Abstract
Fragile X syndrome afflicts 1 in 2,500 individuals and is the leading heritable cause of mental retardation worldwide. The overriding clinical manifestation of this disease is mild to severe cognitive impairment. Age-dependent cognitive decline has been identified in Fragile X patients, although it has not been fully characterized nor examined in animal models. A Drosophila model of this disease has been shown to display phenotypes bearing similarity to Fragile X symptoms. Most notably, we previously identified naive courtship and memory deficits in young adults with this model that appear to be due to enhanced metabotropic glutamate receptor (mGluR) signaling. Herein we have examined age-related cognitive decline in the Drosophila Fragile X model and found an age-dependent loss of learning during training. We demonstrate that treatment with mGluR antagonists or lithium can prevent this age-dependent cognitive impairment. We also show that treatment with mGluR antagonists or lithium during development alone displays differential efficacy in its ability to rescue naive courtship, learning during training and memory in aged flies. Furthermore, we show that continuous treatment during aging effectively rescues all of these phenotypes. These results indicate that the Drosophila model recapitulates the age-dependent cognitive decline observed in humans. This places Fragile X in a category with several other diseases that result in age-dependent cognitive decline. This demonstrates a role for the Drosophila Fragile X Mental Retardation Protein (dFMR1) in neuronal physiology with regard to cognition during the aging process. Our results indicate that misregulation of mGluR activity may be causative of this age onset decline and strengthens the possibility that mGluR antagonists and lithium may be potential pharmacologic compounds for counteracting several Fragile X symptoms.
Keywords: Drosophila, Age onset cognitive deficits, Fragile X, Metabotropic glutamate receptor, Courtship conditioning assays, Pharmacological rescue
Introduction
Fragile X syndrome is the most common single gene disorder associated with mental retardation and in the majority of cases is caused by a (CGG) tri-nucleotide repeat expansion within the 5′ UTR of the Fragile-X Mental Retardation (FMR1) gene (Crawford et al. 2002; Hagerman and Hagerman 2002). In the normal population the number of CGG repeats at the FMR1 locus ranges from 5 to 60. Premutation, or “carriers” contain 150–200 repeats, and Fragile X patients generally have >200 repeats. By some unknown mechanism >200 repeats leads to hypermethylation of the FMR1 locus causing its transcriptional silencing (O’Donnell and Warren 2002).
Fragile X is associated with clinically relevant behaviors including sleep disorders, attention deficit disorder, hyperactivity, epilepsy and autistic behavior (Hagerman and Hagerman 2002; Bakker and Oostra 2003; Bear et al. 2004). The most prominent clinical feature of Fragile X however, is mild to severe mental retardation. Some studies in children and young adults have found progressive cognitive decline and increased autistic features with age, although some have argued that the finding may be related to the types of testing that were performed (Hagerman et al. 1989; Hay 1994; Wright-Talamante et al. 1996; Hatton et al. 2006). In any case, extended longitudinal studies with adult patients have not been done, leaving a critical potential gap in our information regarding performance of Fragile X patients with aging. Also, this issue has not been extensively examined in Fragile X animal models, where it can be studied quickly and cost effectively (Hagerman et al. 1989; Hay 1994; Wright-Talamante et al., 1996; Jacquemont et al. 2007). Furthermore, characterizing the effect that the loss of the protein product of FMR1 (FMRP) in aging may provide clues as to the pathophysiology of fragile X-associated tremor/ataxia syndrome (FXTAS), an age onset disease afflicting some FMR1 premutation carriers.
FXTAS patients suffer from intention tremor, cerebellar gait ataxia and cognitive decline (Berry-Kravis et al. 2007; Brega et al. 2008; Cornish et al. 2008; Cornish et al. 2009). FXTAS has an onset in late middle age and part of the pathophysiology is believed to be mediated by RNA toxicity caused by high level expression of FMR1 mRNA containing an increased number of CGG repeats (Berry-Kravis et al. 2007). However, it is known that FXTAS patients may have FMRP levels that are decreased, indeed FMRP expression is negatively correlated with repeat length in premutation range (Kenneson et al. 2001). Therefore exploring the role of FMRP in aging may be important with regard to normal aging, Fragile X and FXTAS.
The Drosophila gene, dfmr1, shares extensive homology with and has similar biochemical properties to the mammalian fragile X mental retardation protein (FMRP) (Wan et al. 2000). Mutants of the dfmr1 gene have a near normal lifespan, display normal overall activity levels and have intact primary sensory capabilities such as phototaxis and chemotaxis (Zhang et al. 2001; Dockendorff et al. 2002; Morales et al. 2002; McBride et al. 2005). However, dfmr1 mutants display clear deficits in more complex behaviors such as circadian and courtship behavior, and display deficits in memory as well (Dockendorff et al. 2002; Morales et al. 2002; McBride et al. 2005; Bolduc et al. 2008).
In previous studies, we examined the courtship, learning and memory capabilities of dfmr1 mutants in relatively “young” adults (5 days after eclosion). Clearly by that age, the dfmr1 mutants displayed reduced courtship activity and lacked any detectable memory of courtship conditioning (see methods) (Dockendorff et al. 2002; McBride et al. 2005). We also found that these deficits were rescued by treatment with antagonists of metabotropic glutamate receptors (mGluRs) or lithium if they were given during development alone (larval stages), during adulthood alone (post eclosion), or during both time periods (McBride et al. 2005). Additional studies in the fly and mouse, using similar pharmacological treatment, or using genetic reduction of mGluR activity, have also found that several Fragile X related symptoms can be rescued (Chuang et al. 2005; Yan et al. 2005; Dolen et al. 2007; Pan et al. 2008). All of these studies support the hypothesis that several Fragile X symptoms are due to enhanced mGluR signaling (Bear et al. 2004).
To date, the vast majority of studies using Fragile X models have examined defects incurred during development, or that are present in relatively young mice or young adult flies. In fact, we are aware of only two studies that have examined age dependent phenotypes in an animal model of Fragile X. Galvez and Greenough (2005), found that dendritic spine defects in the somatosensory cortex were absent in the brains of young FMR1 KO mice, but were clearly present in older mice (Galvez and Greenough 2005). Larson et al. (2005) found that long-term potentiation was normal in FMR1 KO mice under 6 months of age, but was impaired in older FMR1 KO mice. These findings, as well as those that suggest that Fragile X patients display an increased rate of cognitive decline with age, prompted us to determine if additional impairments of behavioral plasticity occurred in aged dfmr1 mutant flies (Hagerman et al. 1989; Hay 1994; Wright-Talamante et al. 1996). In addition, we wanted to examine the long-term efficacy of drug treatment given during development alone and/or for longer periods during adulthood. These are important points to consider for the long-term care and treatment of patients with Fragile X, particularly with regard to the potential to restore normal social and cognitive function in mentally retarded or autistic patients since some may only receive treatment in adulthood (Ehninger et al. 2008; Walsh et al. 2008).
