Abstract
Importance of the field: Neuronal circuitries are determined by specific synaptic connections and they provide the cellular basis of cognitive processes and behavioral functions. To investigate neuronal circuitries, tracers are typically used to identify the original neurons and their projection targets.
Areas covered in this review: Traditional tracing methods using chemical tracers have major limitations such as nonspecificity. In this review, we will highlight novel genetic tracing approaches which enable visualization of specific neuronal pathways by introducing cDNA encoding a transsynaptic tracer. In contrast to conventional tracing methods, these genetic approaches use cell type specific promoters to express transsynaptic tracers such as WGA and TTC, which allows labeling either the input or output populations and connections of specific neuronal type.
What the reader will gain: Specific neuronal circuit information by these genetic approaches will allow more precise, comprehensive, and novel information about individual neural circuits and their function in normal and diseased brains.
Take home message: Using the tracer gene transfer, neuronal circuit plasticity after traumatic injury or neurodegenerative diseases can be visualized. Also, this can provide a good marker for evaluation of therapeutic effects of neuroprotective or neurotrophic agents.
Keywords: gene transfer, neuronal circuitry, transsynaptic tracer, cell type specific promoter
1. Introduction
The central nervous system (CNS) is composed of billions of neuronal cells interconnected with each other to form neuronal circuits [1]. Visualization of specific neural pathways is essential for understanding the relationship between structure and function in the CNS. Neuronal tracing allows identification and characterization of neuronal pathways in diverse and specific neuronal circuits of the complex nervous system [2]. Advanced neuronal tracing techniques with cutting edge methodologies provide more precise, comprehensive, and even novel information about neuronal connections. In this paper, we will review neuronal tracing techniques using genetic approaches, which can overcome the limitations of nonspecific conventional tracing methods, with a specific focus on transneuronal/transsynaptic tracers such as wheat germ agglutinin (WGA) or a nontoxic C-terminal fragment of tetanus toxin (TTC).
2. Conventional tracing methods
To study neuronal circuitry, tracers are typically used to find the cells of origin that innervate a certain brain structure, and to identify the projection target of the axons of a given population of neurons. Traditionally, neuronal tracing was based on axonal transport by general procedures consisting of tracer application, uptake of tracer(s), transport within the cell, and signal detection. With regard to the direction of transport, depending on the tracer molecules employed anterograde and/or retrograde transport may take place [3]. Anterograde tracing is used to identify the anatomical targets of a particular population of neuronal cell bodies towards the axon terminals, which allows the visualization of the projection fields of neurons. In anterograde tracing, the tracers are taken up by neurons and are transported back to the axon terminals along their axonal tracts. Rhodamine-isothiocyanate (RITC) [4], 1,1'-dioctadecyl-3,3,3',3'-tetramethyl-indocarbocyanine perchlorate (DiI) [5–7], phaseolus vulgaris leucoagglutinin (PHA-L) [8, 9], biotinylated dextran amines (BDA) [10, 11], and herpes simplex virus-1 (HSV-1) [12] are commonly used anterograde tracers. Retrograde tracing is used to identify the origin of axonal projection from the axon terminal to the cell body, which determines location of the soma of the labeled afferent nerve fibers. In retrograde tracing, the tracer molecules are applied to a terminal field of innervations, where they are incorporated into the cell axons, and then carried back to the cell bodies. The plant enzyme horseradish peroxidase (HRP) [13–15], Evans blue dye (EB) [16], fluoro-gold (FG) [17], fluoro-ruby (FR) [18], and pseudorabies virus [19] are commonly used retrograde tracers. Plant lectins, including WGA [20], biocytin and biotinamide [21] and some common fluorescent tracers like carbocyanines [22] and rhodamine [23], are bidirectional tracers.
3. Genetic transsynaptic tracing
3.1 Conventional tracing versus genetic tracing
Conventional tracing methods use axonal transport of tracers, such as HRP or WGA, through the microtubular systems of neurons, followed by immunohistochemical detection. In conventional tracing, it is difficult to selectively label small populations of neurons of a given phenotype, because the tracers must be delivered by microinjection or local application. Moreover, axons passing through the application region can be damaged and/or labeled, leading to non-specific results. Thus, using conventional tracing methods it is likely that tracers can be taken up by all the neurons at the injection sites, resulting in nonspecific labeling of unrelated pathways [24]. To overcome these problems, novel genetic approaches have been developed by introducing cDNA encoding a transsynaptic protein (e.g., WGA) as a transgene under the control of cell-type specific promoter elements for visualization of specific trans-synaptic neuronal pathways [25, 26]. This genetic tracing method allows selective expression of tracers in specific neurons using cell-type specific promoters. Therefore, selection of relevant cell-type specific promoters is critical for precise neuronal circuit mapping studies.
3.2 Transsynaptic tracer molecules used in genetic tracing approaches
Numerous tracers have been used for conventional tracing studies. In contrast, only a few tracer molecules are used for genetic tracing. Genes used for viral vector transduction approaches should be sufficiently short in order to be suitable for packaging into viral vectors. Also, the tracer should be relatively harmless to host cells or neurons. WGA and TTC are the most used tracers in genetic tracing.
3.2.1 WGA
WGA is a plant lectin that has been used as a highly sensitive tracer in neuroanatomical and neuronal mapping studies [27–30]. The mature WGA protein is a small 18 kD cysteine-rich lectin which binds to N-acetylglucosamine and sialic acid in the carbohydrate moiety of glycoproteins and glycolipids. Since these WGA receptors are commonly expressed on the surface of plasma membranes of most types of neurons in the brain, WGA has been used as an effective tracer in a variety of neural systems. Neurons efficiently take up WGA by endocytosis, and the latter is transported to axons and dendrites in both anterograde and retrograde directions. In some cases, injection of WGA in well-mapped neural pathways results in labeling of both first- and second order neurons and their processes, indicating that WGA is transneuronally transported.
Recently, fluorescent proteins such as GFP or DsRed have been fused with WGA [25, 31]. The resultant fusion protein WGA:GFP or WGA:DsRed can be easily detected under the fluorescent microscope, allowing direct visualization of neural pathways.
