Abstract
Coenzyme Q10 (CoQ10) and creatine are promising agents for neuroprotection in neurodegenerative diseases via their effects on improving mitochondrial function and cellular bioenergetics and their properties as antioxidants. We examined whether a combination of CoQ10 with creatine can exert additive neuroprotective effects in a MPTP mouse model of Parkinson’s disease (PD), a 3-NP rat model of Huntington’s disease (HD) and the R6/2 transgenic mouse model of HD. The combination of the two agents produced additive neuroprotective effects against dopamine depletion in the striatum and loss of tyrosine hydroxylase neurons in the substantia nigra pars compacta (SNpc) following chronic subcutaneous administration of MPTP. The combination treatment resulted in significant reduction in lipid peroxidation and pathologic α-synuclein accumulation in the SNpc neurons of the MPTP-treated mice. We also observed additive neuroprotective effects in reducing striatal lesion volumes produced by chronic subcutaneous administration of 3-NP to rats. The combination treatment showed significant effects on blocking 3-NP-induced impairment of glutathione homeostasis and reducing lipid peroxidation and DNA oxidative damage in the striatum. Lastly, the combination of CoQ10 and creatine produced additive neuroprotective effects on improving motor performance and extending survival in the transgenic R6/2 HD mice. These findings suggest that combination therapy using CoQ10 and creatine may be useful in the treatment of neurodegenerative diseases such as PD and HD.
Keywords: oxidative damage, Parkinson’s disease, Huntington’s disease, 3-nitropropionic acid, MPTP, R6/2 HD mice
Introduction
There is substantial evidence that mitochondrial dysfunction and bioenergetic abnormalities play a role in the pathogenesis of neurodegenerative disease (Lin and Beal 2006). It is, therefore, possible that agents, which improve mitochondrial and cellular bioenergetics may be useful in the treatment of neurodegenerative disease. Two agents which show particular promise are Coenzyme Q10 (CoQ10) and creatine.
CoQ10 is an essential cofactor of the electron transport chain where it accepts electrons from complex I and complex II (Ernster and Dallner 1995; Turunen et al. 2004). CoQ10, which is also known as ubiquinone, is composed of a redox active quinoid moiety and a hydrophobic ‘tail’. It is soluble and mobile in the hydrophobic core of the phospholipid bilayer of the inner membrane of mitochondria, where it transfers electrons one at a time, to complex III of the electron transport chain. CoQ10 also serves as an important antioxidant in both mitochondria and in lipid membranes (Noack et al. 1994; Forsmark-Andree et al. 1997). In the inner mitochondrial membranes and microsomal lipid membranes, it reduces α-tocopheroxyl radical and regenerates α-tocopherol (Kagan et al. 1990). CoQ10 is also an obligatory cofactor of mitochondrial uncoupling proteins, which regulate ATP production and reduce free radical generation (Echtay et al. 2002).
Creatine is a guanidine compound, which plays a key role in energy buffering within the cell, which is thought to be particularly important in tissues with high and fluctuating energy requirements such as brain and muscle (Burklen et al. 2006). The creatine/phosphocreatine (PCr) system functions as a spatial energy buffer between the cytosol and mitochondria using a unique mitochondrial creatine kinase (CK) isoform, which is found in the intermembrane space of mitochondria. Creatine kinase can generate ATP from phosphocreatine and adenosine diphosphate (ADP) at the sites of high energy demand and restore phosphocreatine for energy storage (Burklen et al. 2006).
We and others showed that both CoQ10 and creatine exert neuroprotective effects both in vitro and in vivo in animal models of neurodegenerative diseases (Beal and Shults 2003). This has led to clinical trials in both Parkinson’s disease (PD) and Huntington’s disease (HD) (Huntington-Study-Group 2001; Shults et al. 2002). Due to initial promising results, both CoQ10 and creatine are entering phase III trials for the treatment of PD and HD. It is possible that combinations of agents targeting different disease mechanisms may show improved efficacy, and allow agents to be utilized at lower doses to minimize side effects.
Although both CoQ10 and creatine have effects on bioenergetics, they act on different pathways. In the present study, we, therefore, examined whether CoQ10 and creatine can exert additive neuroprotective effects. We examined the combination of CoQ10 and creatine in the 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) model of PD, the 3-nitropropionic acid (3-NP) model of HD, and in the R6/2 transgenic mouse model of HD.