Materials and methods
Drosophila strains, maintenance and drug treatment
A thorough explanation of the relevant genetics of the Drosophila strains used in the study can be found in Dockendorff et al. (2002). The strains utilized in here are isogenic and differ only in their ability to express dfmr1. The wild type rescue strain “WT” contains a single copy of a genomic transgenic rescue construct in the dfmr1 mutant background. This strain expresses near normal levels of dFMR1 protein. The frame-shift “FS” or mutant strain contains a single copy of the same genomic transgenic rescue construct introduced into the dfmr1 mutant background except that the transgene has a frame-shift mutation introduced into the dfmr1 open reading frame. This strain does not express dFMR1 protein.
The Drosophila strains were cultured at 25°C in 50–70% humidity in a 12 h light; 12 h dark (LD) cycle on cornmeal-sucrose-yeast. Drugs were prepared and added to the food as in McBride et al. (2005). Concentrated drug stock (>50-fold concentrate) or vehicle was added to solidified fly food and was mixed in using a spatula. Flies were treated by placing flies in vials containing the vehicle or vehicle with drug at the final concentration. Drugs used in our study are the following: (2-Methyl-6-(phenylethynyl) pyridine hydrochloride) MPEP ((RS)-a-Methyl-4-tetrazolylphenylglycine) MTPG ((RS)-a-Methyl-4-phosphonophenylglycine) MPPG ((2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid) LY341495 and Lithium (LiCl).
Behavioral training and testing and aging
Isolation of naive males and target females
Virgin “naive” male flies were collected under ether anesthesia within 4 h of eclosion (hatching from the pupal case). 10 male flies were placed in a vial of the appropriate treatment food. Flies were switched to fresh vials every 2 days. The females that were used for targets were shibire (shi) kept at 30°C. This simplified their collection as shi males do not hatch at 30°C, insuring that the females are virgin. The females were kept in normal food vials in groups of 10–15. Mated females were 5 days old and observed to have mated the night before being used in the training paradigm or one form of the memory test. The virgin females that were used as targets for naive courtship and for memory post-training were 4 days old. Male flies were aged for 20 days after eclosion in a 12:12 LD at 25°C before behavioral training and testing. All testing was performed during the relative light phase of day. Male flies were assigned to random groups and blinded training and testing was performed for all assays (Siegel and Hall 1979; Hall 1994; Sokal and Rohlf 1995; van Swinderen and Hall 1995; Villella and Hall 1996; Joiner Ml and Griffith 1997; Kane et al. 1997; McBride et al. 1999; McBride et al. 2005).
Activity and simple behavior testing
Because the performance of males in the courtship-based learning and memory tests can be affected by the overall activity of the males, and/or their visual and chemosensory capabilities, these properties were tested in young and aged males. Locomotor testing was done as in Griffith et al. (1993). Individual flies were placed in the courtship chambers with a line drawn down the center of the covering microscope slide. The flies were placed in the chamber and the activity was then measured immediately, since this best recapitulates the situation during testing when the measurements of courtship activity begin as soon as the male is placed into the chamber with a target female. Every time a fly crossed the line in a 2 min test period was scored to measure their activity levels. For each genotype and treatment group we tested 20–25 flies. Olfactory and visual assays were performed as described in Orgad et al. (2000). For the olfactory assay, flies were loaded into the olfactory chamber containing the trap in 4 groups of 10 per genotype and drug treatment. The number of flies that were caught in the trap at 36 and 60 h afterwards was then scored (Orgad et al. 2000). To test visual acuity for each genotype and treatment group, four groups of twenty flies were loaded into a Y maze that was covered in foil (in total darkness) except for the last inch of one branch of the Y maze (Orgad et al. 2000). Flies were given 2 min in the Y-maze. The number of the flies that entered the lighted portion of the chamber was scored.
Measuring naive courtship
During normal courtship (naive male paired with a virgin female in a courtship chamber) a male fly performs a characteristic sequence of behaviors that are repeated with some variation until successful copulation occurs (Sturtevant 1915; Bastock 1955, 1956). In our assays we measured the amount of time the males actively engaged in any of the six steps of courtship (orienting toward the female target, following the female, tapping a leg on the female abdomen, licking the female genitalia, vibrating a wing, and attempting copulation) toward a virgin (receptive) female during a 10 min courtship assay and expressed the percentage of time engaged in courtship activity as a courtship index (CI).
Testing for learning and memory
Learning and memory were tested by using the courtship-conditioning paradigm, which involves pairing a naive male with a mated (unreceptive) female in a courtship chamber. Although virgin females most commonly react to male courtship advances by mating, females that have recently mated with a male are not receptive to male courtship attempts (Spieth 1974). In the conditioned courtship paradigm a male fly learns to modify his courtship behavior after experience with an unreceptive female. Learning (also known as learning during training) is assayed by comparing the courtship index of the first 10 min and last 10 min of a 1 h training session. Control flies generally display a 40% or greater drop in courtship activity, indicative of learning during training (LDT) (Siegel and Hall 1979; Joiner Ml and Griffith 1997; Kane et al. 1997) (for review see Hall 1994). After 1 h of experience with the mated female, the now “trained-male”, when subsequently paired with a virgin female, will display reduced courtship activity for 2–3 h, indicating memory of the training with the mated female (Siegel and Hall 1979). To test for immediate recall memory (0–2 min memory) the freshly trained male was transferred to a new testing chamber containing a virgin female and the courtship activity was measured for a 10 min courtship interval. To measure short-term memory (60 min memory) the freshly trained male was placed in a holding chamber and kept in isolation for 1 h, then was transferred to a fresh testing chamber containing a virgin female target. Courtship activity was then measured for a 10 min interval and compared to the level of naive courtship. In a modified form of the memory test a trained male was placed in a holding chamber for 60 min and was then transferred to a fresh testing chamber containing a novel mated female target. In this test the level of post training courtship was compared to the courtship activity measured in the first 10 min of training with a mated female.
Histological analysis of aged Drosophila brains for cell death
Tunel assays were performed according to Jaffe and Jongens (2001), using a S7110 kit from Oncor. Acridine Orange stainings were performed according to Heriche et al. (2003). Analysis of stained brains was performed by 3D reconstruction of optic stacks taken at 1 µm using a Leica scanning confocal microscope. The number of cell death foci and their relative position were tabulated for each genotype and drug treatment.
Statistics
The CIs of tested males were subjected to arcsin square root transformations to approximate normal distributions (Sokal and Rohlf 1995; van Swinderen and Hall 1995; Villella and Hall 1996; Joiner Ml and Griffith 1997; Kamyshev et al. 1999; McBride et al. 1999; McBride et al. 2005). ANOVAs were performed on arcsin transformed data to get critical p-values. Chi squared analysis was performed on the binning of courtship, olfactory acuity and visual acuity assays. All statistics were performed using Statview 3.0.