3.2.2 TTC
Tetanus toxin is a potent neurotoxin that is taken up by nerve endings at the neuromuscular junction, travels along motor axons retrogradely, and is transported transsynaptically to spinal cord interneurons [32]. TTC is the nontoxic fragment of the neurotoxin and is responsible for binding to neurons. TTC can be transported retrogradely and transsynaptically. It was shown that the TTC:GFP fusion protein can be taken up by nerve endings [33, 34]. TTC:GFP is then retrogradely transported to the soma and dendrites of motor neurons where it is transsynaptically transferred to interconnected higher-order neurons [33, 35, 36]. Transgenic expression of TTC:GFP has the added advantage of allowing large amounts of label to be transferred, and it is consequently more easily detectable, over time.
3.3 Genetic tracing methods based on promoters
Gene promoter systems that drive high-level and cell type specific tracer gene expression are of great value because they allow specific cell types to endogenously express tracers, leading to visualization of specific neural circuitry of the brain. Once the tracer gene has been delivered into the target neuron, its expression level and specificity will depend on the activity of the promoter elements incorporated into the gene delivering vector.
3.3.1 Nonspecific promoters
Previously, several types of nonspecific promoters were used for gene delivery. The CMV promoter has been widely used to drive expression of various transgenes. Kissa et al., used the CMV promoter in an adenoviral vector for GFP-TTC gene delivery [35]. They also developed a β-galactosidase (β-gal)-TTC fusion protein as a genetic marker to map neuronal pathways [33]. The CMV immediate enhancer/β-actin (CAG) promoter was shown to give higher levels of transgene expression in several cell lines compared to the CMV and β-actin promoters [37]. Infusion of the WGA-expressing adenovirus under the CAG promoter through the mouse nostrils lead to infection, throughout the olfactory epithelium, of various cell types expressing WGA [38]. Subsequently, WGA expressed in olfactory neurons was transported along their axons to the olfactory bulb, and transsynaptically transferred to cholinergic neurons in the horizontal limb of diagonal band, serotonergic neurons in the median raphe nucleus, and noradrenergic neurons in the locus coeruleus. Braz et al. [39] combined the CAG promoter and the Cre/loxP site-specific recombination system with the transgenic approach of Yoshihara et al. [26] to produce transgenic mice in which induction of WGA expression can be controlled. EF1α and β-actin promoters are also commonly used to drive various transgene expressions in the brain [40]. Some promoters, such as the NSE promoter [41], synapsin-1 promoter [42], and the human PDGF β-chain promoter combined with the CMV enhancer [43], have been used for neuronal expression of transgenes. These promoters are useful for robust expression of tracers in target regions, but their utility is limited due to their lack of specificity. Feng et al. [44] used the Thy1 promoter for generating transgenic mice expressing multiple spectral variants of GFP (XFP) in neurons. In addition, Livet et al. [45] generated Thy1-Brainbow mice lines, which can yield multiple colors in individual cells to uniquely label many individual cells within a population.
3.3.2 Cell type specific promoters
3.3.2.1 Taste receptor
Sugita and Shiba applied a genetic approach to visualize the neuronal circuits of bitter and sweet-umami taste by using the taste receptor genes and WGA as molecular tools [31]. They used transgenic mice in which WGA was fused to a fluorescent protein (tWGA-DsRed) and was coexpressed with selected taste receptors. They selected the promoter element of the mT2R5 gene, a receptor for cycloheximide, to drive tWGA-DsRed expression in bitter receptor–expressing cells. In phospholipase Cb2 (PLCb2)–deficient mice, which lack sweet, amino acid, and bitter taste reception, the PLCb2 transgene, expressed under the control of the mT2R5 promoter, rescued the response to multiple bitter compounds, but not to sweet or amino acid taste. Ohmoto et al. generated transgenic mouse lines in which WGA was specifically expressed in T1R3-positive sweet/umami taste receptor cells in taste buds [46]. The WGA protein was not transferred laterally to synapse-bearing sour-responsive type III cells in taste buds but directly to a subset of neurons in the geniculate and nodose/petrosal ganglia, and further conveyed to a rostro-central region of the nucleus of the solitary tract. Also, Damak et al. reported that the T1R3 promoter produced WGA that was detected in the nucleus of the solitary tract and in the nucleus ambiguus, the vestibular nucleus, the trigeminal nucleus and in the gigantocellular reticular nucleus of the medulla oblongata [47].
3.3.2.2 Purkinje cell
The WGA transgene was expressed in the cerebellar Purkinje cells under the control of the mouse L7 gene promoter (3 kb) [48]. Purkinje cells from several lines of L7-WGA transgenic mice showed robust expression of WGA mRNA [2]. WGA was detected in Purkinje cells as well as in several brain regions that have anatomical and functional relationships to Purkinje cells such as the deep cerebellar nuclei, the vestibular nucleus, the inferior olive, the red nucleus, and the thalamic ventrolateral nucleus.
3.3.2.3 Olfactory neurons
In one line of transgenic mice using the cDNA of the olfactory marker protein (OMP) fused to WGA (OMP-WGA), WGA mRNA and protein were detected in all the mature olfactory and vomeronasal sensory neurons, faithfully mimicking the endogenous OMP expression pattern. WGA was transported through the axons of these sensory neurons to the olfactory bulb and transsynaptically transferred to dendrites of the second-order neurons, mitral cells and tufted cells, and in glomeruli of the olfactory bulb. Furthermore, WGA was conveyed through the axons of mitral/tufted cells along the lateral olfactory tract and reached the olfactory l areas of the brain such as the anterior olfactory nucleus, the olfactory tubercle, the piriform cortex, the lateral entorhinal cortex, and the medial and lateral amygdaloid nuclei. Many neurons in these areas of the central olfactory system contained WGA-positive granule-like profiles, suggesting transsynaptic labeling of third-order neurons [2].
