Abstract
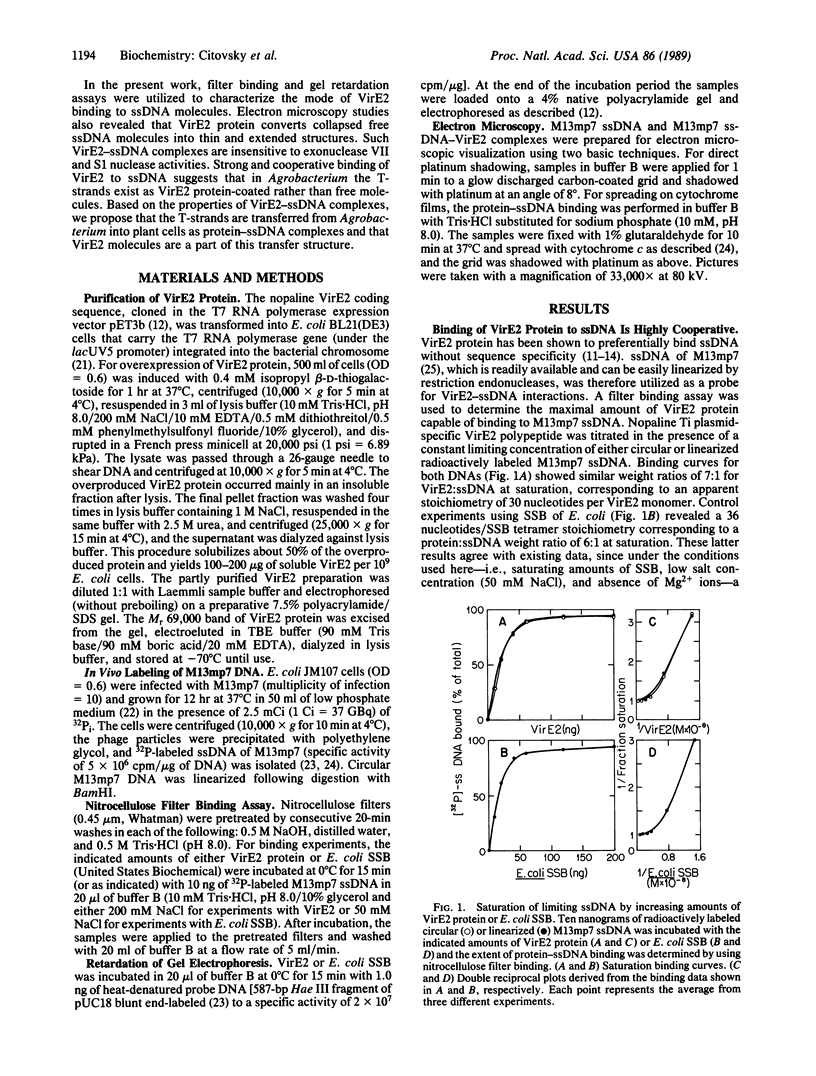
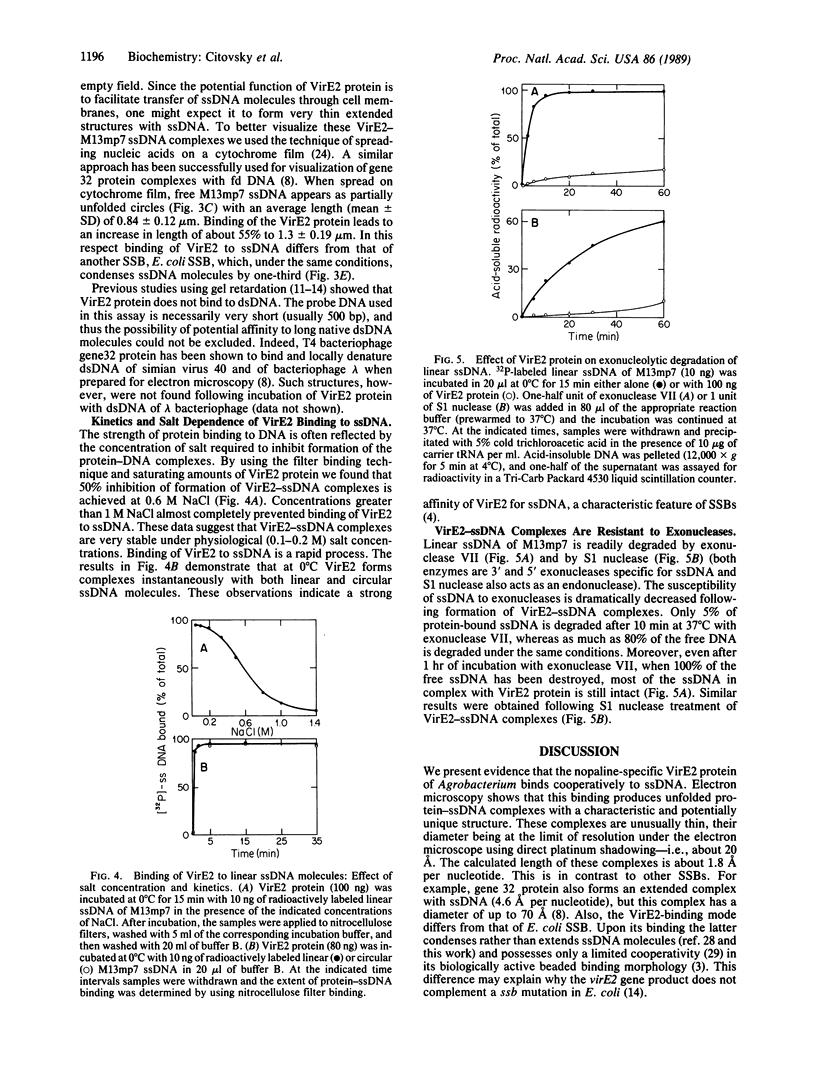
Induction of Agrobacterium tumefaciens vir gene expression by wounded plant cells results in production of a free transferable single-stranded (ss) copy of T-DNA, the T-strand. One of the Vir proteins, the VirE2 polypeptide, is a ssDNA-binding protein. In the present work, interaction of nopaline-specific VirE2 protein (Mr 69,000) with ssDNA was studied by using nitrocellulose filter binding, gel retardation, and electron microscopy techniques. The VirE2 protein was found to bind to ssDNA molecules with strong cooperativity, forming VirE2-ssDNA complexes with a binding site of 28-30 nucleotides. The VirE2-ssDNA complexes are stable at high salt concentrations and resistant to exonucleolytic activity. When examined under the electron microscope, the VirE2 protein converted collapsed free ssDNA molecules into unfolded and extended structures. The structure and properties of VirE2-ssDNA complexes predict possible functions in Agrobacterium virulence to (i) protect the T-strands from cellular nucleases and (ii) facilitate transfer of the T-strands through bacterial membranes possibly by specific interaction with putative membrane pores formed in plant-induced Agrobacterium cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Bujalowski W., Lohman T. M. Limited co-operativity in protein-nucleic acid interactions. A thermodynamic model for the interactions of Escherichia coli single strand binding protein with single-stranded nucleic acids in the "beaded", (SSB)65 mode. J Mol Biol. 1987 Jun 20;195(4):897–907. doi: 10.1016/0022-2836(87)90493-1. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Merrill B. M., Williams K. R. F sex factor encodes a single-stranded DNA binding protein (SSB) with extensive sequence homology to Escherichia coli SSB. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5480–5484. doi: 10.1073/pnas.80.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Ward J. E., Winans S. C., Nester E. W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988 Jun;170(6):2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysogelos S., Griffith J. Escherichia coli single-strand binding protein organizes single-stranded DNA in nucleosome-like units. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5803–5807. doi: 10.1073/pnas.79.19.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., DE Vos G., Zambryski P. Single-Stranded DNA Binding Protein Encoded by the virE Locus of Agrobacterium tumefaciens. Science. 1988 Apr 22;240(4851):501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- Das A. Agrobacterium tumefaciens virE operon encodes a single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1988 May;85(9):2909–2913. doi: 10.1073/pnas.85.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Engström P., Zambryski P., Van Montagu M., Stachel S. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J Mol Biol. 1987 Oct 20;197(4):635–645. doi: 10.1016/0022-2836(87)90470-0. [DOI] [PubMed] [Google Scholar]
- Gietl C., Koukolíková-Nicola Z., Hohn B. Mobilization of T-DNA from Agrobacterium to plant cells involves a protein that binds single-stranded DNA. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9006–9010. doi: 10.1073/pnas.84.24.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D., Harris L. D., Register J., 3rd Visualization of SSB-ssDNA complexes active in the assembly of stable RecA-DNA filaments. Cold Spring Harb Symp Quant Biol. 1984;49:553–559. doi: 10.1101/sqb.1984.049.01.062. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lohman T. M., Overman L. B. Two binding modes in Escherichia coli single strand binding protein-single stranded DNA complexes. Modulation by NaCl concentration. J Biol Chem. 1985 Mar 25;260(6):3594–3603. [PubMed] [Google Scholar]
- McBride K. E., Knauf V. C. Genetic analysis of the virE operon of the Agrobacterium Ti plasmid pTiA6. J Bacteriol. 1988 Apr;170(4):1430–1437. doi: 10.1128/jb.170.4.1430-1437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Timmerman B., Zambryski P. Activation of Agrobacterium tumefaciens vir gene expression generates multiple single-stranded T-strand molecules from the pTiA6 T-region: requirement for 5' virD gene products. EMBO J. 1987 Apr;6(4):857–863. doi: 10.1002/j.1460-2075.1987.tb04831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]