Results
In previous studies, we found deficits in the naive courtship and memory capabilities of relatively young dfmr1 mutants (5 days after eclosion) (Dockendorff et al. 2002; McBride et al. 2005). We also found that these deficits were rescued by treatment with antagonists of metabotropic glutamate receptors (mGluRs) or lithium if they were given during development alone (larval stages), during adulthood alone (post eclosion), or during both time periods (McBride et al. 2005). To expand on these studies we set out to examine the stability of the rescue obtained from treatment during development alone as well as to examine the efficacy of longer treatments during adulthood. To determine if treatment of dfmr1 mutants during development alone, with mGluR antagonists or lithium, could continue to rescue naive courtship in older adults, we tested naive courtship in dfmr1 mutant and control flies that had undergone drug treatment during development, but were aged to 20 days post eclosion (aged adults) on control food. In order to test the effect of prolonged treatment during adulthood, as well as the effect of combined treatment during development and throughout adulthood, we tested naive courtship in dfmr1 mutant and control flies that were treated with mGluR antagonists or lithium during adulthood alone, or during development and adulthood, respectively. We used both WT (control) and FS (dfmr1 mutant) males for these studies. These two fly lines differ only in their ability to express functional dFMR1 protein as they are both derived from the original dfmr13 mutant allele (see methods). This creates a model that recapitulates the cause of fragile X in humans, which is a complete lack of the protein product, FMRP or dFMRP in this case. By utilizing the WT as controls, this model further ensures that the phenotypes that we are examining are solely due to the lack of dFMRP.
The drugs used in our study include the non-competitive mGluR antagonist MPEP, the competitive mGluR antagonists MPPG, MTPG and LY341495 as well as lithium (see methods). The specificities of these drugs, the reasons for using them and the doses used, are described in detail in McBride et al. (2005). Briefly, previous studies had indicated that loss of FMRP led to enhanced mGluR signaling. In our fly fragile X model this enhanced signaling would lower cAMP signaling and increase inositol trisphosphate receptor (InsP3R) mediated calcium signaling. Treatment with mGluR antagonists should ameliorate this dysregulation by reducing mGluR signaling. Lithium acts downstream of the mGluRs. By inhibiting GSK-3B activity lithium increases cAMP mediated signaling and lithium decreases InsP3R mediated calcium signaling by inhibiting inositol trisphosphate (InsP3) synthesis and recycling (Hallcher and Sherman 1980; Klein and Melton 1996; Acharya et al. 1998).
During normal or “naive” courtship (a naive male paired with a virgin female in a courtship chamber), male flies perform a characteristic sequence of behaviors that are repeated with some variation until successful copulation occurs (Sturtevant 1915; Bastock 1955, 1956). In our assays we measured the amount of time the males actively engaged in any of the six steps of courtship toward a virgin (receptive) female during a 10 min courtship assay and expressed the percentage of time engaged in courtship activity as a courtship index (CI) (see methods). In the “aged” flies, we found that naive courtship activity was lower in FS versus WT flies and a lower percentage of FS flies entered the advanced stages of courtship (Figs. 1A and S1, S2). This deficit is similar to what we observed in young adult male FS flies (McBride et al. 2005), however, the difference between WT and FS was less in the aged adults compared to young adults. This is because the control WT flies displayed a reduction in naive courtship activity during aging, whereas the mutant FS flies showed no further decline in activity from what was observed in young mutant flies (Figure S1).
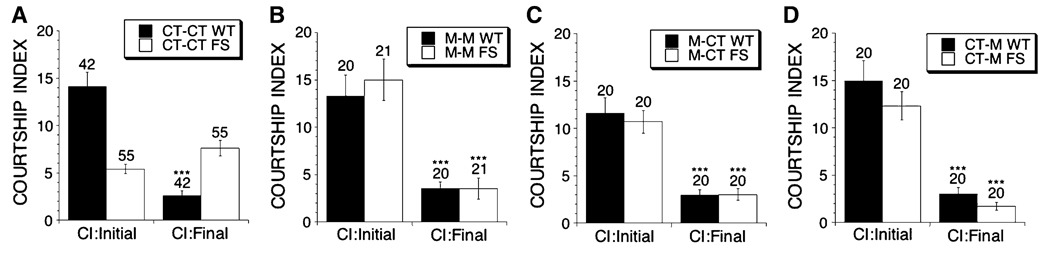
Fig. 1.
Naive courtship in aged dfmr1 mutant and control flies treated with MPEP. Flies are either WT (dFMR13 + wild type rescue fragment) or FS (dFMR13 9 the rescue fragment with an engineered frame shift mutation in the dfmr1 open reading frame) males. Mean CIs (±SEM) are plotted, Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). The food was either control (CT) or exactly the same control food with the addition of a drug. The first abbreviation indicates the food type that was given during the larval stages, and the second abbreviation indicates the food type that the adult flies were placed on within 4 h of eclosion for 19 days. Abbreviations are CT-control and M-86 µM MPEP. (A–D) Naive males were placed in the training chamber for 1 h with no female, and then placed with a virgin target female for a 10 min interval. (A) Without drug treatment (CT–CT) FS males courted virgin females less vigorously than WT flies p < 0.05 (the asterisk is omitted from panel A for clarity). Comparisons in panels B–D were made relative to the (CT–CT) mean of the same genotype in panel (A). (B) When raised on MPEP-containing food during both development and adulthood (M–M) FS flies courted virgin females more actively then (CT–CT) FS flies and as well as untreated WT males shown in panel A. The naive courtship levels of (M-M) WT and (CT–CT) WT flies did not differ significantly. (C) WT and FS flies treated with MPEP as larvae and then placed on CT food as adults (M–CT) courted similarly to (CT–CT) WT and (CT–CT) FS flies, respectively. (D) When treated with MPEP only as adults (CT–M), FS flies displayed significantly greater courtship activity relative to (CT–CT) FS flies, whereas courtship activity of the WT flies was not altered relative to (CT–CT) WT flies
To test the effect of mGluR antagonist treatment on the naive courtship of dfmr1 mutants we first used the non-competitive antagonist MPEP (see methods). When FS flies were given MPEP (86 µM) during development and adulthood (for 20 days) or during adulthood alone, naive courtship was significantly increased. However, treatment of FS flies with MPEP during development alone failed to provide a detectable increase in the naive courtship of aged flies (Fig. 1A–D and Figure S1). This is in stark contrast to the rescue that was seen at 5 days of age (McBride et al. 2005), indicating that the rescue obtained by treatment during development alone is not permanent. As observed with young flies, the increases in naive courtship activity in aged FS flies treated with MPEP in adulthood correlated with an increase in the percentage of flies that reached the more advanced stages of courtship (Figure S2). WT flies given MPEP food during development, adulthood alone or during development and adulthood courted as vigorously as untreated WT flies (Fig. 1A–D).