3.3.2.4 Calbindin neurons
Pavlou et al. cloned and described the use of a short upstream sequence (1.0 kb) of the rat calbindin gene that, in transgenic mice, directed strong expression of the lacZ marker in several identified cell types and several regions associated with strong calbindin expression in the brain, notably in Purkinje cells of the cerebellum, neurons of the olfactory bulb, and parts of the cortex, the hippocampus, and the striatum [49]. Subsequently, Maskos et al. used the calbindin promoter and TTC:GFP transgene for studying cell type specific neural circuits [34]. They showed the connectivity and retrograde transsynaptic transfer of TTC:GFP in the cerebellar system, from TTC:GFP-positive Purkinje neurons to cerebellar basket, Golgi, and granule cells, and to the inferior olive via the climbing fiber projection. The expression of TTC:GFP in a number of typical calbindin-positive cell types, such as olfactory bulb, cortex, hippocampus, hypothalamus, and striatum, was reproducibly very robust.
3.3.2.5 Visual pathway
The WGA protein was introduced transgenically into Drosophila photoreceptor cells (R1-R6) under the control of the rhodopsin 1 (Rh1) promoter [26]. WGA immunoreactivity was detected not only in the retina and the lamina, but also in the medulla of the optic lobe, suggesting that WGA was transsynaptically transferred from the R1-R6 photoreceptor cells to second-order neurons. Also, several studies used the Rh1 promoter and WGA expression in the Drosophila visual system [50, 51]. They used WGA as a marker of the formation of mature synapse and synaptic transmission. When WGA was expressed in retinal rod bipolar cells in Drosophila under the mouse L7 promoter, it was transferred from the bipolar cells to the amacrine cells and transsynaptically to the ganglion cells in the retinal neural circuitry and further conveyed along the optic nerve to the visual centers such as the suprachiasmatic nucleus, the lateral geniculate nucleus, the pretectal nucleus and the superior colliculus [52].
3.3.2.6 GnRH neurons
Boehm et al. introduced transgenic mice in which the Gnrh1 gene promoter controls the expression of two proteins: barley lectin (BL), a transneuronal tracer, and GFP [53]. Based on the location of BL positive neurons versus GnRH positive fibers in BL-IRES-GFP (BIG) mice, it appears that some of the BL positive neurons in both odor- and pheromone-processing areas transmit signals directly to GnRH neurons. Barley lectin shares 95% sequence identity with the antigenically indistinguishable WGA allowing Boehm et al. to successfully use anti-WGA antibodies to detect BL [54].
3.3.2.7 Orexin neurons
Sakurai et al. used the human prepro-orexin gene promoter to drive expression of TTC:GFP [36]. This promoter was previously shown to direct gene expression specifically in orexin neurons [55]. Using this transgenic method, they demonstrated that orexin neurons receive inputs from several brain areas including the amygdala, basal forebrain cholinergic neurons, GABAergic neurons in the preoptic area, and serotonergic neurons in the median/paramedian raphe nuclei.
3.3.2.8 Serotonin neurons
Braz and Basbaum [56] induced transgene expression by crossing ZW mice with mice in which Cre recombinase is regulated by the ePet-1 promoter (ePet-Cre), a transcription factor that defines serotonergic neurons [57]. In double transgenic ePet-ZW mice, Cre-mediated excision of the floxed-LacZ cDNA results in WGA induction exclusively in 5HT neurons. To identify the neurons that synthesize the tracer (so called, first-order neurons), they double-labeled sections for WGA and serotonin in ePet-ZW mice. As expected, serotonergic neurons in all raphe nuclei of the brainstem contained WGA-positive neurons. Double-labeled neurons were particularly numerous in the dorsal and median raphe. Most were intensely labeled, consistent with these being first-order neurons expressing the transgenes [57]. In addition, Benzekhroufa et al. have developed lenti and adenoviral vectors which drive visible levels of EGFP in raphe serotonin neurons, which allowed labeling of these cells and their axons in rats and various strains of mice [58–60]. It will be of interest if this approach can be expanded by using the WGA gene as a transsynaptic molecule.
3.3.2.9 Noradrenaline neurons
The study of noradrenergic neurons has been helped by the development of vectors using the noradrenergic neuron-specific transcription factors, Phox2a/2b, efficient synthetic promoters as well as a modified dopamine β-hydroxylase gene promoter [61, 62]. Using these promoters in adenoviral vectors it was possible to selectively transduce descending noradrenergic projections from the brainstem noradrenergic clusters, such as LC or ventral catecholaminergic groups [63, 64]. Furthermore, Howorth et al. selectively targeted the pontospinal noradrenergic neurons that seem to be involved in the descending control of nociception [63].
3.3.2.10 Dopamine neurons
Oh et al. used the 2.5 kb rat tyrosine hydroxylase (TH) upstream promoter in adenovirus or adeno-associated virus (AAV) to express tracers specifically in dopamine neurons [25]. This promoter was previously shown to drive cell-type-specific gene expression in midbrain dopamine neurons using transgenic founder analysis [65]. Owing to its relatively short length, this promoter fragment could be tested in various viral vectors including AAV and adenovirus. Following stereotaxic injection of this viral vector, expression of WGA was predominantly detected in the LC area where TH-positive noradrenergic neurons are predominant. Moreover, this vector allowed prominent transgene expression in DA neurons of the substantia nigra.
3.3.2.11 Sensory neurons
Braz et al. [66] developed a LacZ-WGA (ZW) mouse line that can be used to trace pathways originating from CNS neurons and in particular the neuronal pathways engaged by primary afferent neurons. In this mouse, WGA expression can be triggered either by crossing the mice with Cre-expressing transgenic mice or by microinjecting a Cre-expressing adeno-associated virus. Furthermore, Braz and Basbaum [67] recently developed a ZWX mouse in which WGA expression can be controlled by conditional Cre recombination in primary somatic or visceral afferent neurons, allowing both regional and temporal control of tracer expression, even in the adult. This ZWX mouse line illustrated how it can be used to analyze the neural circuits engaged by sensory neurons.
3.4 Genetic tracing using transgenic mice versus viral vectors
3.4.1 Transgenic mouse approach
The genetic approach using the WGA transgene, expressed under the control of cell type specific promoter elements, showed neuronal circuitries originating from a specific type of neurons. Other transgenic mice studies used the TTC transgene as a retrograde tracer [34]. In these transgenic mice, the tracer protein becomes an endogenous product recognized as self by the immune system. In addition, if the tracer transgene is stably transmitted to offspring and transgenic lines are established, the selective neural pathways can be visualized in all animals with high reproducibility.
