used to calculate basal release as a single exponential decay (SigmaPlot 2001; Systat Software, Inc., Point Richmond, CA). This basal release was then subtracted from the values in the peak (fractions that exceeded 10% of baseline). Peak values were then summed and expressed as a percent of total counts (sum of all fractions collected plus filter radioactivity). Nicotine-stimulated release data for α-CtxMII-resistant (release remaining in presence of 50 nM α-CtxMII) and total release (no α-CtxMII added) were analyzed using the nonlinear least-squares algorithm in SigmaPlot 2001 (Systat Software, Inc., Point Richmond, CA) as described above for 86Rb efflux. Nicotine-stimulated dopamine release curves for total and αCtxMII-resistant release for all rats in the 6 treatment groups were fit to the Hill equation (as described above for 86Rb efflux) providing separate determinations of Etot or Eres and EC50tot or EC50res. When differences (p<0.05) were detected, the means of the EC50 and Emax values for the six treatment groups were further compared by two-way and subsequent one-way ANOVA as described.
The α-CtxMII-resistant dopamine release is mediated largely or entirely by α4β2* nAChRs, whereas release inhibited by α-CtxMII is mediated by α6* nAChRs (Salminen et al. 2004) (Kulak et al. 1997) (Perry et al. 2007). The α-CtxMII-sensitive release was determined by the difference between total and α-CtxMII-resistant release (Etot - Eres = Esen). EC50sen values were determined by the difference between total and α-CtxMII-resistant values (EC50tot – EC50res = EC50sen). Therefore, to accurately calculate the α-CtxMII-sensitive portion of the total dopamine release (Esen; EC50sen), the difference between the Eres; EC50res values determined for each individual α-CtxMII-resistant release curve was taken from the individual total Etot; EC50tot values obtained in the absence of α-CtxMII (Salminen et al. 2004) (Salminen et al. 2007).
Results were evaluated by analysis of variance (ANOVA) using SPSS (SPSS, Inc. Chicago, IL). Two-way ANOVAs (prenatal treatment v. adolescent treatment, nicotine treatment v. age)] were used to analyze the effect of prenatal treatment on adolescent treatment and the effect of age or nicotine treatment on Emax, Eres, and Esen and EC50tot, EC50res, and EC50sen in striatal synaptosomes. The effects on each individual treatment group (N=9–11) were evaluated with one-way ANOVA followed by Tukey’s post-test to compare the effects of prenatal nicotine exposure in adolescence (PreSal/AdolSal, PreSal/AdolNic, PreNic/AdolSal, PreNic/AdolNic) or to compare the effects of age (PreSal, PreSal/AdolSal) and age at nicotine exposure (PreNic, PreSal/AdolNic) on Emax, Eres, and Esen and EC50tot, EC50res, and EC50sen. Differences were deemed significant at p<0.05.
ACKNOWLEDGMENTS
This work was supported by NIH grant DA015767 (DCP), and is in part from a dissertation by Ms. Gold to be presented to the Neuroscience Program of the George Washington University Institute for Biomedical Sciences in partial fulfillment of the requirements for the PhD degree. The authors are grateful to Christopher Gorini for his help with pregnant rats, and to Drs. Michael Marks and Sharon Grady for their generous help with data analysis.
Non-standard abbreviations
- α-CtxMII
α-conotoxin MII
- nAChR
neuronal nicotinic acetylcholine receptor
- PN
postnatal day
References
- Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Res. 2003;979:114–128. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- AlMamun A, O'Callaghan FV, Alati R, O'Callaghan M, Najman JM, Williams GM, Bor W. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15:452–457. doi: 10.1136/tc.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, Surowy CS. Untranslated region-dependent exclusive expression of high-sensitivity subforms of alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;70:227–240. doi: 10.1124/mol.105.020198. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [(3)H]dopamine release. Neuropharmacology. 2005;48:72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychol. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor alpha4 or beta2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha4 and beta2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Brooks AR, Coyle JT. Neonatal lesions of the basal forebrain cholinergic neurons result in abnormal cortical development. Brain Res. 1988;470:253–264. doi: 10.1016/0165-3806(88)90244-1. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Wang X, Dergacheva O, Mendelowitz D. Prenatal nicotine exposure recruits an excitatory pathway to brainstem parasympathetic cardioinhibitory neurons during hypoxia/hypercapnia in the rat: implications for sudden infant death syndrome. Pediatr Res. 2005;58:562–567. doi: 10.1203/01.PDR.0000179380.41355.FC. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by α-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Farnham DA, Grady SR, Collins AC. Nicotinic receptor function determined by stimulation of rubidium efflux from mouse brain synaptosomes. J Pharmacol Exp Ther. 1993a;264:542–552. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993b;266:1268–1276. [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor- mediated rubidium efflux in mouse brain require the β2 subunit. J Pharmacol Exp Ther. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, McIntosh JM, Quik M. Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006;96:1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- Meyer EL, Xiao Y, Kellar KJ. Agonist regulation of rat α3β4 nicotinic acetylcholine receptors stably expressed in human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:568–576. [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. {alpha}4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [(125)I]Y(0)-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Zeng WY, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40:1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J Pharmacol Exp Ther. 1988;244:940–944. [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates {alpha}6- and {beta}3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, McCracken JT, Maas JW. Catecholamines in attention-deficit hyperactivity disorder: current perspectives. J Am Acad Child Adolesc Psychiatry. 1996;35:264–272. doi: 10.1097/00004583-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of {alpha}-Conotoxin MII-Sensitive Subtypes of Nicotinic Acetylcholine Receptors Isolated by Breeding of Null Mutant Mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]