To determine if similar results were obtained with additional mGluR antagonists, we tested the effect of MTPG, MPPG and lithium treatment on naive courtship. Adulthood treatment with the mGluR antagonist MTPG (348 µM) as well as lithium (5 mM) significantly increased naive courtship in FS flies, while adult treatment with NaCl (a control for lithium) and the mGluR antagonist MPPG (573 µM) failed to increase naive courtship activity (Fig. 2B, D) (McBride et al. 2005). When tested in young adults, MPPG was capable of rescuing naive courtship (McBride et al. 2005). Failure of MPPG to work with longer treatment may be because flies build up a tolerance to this drug, or that this drug has toxic side effects that eventually affect naive courtship. Adulthood treatment alone of WT flies with MTPG, MPPG and lithium decreased naive courtship activity (Fig. 2A, C). This negative effect on naive courtship was previously observed with young WT flies that were treated with lithium or the mGluR antagonists during adulthood alone (McBride et al. 2005). Thus the control flies are unable to adjust to the negative effect of treatment with mGluR antagonists or lithium, even when given during adulthood alone for prolonged periods of time.
Fig. 2.
Naive courtship in aged dfmr1 mutant and control flies with and without additional mGluR antagonists and LiCl. Flies are either WT or FS males. Mean CIs (±SEM) are plotted, Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). The food was either control (CT) or exactly the same control food with the addition of a drug. All flies were placed on drug containing within 4 h of eclosion for 19 days. Abbreviations are CT-control, MPPG-573 µM MPPG, MTPG-348 µM MTPG, Li-5 mM lithium and Na-5 mM NaCl. (A–D) The naive courtship levels of WT flies (A and C) and FS flies (B and D) were tested after being raised on CT food during development, then given food containing either NaCl or a test drug during adulthood for 19 days. The naive courtship levels shown for CT–CT FS flies and CT–CT WT flies in Fig. 2A–D are replicated from Fig. 1A as a reference point to compare to naive courtship of the various treatment groups. (A) Treatment with MPPG or MTPG suppressed the courtship of WT flies. (B) Treatment with MTPG increased naive courtship in FS flies, whereas treatment with MPPG did not. (C) Treatment with lithium suppressed naive courtship in WT flies, whereas treatment with NaCl did not. (D) Treatment with lithium increased naive courtship in FS flies, whereas treatment with NaCl did not
Prior to testing the effects of the different drug treatment paradigms on memory, we first evaluated the learning during training capabilities of aged flies in courtship conditioning. Virgin females most commonly react to male courtship advances by mating; in contrast, females that have recently mated with a male are not receptive to male courtship attempts (Spieth 1974). In the conditioned courtship paradigm a male fly learns to modify his courtship behavior after experience with an unreceptive female. The naive male will find a previously mated female to produce a pheromone repertoire that is less provocative than that of a virgin female target. The previously mated female will also rebuff courtship advances. When initially paired with a previously mated female, a naive male will court her. However, his courtship activity soon decreases which is indicative of learning, e.g. “learning during training (LDT)”. After 1 h of experience with the mated female, the now “trained-male”, when subsequently paired with a receptive virgin female, will display reduced courtship activity for 2–3 h, indicating memory of the training that occurred with the mated female (Siegel and Hall 1979).
In our previous studies we found that young dfmr1 mutant males displayed normal learning during training (McBride et al. 2005). To assess learning during training capabilities of aged flies, adult FS and WT male flies that were 20 days post-eclosion were placed in a training chamber with a previously mated female for 1 h. The amount of time the male spent courting in the first 10 min interval was compared to the amount of time the male spent courting the female target in the last 10 min interval. In this paradigm, control flies typically show a 40% or more decrease in courtship activity (Joiner Ml and Griffith 1997; Kane et al. 1997). We found that the untreated FS flies did not show a decrease in time spent courting during the training period, indicating that no learning during training was occurring (Fig. 3A). This is in contrast to the normal learning during training observed with young FS flies (McBride et al. 2005). The WT flies continued to display intact learning during training at 20 days of age (Fig. 3A), so the loss of learning during training for the FS flies cannot be attributed to normal aging.
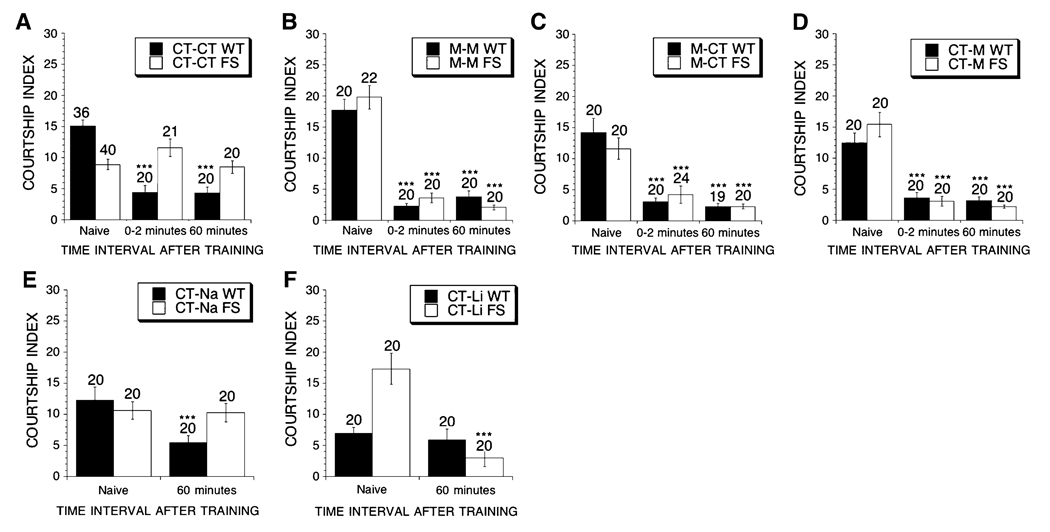
Fig. 3.