3.4.2 Viral vectors
While the transgenic mouse approach provided an efficient system to study gene function in a variety of physiological and pathological circumstances, this technology initially takes time to screen positive transgenic founder lines [68]. An alternate and complementary approach is direct gene transfer using stereotaxic injection of recombinant viral vectors. This approach represents a useful method that permits convenient spatio-temporal control of transgene expression [69]. Indeed, several recent studies used this virally mediated gene delivery method for neuronal tracing and demonstrated its effectiveness. Kinoshita et al. visualized olfactory pathways using a WGA-expressing adenoviral vector system [38]. They made a recombinant adenovirus expressing WGA under the control of the CAG promoter. In addition, Kissa et al. [35] reported a neuronal tracing study using GFP-TTC gene delivery by an adenoviral vector. The GFP-TTC fusion protein was expressed in neurons and allowed the visualization of neurons’ connectivity. Recently, Oh et al. used cell-type-specific promoters that can direct expression of GFP-WGA to midbrain DA neurons and central noradrenergic neurons using AAV and adenovirus, respectively [25].
In addition, other viruses have been used in tracing studies of neural circuits. For example, swine α-herpes virus known as pseurorabies virus (PRV) have been used for tracing studies [70–72]. More recently, a Cre-dependent PRV was developed and used in many tracing studies [73–77]. Using the Cre-dependent PRV, cell type specific PRV tracing can be achieved. Also rabies virus has been used with great success in its intact form as a transsynaptic tracer, crossing synapses exclusively in the retrograde direction [78–80]. Recently, Wickersham et al. [81, 82] used genetically modified rabies virus for monosynaptic restriction of viral transsynaptic tracing.
4. Conclusion
Neuroanatomical mapping studies using tracer molecules have tremendously advanced our understanding of the structure and function of specific and diverse neural circuits. Compared to conventional tracing methods, the more recent genetic tracing approach has many advantages including highly specific determination of neural pathway and avoidance of undesirable immune reactions. Both nonspecific and cell type specific promoters have been used in genetic tracing methods for the analysis of neural circuitries. Since cell type specific promoters allow tracer expression in a selective population, they have tremendous potential in the investigation of specific neural circuits and their function in normal and diseased brains. WGA and TTC have been mostly used as tracers in genetic tracing for visualization of neural circuitry because they can be transferred transsynaptically. WGA is transferred mainly anterogradely and, to a lesser degree, retrogradely to adjacent or remote neurons. In contrast, TTC is transported in a retrograde manner. Therefore, these tracers can be used to label either the input (TTC, WGA) or output (WGA) populations of specific neuronal type expressing the tracer. Expression of WGA and TTC under the same promoter may allow the differentiation between anterograde and retrograde transport of the tracers. Indeed, a recent study elegantly expressed both WGA and TTC:GFP under cell type specific promoters using transgenic mice [67], demonstrating co-expression of WGA and TTC:GFP in the same dorsal root ganglion neurons. With the availability of more cell type-specific promoters, this genetic tracing method will further provide us with important insights with regard to specific neural pathways and their functions. Using the tract tracing method, neuronal circuit plasticity after traumatic injury or neurodegenative diseases can be visualized and functionally studied. This visualization of neuronal circuit change after neuronal injury can give novel and important insights on functional changes of the nervous system after injury. Also, cell type specific visualization of neural circuit can be a good marker for further evaluation of therapeutic effects of neuroprotective or neurotrophic agents on specific neuron phenotypes.
5. Expert opinion
While the genetic tracing method is a very powerful approach for the identification and characterization of specific neuronal pathways, there are limitations to be overcome. For instance, given that one neuron has numerous synaptic contacts with adjacent and remote neurons, endogenously expressed tracers can be rapidly transported and diluted through the multitudes of synaptic connections. The resulting “absence” or poor detection of tracer could be misinterpreted as a lack of synaptic connectivity [83]. Amplification of the signal by fusing tracers with fluorescent proteins or using more sensitive staining methods such as tyramide signal amplification may be necessary to address this limitation. In addition, it is important to use both WGA and TTC for clear determination of the directionality of neuronal pathways. Finally, two different genetic tracing methods, transgenic mouse and viral injection, have also different advantages and disadvantages. From the viewpoint of region specific tracer expression, the transgenic mouse approach allows reproducible visualization of specific neural circuits in all descendants, but the mosaic transgene expression can be a limiting factor for tracing pathways over multiple synapses. In addition, it has the disadvantage of tracer expression in all related cell types. For example, if the catecholamine neuron-specific TH promoter is used, the tracer will be expressed in all DA neurons as well as noradrenergic and adrenergic neurons. In contrast, if the same promoter is used in the context of a viral vector that is stereotaxically injected, the tracer can be expressed only in a lateral part of a subset of catecholamine neurons (e.g., A9 DA neurons in the substantia nigra). Thus, this allows a unique opportunity to address the differential structure and function of lateral versus contralateral DA pathway from A9 neurons in normal and diseased brains. However, it is worthwhile to note that viral vectors also have several disadvantages for neural circuit tracing studies. First, viral vectors can induce inflammatory reaction and necrosis at the injection site. Second, viruses can infect a limited number of cells that may result in limited tracer production and thus difficulty in tracing discrete populations. Also, there is possibility of false negative given that some viruses do not infect all types of neurons. For example, adenoviruses do not infect neurons that do not have coxsackie and adenovirus (CAR) receptor.
Article highlights.
Conventional and genetic transsynaptic tracing methods are compared for neuronal circuit tracing.
WGA and TTC are used as transsynaptic tracer molecules in genetic tracing approaches.
Genetic tracing uses cell type specific and nonspecific tracer gene expression.
Advantages and disadvantages of transgenic mouse and viral transduction approaches are compared for neural circuit tracing.
Neural circuit tracing analyses can be useful for evaluation of the effect of therapeutic agents on neuronal injury and neurodegenerative diseases.