Learning during training in aged dfmr1 mutant and control flies that were treated with MPEP. Flies are either WT or FS. Mean CIs (±SEM) are plotted, Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). The food was either control (CT) or exactly the same control food with the addition of a drug. The first abbreviation indicates the food type that was given during the larval stages, and the second abbreviation indicates the food type that the adult flies were placed on in adulthood for 19 days. Abbreviations are CT-control and M-86 µM MPEP. For Panels A–D: filled bars indicate C.I. values for WT flies; open bars indicate C.I. values for FS flies. The male flies were placed in a training chamber with a previously mated female for 1 h. The amount of time the male spent courting in the first 10 mi interval was compared to the amount of time the male spent courting the female target in the last 10 min interval to assess learning during training. (A) WT and FS flies on control food during development and as adults. (B) WT and FS flies on MPEP containing food during development and as adults. (C) WT and FS flies on MPEP containing food during development and control food as adults. (D) WT and FS flies on control food during development and MPEP containing food as adults. There was no difference between the courtship activity in the two intervals of the (CT–CT) FS group, indicating that no learning during training occurred. The initial and final courtship levels of all other groups showed significant depression from the initial to final intervals indicating that all other groups demonstrated learning during training. This demonstrates that treatment of FS flies with MPEP in development, adulthood or both is sufficient to restore learning during training in FS flies. WT flies demonstrated learning during training in all treatment groups
Since we previously found that naive courtship and memory could be rescued by treatment with mGluR antagonists and lithium, we investigated whether the age onset deficit in learning during training could be the result of the continued enhancement of mGluR signaling. We first tested the efficacy of MPEP treatment on the dfmr1 mutants and found that treatment in development, adulthood or development and adulthood rescued learning during training in the aged FS flies (Fig. 3B–D). This demonstrated that treatment in development can prevent impairment in LDT that occurs in late adulthood (Fig. 3C). The drug treatments had no negative effects on control flies as the WT flies that were treated with MPEP in development, adulthood or both displayed normal learning (Fig. 3B–D).
To confirm that the rescue was occurring through downregulation of mGluR activity, we tested whether similar results were obtained with a lower dose of MPEP, to avoid possible offsite antagonism of NMDA receptors (see McBride et al. 2005), other mGluR antagonists and lithium. FS flies treated during adulthood with a low dose of MPEP (LM), continued to restore learning during training. Additionally, the mGluR antagonists LY341495 (LY), MPPG and MTPG also restored learning during training (Fig. 4A, B, E, F). Lithium treatment also rescued learning during training in FS flies, whereas NaCl treatment did not (Fig. 4C–D). Again the drug treatments had no negative effect on WT flies as they displayed significant learning during training with all treatments (Fig. 4A–F). This indicates that FS flies have an age-dependent impairment in learning during training that is rescued by treatment with mGluR antagonists and lithium.
Fig. 4.
Learning during training in aged dfmr1 mutant and control flies that were untreated or treated with mGluR antagonists or LiCl. Flies are either WT (filled bars) or FS (open bars). Mean CIs (±SEM) are plotted, Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). Abbreviations are CT-control, MPPG-573 µM MPPG, MTPG-348 µM MTPG, Li-5 mM lithium, Na-5 mM NaCl, LM-8.6 µM MPEP, and LY-400nM LY341495. FS flies on LM (A), LY (B), Li (D), MPPG (E) or MTPG (F) as adults demonstrated learning during training, whereas FS flies treated with NaCl (C) did not. WT flies demonstrated learning during training in all treatment groups
To examine the effect of aging on memory as well as the efficacy of the various drug treatment paradigms on the rescue of memory, we first examined memory in untreated aged males. To assay memory we placed 20 day-old adult “trained” males with a virgin target female, immediately after the 1 h training with a mated female or after 1 h in isolation to measure immediate recall memory (0 min) or short-term memory (60 min), respectively. The level of courtship activity measured in these 10 min intervals was compared to the courtship of naive aged males placed in isolation for 1 h, then placed with a virgin target female for a 10 min interval. Similar to what was observed with young flies, untreated aged FS flies displayed no detectable memory at 0 or 60 min after training (Fig. 5A) (McBride et al. 2005).
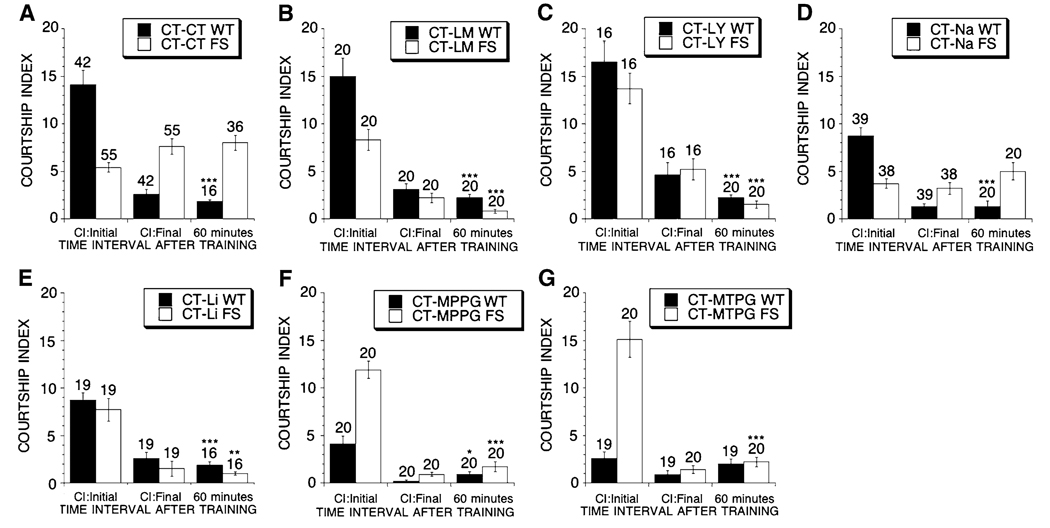
Fig. 5.
Memory in aged dfmr1 mutant and control flies that were untreated or treated with MPEP or LiCl. Flies are either WT (filled bars) or FS (open bars). Mean CIs (±SEM) are plotted; Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). Abbreviations are CT-control, M-86 µM MPEP, Li-5 mM lithium and Na–5 mM NaCl. 0–2 min is immediate recall memory and 60 min is short-term memory. (A) The courtship activity of (CT–CT) WT flies was significantly reduced at 0 and 60 min post training when compared to the level of naive courtship (p < 0.0001). (CT–CT) FS flies at 0 and 60 min post training failed to display any reduction in courtship levels when compared to naive courtship levels. Thus FS flies lacked any detectable immediate recall and short-term memory in this assay. (B–D) WT and FS flies whether fed (B) 86 µM MPEP during development and adulthood ((M–M) WT and (M–M) FS), (C) during development alone ((M–CT) WT and (M–CT) FS), or (D) adulthood alone ((CT–M) WT and (CT–M) FS) all displayed a significant reduction in courtship activity toward a virgin female at 0 and 60 min after training when compared to the naive courtship levels obtained for similarly fed flies (p < 0.0001 for all). FS flies treated with NaCl in adulthood did not exhibit short-term memory (E), while FS flies treated with Li did exhibit short-term memory (F). WT flies treated with NaCl in adulthood did exhibit short-term memory (E), while WT flies treated with Li did not exhibit short-term memory (F)
Since we observed that all of the treatment paradigms with mGluR antagonists and lithium rescued learning during training, we tested if they also rescue memory in the aged males. Aged FS flies that were treated with MPEP during development, as adults or during both periods, all display experience dependent reduction of courtship activity immediately (immediate recall memory) after training when compared to group matched naive males (Fig. 5B–D). All FS flies that underwent MPEP treatment also demonstrated short-term memory (Fig. 5B–D). Therefore, not only can adult treatment with MPEP restore memory in aged flies, as has been shown for young adult flies (McBride et al. 2005), treatment during development alone continues to be effective for 20 days post-eclosion (Fig. 5C).
Untreated and MPEP treated aged WT flies demonstrate normal depression of courtship activity immediately or 1 h after training relative to group matched naive flies, indicating that none of the MPEP treatment paradigms affected immediate recall or short term memory in the control flies (Fig. 5A–D). Additionally, WT and FS flies were treated throughout adulthood with 5 mM lithium or 5 mM NaCl and tested for short-term memory. Prolonged lithium treatment restored short-term memory in the aged FS flies and impaired short-term memory in WT flies. As expected no rescue was observed in FS flies fed food containing 5 mM NaCl throughout adulthood (Fig. 5E–F).
To verify that the deficit in memory was not due to a sensory deficit in FS flies that prevented them from recognizing appropriate sensory cues from the mated and virgin female targets, thereby preventing the trained male from courting a virgin female, we utilized a slightly different memory paradigm. We tested the memory of trained male flies using mated females in the post training courtship test, instead of virgin females, as courtship targets. This modified form of the courtship conditioning memory test has been used in previous studies to test memory, including our initial characterization of the memory phenotype in young dfmr1 mutants (Kamyshev et al. 1999; Savvateeva et al. 2000; McBride et al. 2005). In this modified memory test, aged WT flies demonstrated intact memory, in contrast to the aged FS flies that lacked any detectable memory (Fig. 6A). Using this modified memory test, we also observed that treatment with a low dose of MPEP (LM), LY341495, lithium, MPPG and MTPG, during adulthood alone, all restored short-term memory in aged FS flies, whereas NaCl treatment did not (Fig. 6B–G).
Fig. 6.
Testing short-term memory in aged dfmr1 mutant and control flies that were untreated or treated with mGluR antagonists or LiCl, using an alternative memory paradigm. Flies are either WT (filled bars) or FS (open bars) males. Mean CIs (±SEM) are plotted; Ns are indicated above each bar for all groups. The levels of significance are indicated (one asterisk * indicates p < 0.05, two asterisks ** indicates p < 0.005, three asterisks *** indicates p < 0.0001). Abbreviations are CT-control, MPPG-573 µM MPPG, MTPG-348 µM MTPG, Li-5 mM lithium, Na-5 mM NaCl, LM-8.6 µM MPEP, and LY-400nM LY341495. (A–G) After the one training session with a previously mated female, the male was placed in a holding chamber for 60 min and subsequently placed in a testing chamber with a different previously mated female target to assess short-term memory. The comparisons that indicate memory in Fig. 6, panels A–G are between CI: Initial and 60 min post training. CI: Final values are shown on the graphs as a reference, therefore no asterisks are placed above these values since this is not the comparison of interest in these graphs. A decrease in CI at 60 min post training versus CI: Initial indicates memory. CT–CT WT flies demonstrated intact memory as indicated by a significant decrease in courtship index at 60 min post training compared to CI: Initial, whereas CT–CT FS flies did not (A). In panels (B–G) all flies were raised on CT food and then placed on drug containing food. FS flies on LM (B), LY (C), Li (E), MPPG (F) or MTPG (G) in adulthood demonstrated intact memory at 60 min, whereas FS flies treated with NaCl (D) did not. WT flies on LM (B), LY (C), Li (E), MPPG (F) or NaCl (D) as adults demonstrated intact memory at 60 min, whereas WT flies treated with MTPG (G) did not
The appearance of a deficit in learning during training upon aging in FS flies may be analogous to the progressive cognitive decline that appears to occur in humans afflicted with Fragile X syndrome (Hagerman et al. 1989; Hay 1994; Wright-Talamante et al. 1996). Therefore, we investigated possible causes for the loss of learning during aging. Examination of the aged dfmr1 mutants via locomotor, phototactic and olfactory assays, however, failed to reveal any explanation for this loss of learning. In all assays performed, comparison of the performance of the aged dfmr1 mutants, with or without drug treatment, to similarly treated control flies failed to provide a correlation to loss of learning during training (Fig. 7A–C).
Fig. 7.
Examination of locomotor activity, sensory capabilities and cell death in aged dfmr1 mutant and control flies. (A) The locomotor function of 20–22 day post eclosion FS and WT flies that were either untreated or given drugs was measured using a line crossing assay (McBride et al. 2005). The position of the CT or M is indicative of the point at which the group was on the particular food. Black bars (CT–CT) WT (dFMR13 + wild type rescue fragment); hatched bars (CT–M) WT; blue bars (CT–CT) FS (dFMR13 + frame shifted rescue fragment); open bars (CT–M) FS; gray bars (M–M) WT; green bars (M–CT) WT; yellow bars (M–M) FS; red (M–CT) FS. Untreated FS and WT flies performed similarly and only the M–M WT flies showed a significant increase in activity compared to untreated flies. (B) The olfactory capabilities of the flies were measured using an olfactory trap assay (McBride et al. 2005). The number of flies that were caught in the trap at 36 and 60 h afterwards was then scored. The drug treatments and genotypes are as listed for panel A. Drug treatment did not improve performance in any group at 36 h and no significant differences were found between any of the groups at 60 h. (C) The visual acuity of the flies was measured using a Y maze test (McBride et al. 2005). The number of flies that entered the chamber having the light shown into it was scored. The genotypes and drug treatments are as described for panel A. There was no difference in the ability of the flies to detect light. Untreated FS and WT flies performed similarly, and drug treatment did not improve the performance of FS or WT flies. (D–F) Acridine Orange staining of (D) FS (E) WT and (F) ATP alpha [EY02875] brains removed from flies that were 20 days post eclosion. The ATP alpha mutants have previously been shown to undergo cell death in the brain and were included in the assay as a positive control (Palladino et al. 2000). Arrow in E and F indicate cells undergoing cell death. (G) The number of cell death foci detected with Acridine Orange staining for each brain was scored. A minimum of 15 brains were scored for each group and plotted. Most brains lacked any detectable cell death and there was no correlation between level of cell death and a reduction in cognitive capabilities
Another possible explanation for the loss of learning we investigated was an increased rate of neuronal cell death in the dfmr1 mutant brains, compared to aged matched controls. To determine if this was a likely explanation, we assayed aged FS and WT brains for cell death using tunel staining (not shown) and Acridine Orange staining (Fig. 7D–G). This histological analysis however, did not indicate cell death as the cause of cognitive dysfunction in young or old FS flies. The majority of the FS and WT brains lacked any detectable cell death (Fig. 7G). In the absence of detectable cell death, another possible explanation is that of synaptic silencing. In this case, dysfunctional neuronal signaling at synapses would be occurring before any detectable histological or morphological changes in the neurons (Landis 1976).