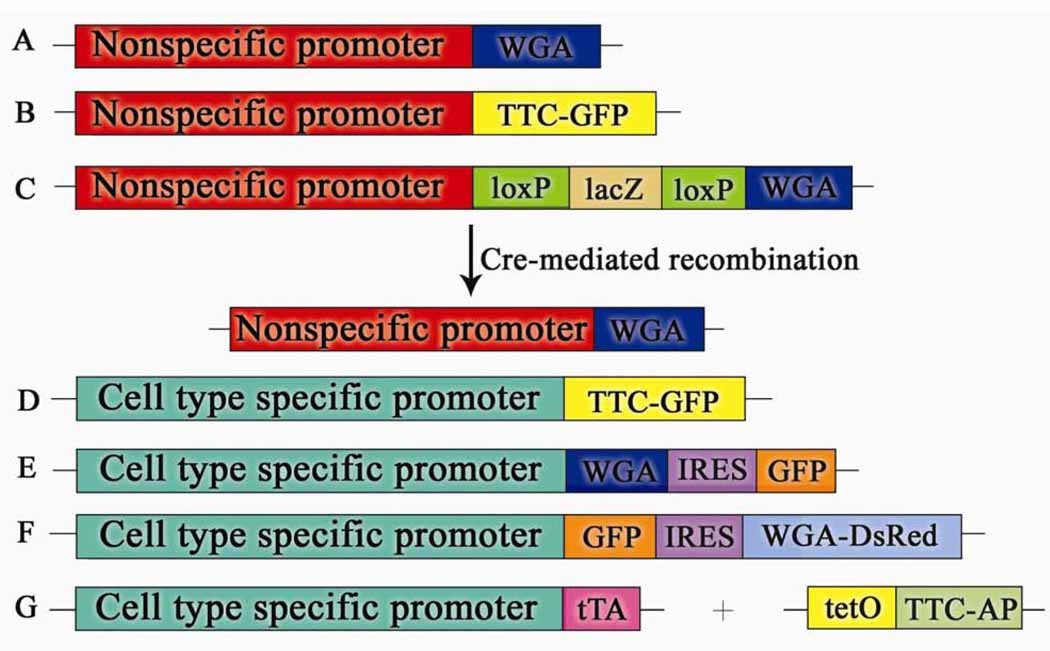
Figure 1.
Schematic diagram indicating the structure of transgene to trace neuronal circuitries. A. A nonspecific promoter drives expression of WGA. B. A nonspecific promoter drives expression of TTC-GFP. C. A nonspecific promoter drives WGA using the Cre-loxP system. D. A cell type specific promoter drives expression of TTC-GFP. E. A cell type specific promoter drives expression of WGA and GFP using the IRES gene. F. A cell type specific promoter drives expression of GFP and WGA-DsRed using the IRES gene. G. A cell type specific promoter drives expression of TTC-AP using the tTA-responsive promoter tetO.
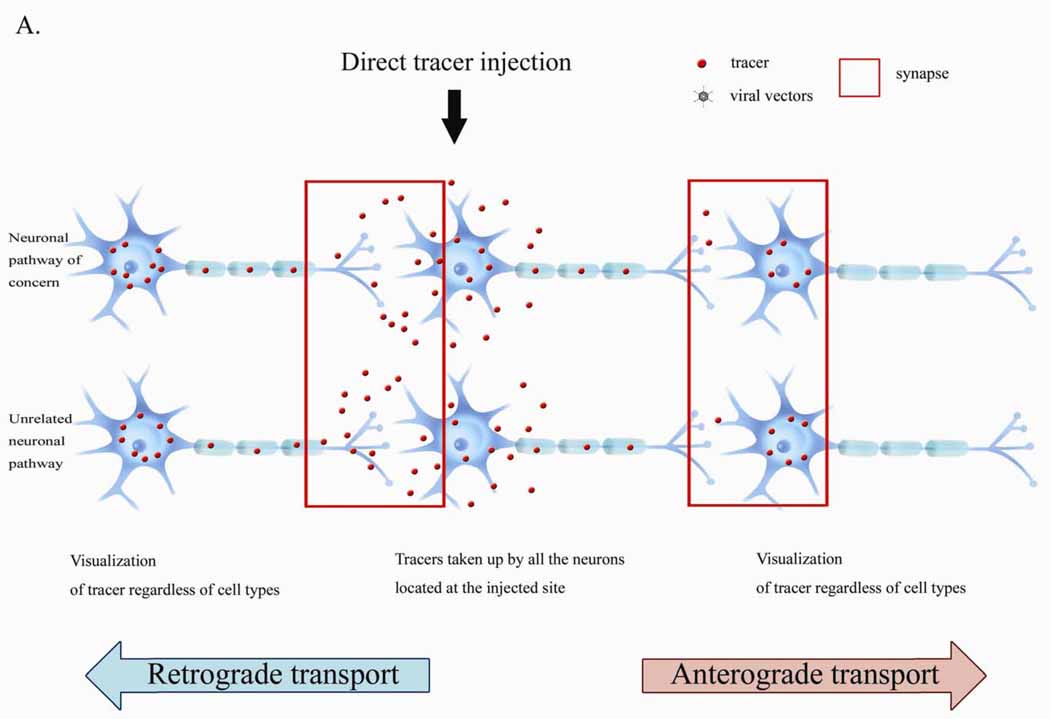
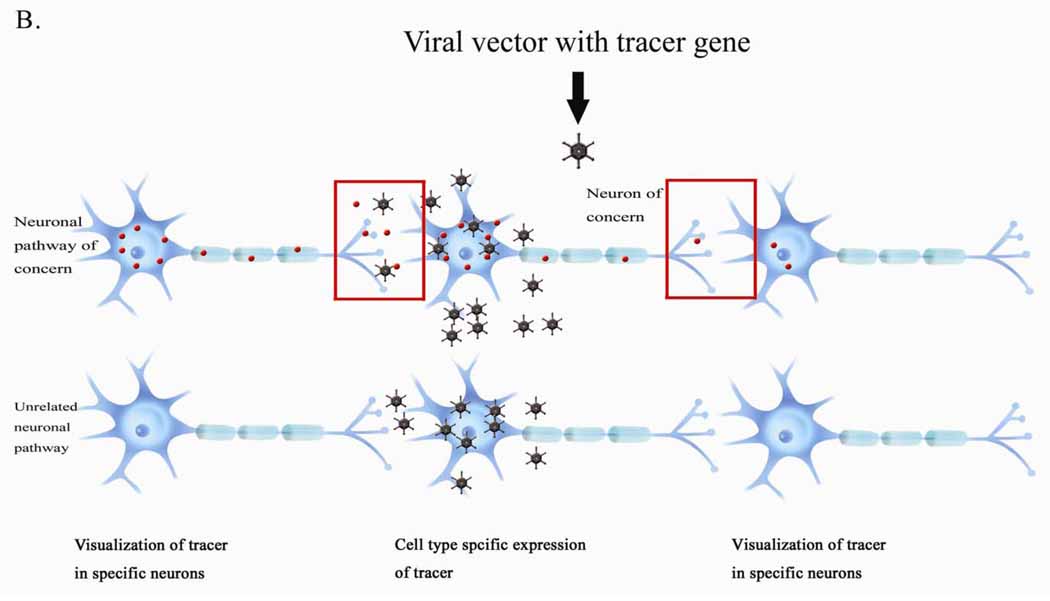
Figure 2.
Transneuronal transfer of tracer using conventional and viral tracer gene delivery. A. Conventional tracing. Tracers can be taken up by all the neurons located at the injection sites, resulting in nonspecific labeling of unrelated pathways. B. Viral tracer gene delivery. Tracers can be expressed selectively in specific neurons, resulting in visualization of specific trans-synaptic neuronal pathways.
Table 1.
Promoters for genetic tracing of neural circuits
| Promoters | Tracers | Vehicle | Phenotype of cells | Ref. |
|---|---|---|---|---|
| Nonspecific promoters | ||||
| CMV | TTC:GFP | Adenovirus | Nonspecific | 35 |
| CAG | WGA | Adenovirus, Transgenic | Nonspecific | 26, 38, 39 |
| Thy1 | XFP | Transgenic | Neurons, thymocyte | 44, 45 |
| Specific promoters | ||||
| T2R5 | WGA:DsRed | Transgenic | Bitter taste | 31 |
| T1R3 | WGA | Transgenic | Sweet/umami taste | 31, 46, 47 |
| L7 | WGA | Transgenic | Purkinje cell, rod bipolar cell | 2, 48 |
| OMP | WGA | Transgenic | Olfactory cell | 2 |
| Calbindin | TTC:GFP | Transgenic | Calbindin neuron | 34 |
| Rh1 | WGA | Transgenic | Photoreceptor cell | 26, 50, 51, 52 |
| Gnrh1 | WGA | Transgenic | GnRH neuron | 53 |
| Prepro-orexin | TTC:GFP | Transgenic | Orexin neuron | 36 |
| ePet-1 | WGA | Transgenic | Serotonin neuron | 56 |
| TH | WGA:GFP, WGA:DsRed2 | Adenovirus, AAV | Dopamine neuron | 25 |
| Nav1.8 | WGA, TTC:GFP | Transgenic | Sensory neuron | 66, 67 |
| NPY | WGA, TTC:GFP | Transgenic | Sensory neuron | 67 |
Acknowledgments
Declaration of interest
This work was supported by NIH grants DC006501, MH48866, and SEOULRNBD program (10524). The authors would like to thank Dr. Sergey Kasparov (University of Bristol, UK) for critical reading of this manuscript.
Bibliography
- 1.Boldogkoi Z, Sik A, Denes A, et al. Novel tracing paradigms-genetically engineered herpes viruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog. Neurobiol. 2004;72(6):417–445. doi: 10.1016/j.pneurobio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Yoshihara Y. Visualizing selective neural pathways with WGA transgene: combination of neuroanatomy with gene technology. Neurosci. Res. 2002;44(2):133–140. doi: 10.1016/s0168-0102(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 3. Kobbert C, Apps R, Bechmann I, et al. Current concepts in neuroanatomical tracing. Prog. Neurobiol. 2000;62(4):327–351. doi: 10.1016/s0301-0082(00)00019-8. · · Review on the conventional tracing method
- 4.Thanos S, Vidal-Sanz M, Aguayo AJ. The use of rhodamine-B-isothiocyanate (RITC) as an anterograde and retrograde tracer in the adult rat visual system. Brain Res. 1987;406(1–2):317–321. doi: 10.1016/0006-8993(87)90799-2. [DOI] [PubMed] [Google Scholar]
- 5.Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- 6.Holmqvist BI, Ostholm T, Ekstrom P. DiI tracing in combination with immunocytochemistry for analysis of connectivities and chemoarchitectonics of specific neural systems in a teleost, the atlantic salmon. J. Neurosci. Methods. 1992;42(1–2):45–63. doi: 10.1016/0165-0270(92)90134-y. [DOI] [PubMed] [Google Scholar]
- 7.Sparks DL, Lue L-F, Martin TA, Rogers J. Neural tract tracing using Di-I: a review and a new method to make fast Di-I faster in human brain. J. Neurosci. Methods. 2000;103(1):3–10. doi: 10.1016/s0165-0270(00)00291-0. [DOI] [PubMed] [Google Scholar]
- 8.Dolleman-Van Der Weel MJ, Wouterlood FG, Witter MP. Multiple anterograde tracing, combining Phaseolus vulgaris leucoagglutinin with rhodamine- and biotin-conjugated dextran amine. J. Neurosci. Methods. 1994;51(1):9–21. doi: 10.1016/0165-0270(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 9.Illig KR, Eudy JD. Contralateral projections of the rat anterior olfactory nucleus. J. Comp. Neurol. 2009;512(1):115–123. doi: 10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halberstadt AL, Balaban CD. Selective anterograde tracing of the individual serotonergic and nonserotonergic components of the dorsal raphe nucleus projection to the vestibular nuclei. Neuroscience. 2007;147(1):207–223. doi: 10.1016/j.neuroscience.2007.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiner A, Veenman CL, Medina L, et al. Pathway tracing using biotinylated dextran amines. J. Neurosci. Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 12.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(3):R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koliatsos VE, Price DL. Retrograde axonal transport: Applications in trophic factor research. In: Neurotrophic factors, neuromethods. vol. 27. Clifton, USA: Humana Press; 1993. [Google Scholar]
- 14.Lavail JH. The retrograde transport method. Fed. Proc. 1975;34(7):1618–1624. [PubMed] [Google Scholar]
- 15. Mesulam MM. Tracing neural connections with horseradish peroxidase. New York, USA: Wiley; 1982. · Review on retrograde neural tracing with horseradish peroxidase
- 16.Kuypers HGJM, Huisman AM. Fluorescent neuronal tracers. Adv. Cell. Neurobiol. 1984;5:307–340. [Google Scholar]
- 17.Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377(1):147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed F, Macarthur L, De Bernardi MA, Mocchetti I. Retrograde and anterograde transport of HIV protein gp120 in the nervous system. Brain Behav. Immun. 2009;23(3):355–364. doi: 10.1016/j.bbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curanovic D, Lyman MG, Bou-Abboud C, Card JP, Enquist LW. Repair of the UL21 locus in pseudorabies virus Bartha enhances the kinetics of retrograde, transneuronal infection in vitro and in vivo. J. Virol. 2009;83(3):1173–1183. doi: 10.1128/JVI.02102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab ME, Javoy-Agid F, Agid Y. Labeled wheat germ agglutinin (WGA) as a new, highly sensitive retrograde tracer in the rat brain hippocampal system. Brain Res. 1978;152(1):145–150. doi: 10.1016/0006-8993(78)90140-3. [DOI] [PubMed] [Google Scholar]