Discussion
In a previous study we examined the courtship capabilities (social interactions), learning during training and memory of relatively young (5 day-old) dfmr1 mutant flies (McBride et al. 2005). These studies revealed that flies lacking dfmr1 function display reduced levels of naive courtship activity relative to controls and failed to display any detectable memory of courtship conditioning. The learning during training profile of these mutant flies, however, was essentially identical to that of controls, indicating that there was no deficit in this cognitive task at a young age.
In this study, we have expanded on our previous findings by examining the effects of aging on dfmr1 mutants. This analysis has revealed that with age, dfmr1 mutants selectively lose the ability to learn during training in the courtship-conditioning paradigm. This loss of learning occurs without any significant change in their sensory perception or locomotor activity and cannot be explained by elevated levels of cell death in the brain. Furthermore, the initial level of courtship performed by the aged dfmr1 males during the first 10 min of the training session is similar to that of young dfmr1 mutant flies that do display normal learning during training (McBride et al. 2005). Thus the learning deficit qobserved in aged males cannot be due to a failure to perform a sufficient level of courtship activity during training. Our results indicate that the loss of learning is clearly age-dependent and indicates that dfmr1 has a physiological role in neuronal aging in addition to its previously documented role in neuronal development (Restifo 2005; Zarnescu et al. 2005). Whether the defects we observed in young dfmr1 males are due to physiological or developmental defects is less clear, as they are present as soon as we can reliably test them at 5 days post-eclosion.
In our analysis of the age-dependent loss of learning during training we have found that although the learning deficit appears at a much later age than the deficits in naive courtship and memory, it too can be rescued by treatment with mGluR antagonists or lithium. These data indicate that the deficits in naive courtship, learning during training and memory can all be ascribed to enhanced mGluR-signaling. These results also suggest that the underlying defect that leads to the cognitive decline observed in the Fragile X patients may be linked to the same defect giving rise to their initial cognitive deficits.
The coupling of different drug treatment paradigms to our aging study has allowed us to examine the stability of the rescue obtained by treating during development alone, as well as efficacy of adult treatments for longer periods. These studies have revealed some interesting similarities and differences in the underlying defects that cause the behavior deficits displayed by the dfmr1 mutants. The most surprising finding is that the age onset loss of learning can be rescued by treatment during development alone. This finding suggests that this age onset phenotype, like naive courtship and memory, is at least in part due to defects incurred during development. These defects must be such that they leave the fly capable of learning during training at 5 days of age, but subsequently deteriorate such that by day 20 the ability to learn during training is lost. This defect must also be comparable to the underlying defect that leads to the deficit in memory, in that it can be stably rescued by treatment with mGluR antagonists or lithium during development alone, or with treatment during adulthood. The defect underlying the reduction in naive courtship activity differs in that it is not stably rescued by treatment during development alone, considering that the rescue that was observed at 5 days of age (McBride et al. 2005) is no longer detected at 20 days of age.
At this time we have not identified the underlying causes of the various behavioral deficits observed with the dfmr1 mutant flies. As mentioned above, our analysis indicates that they are not caused by cell death. Previously described developmental defects in brain neurons of dfmr1 mutants also fail to provide an explanation. The only identified defect with relevance to the learning and memory defects is the finding that the beta-lobes of the mushroom body (MB) in dfmr1 mutant brains display a midline cross-over defect (Michel et al. 2004; McBride et al. 2005). The MB is a bilateral neuropile structure in the fly brain that is required for short-term and long-term memory (McBride et al. 1999; Zars et al. 2000; Pascual and Preat 2001; McBride et al. 2005). The fact that the beta-lobe cross-over defect can be rescued by treatment with mGluR antagonists during development is intriguing (McBride et al. 2005). However, since treatment during adulthood has no effect on this developmental defect, but clearly rescues naive courtship, learning and memory, this defect cannot account for the behavioral defects. We feel that a more likely cause is/are more subtle and reversible defects, possibly at the synapse. The different responses that naive courtship has to drug treatments compared to learning, immediate recall and short-term memory might be due to fundamental differences between the specific synapses or neuronal circuits involved in innate behavior (naive courtship) versus those involved in behavior (learning and memory).
In summary, our findings fit well with the results of initial studies in Fragile X male patients that indicate a general drop in their IQ with age and clearly illustrate a role for FMRP in aging. However, it is important to point out that confounding issues with the human studies have been noted, such as the different requirements for abstract thinking on tests as age ranges increase, making it difficult to clearly delineate the magnitude of the cognitive decline that occurs in these patients (Hagerman et al. 1989; Hay 1994). Also, these studies have only included patients in childhood and adolescence. Long-term studies including adult populations have not been done with Fragile X patients. Nonetheless, the fact that our Drosophila Fragile X model displays cognitive decline with age, suggests that more extensive studies on the effects of aging on the cognitive abilities of Fragile X patients should be performed. This work also clearly illustrates the requirement for FMRP in neurons for proper function with age. Our results suggest that antagonizing mGluRs may be a potential therapeutic target for prolonged correction of the cognitive deficits associated with Fragile X syndrome, as well as the observed progressive cognitive decline. This work adds to a growing body of literature demonstrating that adulthood treatments may be effective in treating aspects of mental retardation and autism (Raymond and Tarpey 2006; Ehninger et al. 2008; Walsh et al. 2008). This demonstration of the need for FMRP with aging may also be important with regard to the pathophysiology of FXTAS, where FMRP levels are decreased. RNA toxicity is thought to be the major cause of this disorder, but our data suggest that the lack of FMRP with aging can in itself have a negative impact on neuronal function. Therefore, it could be that decreased FMRP levels coupled with normal aging also contribute to FXTAS. Additionally, the strategy of modulating the activity of mGluRs to prevent synaptic silencing may be generally applicable to the treatment of other diseases involving progressive cognitive decline due to enhanced glutamatergic signaling such as Alzheimer’s disease, tauopathies and Huntington’s disease.
Supplementary Material
Acknowledgments
We received helpful input on the experiments contained in this manuscript from communications with Mike Tranfaglia, Joseph Hinchey, Sean Campbell, Kathleen Siwicki, and John Jenkins. We are grateful to Randi Hagerman and Evan Braunstein for critically reading the manuscript. Technical assistance was provided by Charles Smith, Oliver Schipper and Edward Carlin. Funding for this work came from grants from the FRAXA Research Foundation to S.M.J.M, C.H.C., and T.A.J. A Medical Scientist Training Program grant through Albert Einstein College of Medicine supported S.M.J.M. This work was also funded by a grant from Autism Speaks to T.V.M and S.M.J.M., as well as funding to E.K. from The National Fragile X Foundation. T.A.J. received a NIH grant (NS046573) to support this work.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10522-009-9259-6) contains supplementary material, which is available to authorized users.