- 21.Wirsig-Wiechmann CR. Biocytin: a neuronal tracer compatible with rapid decalcification procedures. J. Neurosci. Methods. 1994;51(2):213–216. doi: 10.1016/0165-0270(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 22.Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J. Neurosci. Methods. 1992;41(3):239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- 23.Thanos S, Bonhoeffer F. Investigations on the development and topographic order of retinotectal axons: anterograde and retrograde staining of axons and perikarya with rhodamine in vivo. J. Comp. Neurol. 1983;219(4):420–430. doi: 10.1002/cne.902190404. [DOI] [PubMed] [Google Scholar]
- 24. Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr. Opin. Neurobiol. 2008;18(6):617–623. doi: 10.1016/j.conb.2009.03.007. · Review on transsynaptic neuronal tracing with neurotropic virus
- 25. Oh MS, Hong SJ, Huh Y, Kim KS. Expression of transgenes in midbrain dopamine neurons using the tyrosine hydroxylase promoter. Gene Ther. 2009;16(3):437–440. doi: 10.1038/gt.2008.148. · First report on the use of TH promoter for tracing
- 26. Yoshihara Y, Mizuno T, Nakahira M, et al. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron. 1999;22(1):33–41. doi: 10.1016/s0896-6273(00)80676-5. · First report of genetic tracing with WGA and cell type specific promoter
- 27.Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J. Comp. Neurol. 1985;242(4):632–650. doi: 10.1002/cne.902420410. [DOI] [PubMed] [Google Scholar]
- 28.Fabian RH, Coulter JD. Transneuronal transport of lectins. Brain Res. 1985;344(1):41–48. doi: 10.1016/0006-8993(85)91187-4. [DOI] [PubMed] [Google Scholar]
- 29.Gonatas NK, Harper C, Mizutani T, Gonatas JO. Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J. Histochem. Cytochem. 1979;27(3):728–734. doi: 10.1177/27.3.90065. [DOI] [PubMed] [Google Scholar]
- 30.Itaya SK. Anterograde transsynaptic transport of WGA-HRP in rat olfactory pathways. Brain Res. 1987;409(2):205–214. doi: 10.1016/0006-8993(87)90703-7. [DOI] [PubMed] [Google Scholar]
- 31.Sugita M, Shiba Y. Genetic tracing shows segregation of taste neuronal circuitries for bitter and sweet. Science. 2005;309(5735):781–785. doi: 10.1126/science.1110787. [DOI] [PubMed] [Google Scholar]
- 32.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80(2):717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 33.Coen L, Osta R, Maury M, Brulet P. Construction of hybrid proteins that migrate retrogradely and transynaptically into the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 1997;94(17):9400–9405. doi: 10.1073/pnas.94.17.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maskos U, Kissa K, St Cloment C, Brulet P. Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99(15):10120–10125. doi: 10.1073/pnas.152266799. ·· Report on the use of TTC:GFP for retrograde neuronal tracing
- 35.Kissa K, Mordelet E, Soudais C, et al. In vivo neuronal tracing with GFP-TTC gene delivery. Mol. Cell. Neurosci. 2002;20(4):627–637. doi: 10.1006/mcne.2002.1141. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai T, Nagata R, Yamanaka A, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Niwa H, Yamamura K-I, Muyazaki J-I. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 38. Kinoshita N, Mizuno T, Yoshihara Y. Adenovirus-mediated WGA gene delivery for transsynaptic labeling of mouse olfactory pathways. Chem. Senses. 2002;27(3):215–223. doi: 10.1093/chemse/27.3.215. · Review on the tracing with WGA gene delivery with recombinant adenovirus
- 39. Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99(23):15148–15153. doi: 10.1073/pnas.222546999. · Report on the use of Cre-mediated induction of WGA in transgenic mice
- 40.Amsterdam A, Lin S, Moss LG, Hopkins N. Requirements for green fluorescent protein detection in transgenic zebrafish embryos. Gene. 1996;173(1):99–103. doi: 10.1016/0378-1119(95)00719-9. [DOI] [PubMed] [Google Scholar]
- 41.Navarro V, Millecamps S, Geoffroy MC, et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Ther. 1999;6(11):1884–1892. doi: 10.1038/sj.gt.3301008. [DOI] [PubMed] [Google Scholar]
- 42.Kugler S, Meyn L, Holzmuller H, et al. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Mol. Cell. Neurosci. 2001;17(1):78–96. doi: 10.1006/mcne.2000.0929. [DOI] [PubMed] [Google Scholar]
- 43.Liu BH, Wang X, Ma YX, Wang S. CMV enhancer/human PDGF-beta promoter for neuron-specific transgene expression. Gene Ther. 2004;11(1):52–60. doi: 10.1038/sj.gt.3302126. [DOI] [PubMed] [Google Scholar]
- 44. Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. · Report on the use of thy-1 promoter and expression of multiple spectral variants of GFP
- 45. Livet J, Weissman TA, Kang H, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56–62. doi: 10.1038/nature06293. · Report on the use of thy-1 promoter and expression of brainbow fluorescent proteins
- 46.Ohmoto M, Matsumoto I, Yasuoka A, YOSHIHARA Y, ABE K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol. Cell. Neurosci. 2008;38(4):505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96. doi: 10.1186/1471-2202-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberdick J, Schilling K, Smeyne RJ, et al. Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron. 1993;10(6):1007–1018. doi: 10.1016/0896-6273(93)90050-2. [DOI] [PubMed] [Google Scholar]
- 49.Pavlou O, Ehlenfeldt R, Horn S, Orr HT. Isolation, characterization and in vivo analysis of the murine calbindin-D28K upstream regulatory region. Mol. Brain Res. 1996;36(2):268–279. doi: 10.1016/0169-328x(95)00259-u. [DOI] [PubMed] [Google Scholar]
- 50.Iwai Y, Hirota Y, Ozaki K, et al. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol. Cell. Neurosci. 2002;19(3):375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- 51.Tabuchi K, Sawamoto K, Suzuki E, et al. GAL4/UAS-WGA system as a powerful tool for tracing Drosophila transsynaptic neural pathways. J. Neurosci. Res. 2000;59(1):94–99. [PubMed] [Google Scholar]
- 52.Hanno Y, Nakahira M, Jishage K, Noda T, Yoshihara Y. Tracking mouse visual pathways with WGA transgene. Eur. J. Neurosci. 2003;18(10):2910–2914. doi: 10.1111/j.1460-9568.2003.03023.x. [DOI] [PubMed] [Google Scholar]
- 53. Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123(4):683–695. doi: 10.1016/j.cell.2005.09.027. · Report on cell type specific promoter and WGA expression in the hypothalamus
- 54.Lerner DR, Raikhel NV. Cloning and characterization of root-specific barley lectin. Plant Physiol. 1989;91(1):124–129. doi: 10.1104/pp.91.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 56. Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J. Comp. Neurol. 2008;507(6):1990–2003. doi: 10.1002/cne.21665. · · Report on the use of many cell type specific promoter for sensory neuronal tracing
- 57.Scott MM, Wylie CJ, Lerch JK, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. U. S. A. 2005;102(45):16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benzekhroufa K, Liu B, Tang F, Teschemacher AG, Kasparov S. Adenoviral vectors for highly selective gene expression in central serotonergic neurons reveal quantal characteristics of serotonin release in the rat brain. BMC Biotechnol. 2009;9:23. doi: 10.1186/1472-6750-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benzekhroufa K, Liu BH, Teschemacher AG, Kasparov S. Targeting central serotonergic neurons with lentiviral vectors based on a transcriptional amplification strategy. Gene Ther. 2009;16(5):681–688. doi: 10.1038/gt.2009.7. [DOI] [PubMed] [Google Scholar]
- 60.Duale H, Kasparov S, Paton JF, Teschemacher AG. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp. Physiol. 2005;90(1):71–78. doi: 10.1113/expphysiol.2004.029173. [DOI] [PubMed] [Google Scholar]
- 61.Hwang DY, Carlezon WAJ, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum. Gene Ther. 2001;12(14):1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 62.Hwang DY, Hwang MM, Kim HS, Kim KS. Genetically engineered dopamine beta-hydroxylase gene promoters with better PHOX2-binding sites drive significantly enhanced transgene expression in a noradrenergic cell-specific manner. Mol. Ther. 2005;11(1):132–141. doi: 10.1016/j.ymthe.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J. Comp. Neurol. 2009;512(2):141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lonergan T, Teschemacher AG, Hwang DY, et al. Targeting brain stem centers of cardiovascular control using adenoviral vectors: impact of promoters on transgene expression. Physiol. Genomics. 2005;20(2):165–172. doi: 10.1152/physiolgenomics.00120.2004. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Merlie JP, Todd RD, O'Malley KL. Identification of cell type-specific promoter elements associated with the rat tyrosine hydroxylase gene using transgenic founder analysis. Mol. Brain Res. 1997;50(1–2):33–42. doi: 10.1016/s0169-328x(97)00163-0. [DOI] [PubMed] [Google Scholar]
- 66.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47(6):787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163(4):1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hickman-Davis JM, Davis IC. Transgenic mice. Paediatr. Respir. Rev. 2006;7(1):49–53. doi: 10.1016/j.prrv.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Pilpel N, Landeck N, Klugmann M, Seeburg PH, Schwarz MK. Rapid, reproducible transduction of select forebrain regions by targeted recombinant virus injection into the neonatal mouse brain. J. Neurosci. Methods. 2009;182(1):55–63. doi: 10.1016/j.jneumeth.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 70. Card JP, Enquist LW, Moore RY. Neuroinvasiveness of pseudorabies virus injected intracerebrally is dependent on viral concentration and terminal field density. J. Comp. Neurol. 1999;407(3):438–452. doi: 10.1002/(sici)1096-9861(19990510)407:3<438::aid-cne11>3.0.co;2-2. · Report on the use of peudorabies virus for retrograde neuronal tracing
- 71.Ugolini G, Kuypers HG, Simmons A. Retrograde transneuronal transfer of herpes simplex virus type 1 (HSV 1) from motoneurones. Brain Res. 1987;422(2):242–256. doi: 10.1016/0006-8993(87)90931-0. [DOI] [PubMed] [Google Scholar]
- 72.Ugolini G, Kuypers HG, Strick PL. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science. 1989;243(4887):89–91. doi: 10.1126/science.2536188. [DOI] [PubMed] [Google Scholar]
- 73.Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J. Comp. Neurol. 2009;514(2):145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone neurons in the mouse using conditional viral tract tracing. Endocrinology. 2007;148(12):5884–5890. doi: 10.1210/en.2007-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Defalco J, Tomishima M, Liu H, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 76.Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 78.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005;8(11):1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 79.Nassi JJ, Lyon DC, Callaway EM. The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron. 2006;50(2):319–327. doi: 10.1016/j.neuron.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J. Comp. Neurol. 1995;356(3):457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- 81.Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods. 2007;4(1):47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wickersham IR, Lyon DC, Barnard RJ, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53(5):639–647. doi: 10.1016/j.neuron.2007.01.033. ·· Report on the use of modified rabies virus for retrograde tracing
- 83.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57(5):634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]