Contributor Information
Catherine H. Choi, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA Department of Medicine, Lehigh Valley Health Network, Allentown, PA 18105, USA.
Sean M. J. McBride, Email: smcbride@aecom.yu.edu, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Brian P. Schoenfeld, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
David A. Liebelt, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
David Ferreiro, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Neal J. Ferrick, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
Paul Hinchey, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Maria Kollaros, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Rebecca L. Rudominer, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
Allison M. Terlizzi, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
Eric Koenigsberg, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Yan Wang, Department of Genetics, University of Pennsylvania School of Medicine, 538A CRB, 415 Curie Blvd., Philadelphia, PA 19104-6145, USA.
Ai Sumida, Department of Genetics, University of Pennsylvania School of Medicine, 538A CRB, 415 Curie Blvd., Philadelphia, PA 19104-6145, USA.
Hanh T. Nguyen, Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, Bronx, NY 10461, USA
Aaron J. Bell, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
Thomas V. McDonald, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA
Thomas A. Jongens, Email: jongens@mail.med.upenn.edu, Section of Molecular Cardiology and Department of Molecular Pharmacology, Albert Einstein College of Medicine, Forchheimer G35, 1300 Morris Park Avenue, Bronx, NY 10461, USA; Department of Genetics, University of Pennsylvania School of Medicine, 538A CRB, 415 Curie Blvd., Philadelphia, PA 19104-6145, USA.
References
- Acharya JK, Labarca P, Delgado R, Jalink K, Zuker CS. Synaptic defects and compensatory regulation of inositol metabolism in inositol polyphosphate 1-phosphatase mutants. Neuron. 1998;20:1219–1229. doi: 10.1016/s0896-6273(00)80502-4. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Oostra BA. Understanding fragile X syndrome: insights from animal models. Cytogenet Genome Res. 2003;100:111–123. doi: 10.1159/000072845. [DOI] [PubMed] [Google Scholar]
- Bastock MA. The courtship of drosophila melanogaster. Behaviour. 1955;8:86–111. [Google Scholar]
- Bastock MA. A gene mutation which changes a behavior pattern. Evolution. 1956;10:421–439. [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. (quiz 2140) [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega AG, Goodrich G, Bennett RE, Hessl D, Engle K, Leehey MA, Bounds LS, Paulich MJ, Hagerman RJ, Hagerman PJ, Cogswell JB, Tassone F, Reynolds A, Kooken R, Kenny M, Grigsby J. The primary cognitive deficit among males with fragile X-associated tremor/ataxia syndrome (FXTAS) is a dysexecutive syndrome. J Clin Exp Neuropsychol. 2008;31:1–17. doi: 10.1080/13803390701819044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69:551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Shubek L, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: diagnosis, treatment, and research. 3rd edn. Baltimore, MD, USA: John Hopkins University Press; 2002. [Google Scholar]
- Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, Habicht K. Longitudinal IQ changes in fragile X males. Am J Med Genet. 1989;33:513–518. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hallcher LM, Sherman WR. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980;255:10896–10901. [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hay DA. Does IQ decline with age in fragile-X? A methodological critique. Am J Med Genet. 1994;51:358–363. doi: 10.1002/ajmg.1320510412. [DOI] [PubMed] [Google Scholar]
- Heriche JK, Ang D, Bier E, O’Farrell PH. Involvement of an SCFSlmb complex in timely elimination of E2F upon initiation ofDNAreplication in drosophila. MCGenet. 2003;4:9. doi: 10.1186/1471-2156-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Jongens TA. Structure-specific abnormalities associated with mutations in a DNA replication accessory factor in drosophila. Dev Biol. 2001;230:161–176. doi: 10.1006/dbio.2000.0117. [DOI] [PubMed] [Google Scholar]
- Joiner MlA, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J Neurosci. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyshev NG, Iliadi KG, Bragina JV. Drosophila conditioned courtship: two ways of testing memory. Learn Mem. 1999;6:1–20. [PMC free article] [PubMed] [Google Scholar]
- Kane NS, Robichon A, Dickinson JA, Greenspan RJ. Learning without performance in PKC-deficient drosophila. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and permutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC. Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc Natl Acad Sci USA. 1976;73:4220–4224. doi: 10.1073/pnas.73.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Orgad S, Rosenfeld G, Greenspan RJ, Segal D. Court-less, the Drosophila UBC7 homolog, is involved in male courtship behavior and spermatogenesis. Genetics. 2000;155:1267–1280. doi: 10.1093/genetics/155.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I pre-mRNA editing in drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- Pan L, Woodruff E, 3rd, Liang P, Broadie K. Mechanistic relationships between drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 2008;37:747–760. doi: 10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Raymond FL, Tarpey P. The genetics of mental retardation. Hum Mol Genet. 2006;15:R110–R116. doi: 10.1093/hmg/ddl189. (Spec No 2) [DOI] [PubMed] [Google Scholar]
- Restifo LL. Mental retardation genes in drosophila: new approaches to understanding and treating developmental brain disorders. Ment Retard Dev Disabil Res Rev. 2005;11:286–294. doi: 10.1002/mrdd.20083. [DOI] [PubMed] [Google Scholar]
- Savvateeva E, Popov A, Kamyshev N, Bragina J, Heisenberg M, Senitz D, Kornhuber J, Riederer P. Age-dependent memory loss, synaptic pathology and altered brain plasticity in the drosophila mutant cardinal accumulating 3-hydroxykynurenine. J Neural Transm. 2000;107:581–601. doi: 10.1007/s007020070080. [DOI] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant drosophila. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: W.H. Freeman and Co; 1995. [Google Scholar]
- Spieth HT. Courtship behavior in drosophila. Annu Rev Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in drosophila. J Anim Behav. 1915;5:351–366. [Google Scholar]
- van Swinderen B, Hall JC. Analysis of conditioned courtship in dusky-Andante rhythm mutants of drosophila. Learn Mem. 1995;2:49–61. doi: 10.1101/lm.2.2.49. [DOI] [PubMed] [Google Scholar]
- Villella A, Hall JC. Courtship anomalies caused by double sex mutations in drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135:396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright-Talamante C, Cheema A, Riddle JE, Luckey DW, Taylor AK, Hagerman RJ. A controlled study of longitudinal IQ changes in females and males with fragile X syndrome. Am J Med Genet. 1996;64:350–355. doi: 10.1002/(SICI)1096-8628(19960809)64:2<350::AID-AJMG23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zarnescu DC, Jin P, Betschinger J, Nakamoto M, Wang Y, Dockendorff TC, Feng Y, Jongens TA, Sisson JC, Knoblich JA, Warren ST, Moses K. Fragile X protein functions with lgl and the par complex in flies and mice. Dev Cell. 2005;8:43–52. doi: 10.1016/j.devcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.