Materials and Methods
Animals and materials
Male C57BL/6 mice (3-month old, 25–30 g) and male Lewis rats (3-month old, 250–300 g) were obtained from the Jackson Laboratory (Bar Harbor, ME). A stable colony of R6/2 HD mice has been maintained at the Bedford Veterans Affairs Medical Center Laboratories, with founders originally from the Jackson Laboratory (Bar Harbor, ME). Male R6/2 mice were bred with females from their background strain (B6 CBAFI/J). The progeny were genotyped via PCR assay on DNA isolated from tail biopsy. The length of the CAG repeat was monitored by the Core Sequencing Facility at Boston University. All mice used in this study had CAG repeats in the range of 148–153, with corresponding base pairs of 500–550, as determined by PCR. We have standardized criteria to ensure homogeneity of the experimental mice and the cohorts within the testing groups (Hersch and Ferrante 2004). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Weill Cornell Medical College Animal Care Committee or the Veterans Administration and Boston University Animal Care Committees. MPTP and 3-NP manipulations were carried out in a restricted room in the Cornell animal facility specified for neurotoxin manipulation.
MPTP, 3-NP, dopamine, glutathione (GSH), oxidized glutathione (GSSG), malondialdehyde (MDA), deoxyguanosine (dG), 8-hydroxy-2-deoxyguanosine (8OH2dG) were all purchased from Sigma (St. Louis, MO). All of the animal diets, 2% creatine (Sigma Chemical, St Louis, MO), 1% CoQ10 (Enzymatic Therapy, Green Bay, WI), the combination of 2% creatine and 1% CoQ10 along with the control diet, were formulated into rodent chow by Purina (St. Louis, Missouri).
Mouse model of chronic MPTP toxicity and therapeutic treatment
Male C57BL/6 mice (weighing 25-30g) were fed with either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of the two for one week (Cleren et al. 2008) before being implanted subcutaneously with osmotic minipumps (Model 2004, Alzet, Cupertino CA, filled with MPTP 170mg/ml in PBS) that delivered MPTP at a dose of 40mg/kg body weight daily for 28 days. Fifteen mice were placed in each diet group and they were maintained on the same diets under standard conditions with ad libitum access to water and food during the delivery of MPTP by subcutaneously-implanted minipumps. After 28 days mice were sacrificed and fresh striata were dissected for dopamine and 1-methyl-4-phenylpyridinium (MPP+) analysis and midbrains were fixed in 4% paraformaldehyde for immunohistochemistry staining.
Rat model of 3-NP toxicity
Male Lewis rats (weighing 260-300g) fed with either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of the two for one week were implanted subcutaneously with osmotic minipumps (Model 2ML1, Alzet, Cupertino CA, filled with 3-NP 70mg/ml in PBS, pH 7.4) that delivered 3-NP 50mg per kg bodyweight daily for 7 days while remaining on the same diets (Ouary et al. 2000). The rats were sacrificed and one half of the brain was fixed in 4% paraformaldehyde for the striatal lesion volume measurement and the other half was dissected freshly for GSH, GSSG, MDA, dG and 8OH2dG assays.
R6/2 mice motor performance and survival
A total of eighty R6/2 mice were used in these experimental studies. Mice from both genders were equally included in the experimental paradigm. We have not experienced gender differences in survival in this transgenic HD mouse model. Mice dying prematurely (<70 d) were excluded from the study. Mice were housed five per cage under standard conditions with ad libitum access to food and water. Enrichment conditions were not applied to any cages because this is considered a therapeutic treatment that may confound the mouse trials (Smith et al. 2006). At 21 days of age, mice were placed on either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of the two within the same pellet. Twenty mice from the same generation were placed within each of the treatment groups. Groups were randomly pooled from multiple liters (six to eight) to ensure heterogeneity. The amount of food intake per mouse was found to be 4-5 g/d, with no significant difference between control diet and supplemented diets. During the temporal progress of the disease, the food consumed per gram of mouse weight was stable until end stage (12-14 weeks). Mice were observed twice daily. Motor performance was assessed weekly from 28 to 63 days of age and twice weekly from 63 to 90 days of age in the R6/2 and littermate control mice. The mice were given two training sessions to acclimate them to the rotarod apparatus (Columbus Instruments, Columbus, OH). During testing the mice were placed on a rod rotated at a constant speed of 16 rpm. Each mouse had three separate trials at 180 s each. The three results were averaged and recorded. The criterion for euthanization was the point in time at which the mice were unable to initiate movement after being gently prodded for 2 min. Mice had lost ~40-50% of their body weight at this time point. Two independent observers (R.J.F. and K.S.) confirmed the criteria for euthanization. This time point was identified as the time of death.
HPLC analysis for dopamine
The striatal level of dopamine was measured after sonicating and centrifuging fresh mouse strata in chilled 0.1 M perchloric acid (PCA, about 100ul/mg tissue) as modified from our previously described method (Yang et al. 2005). Briefly, 15 ul supernatant was isocratically eluted through an 80 × 4.6 mm C18 column (ESA, Inc Chelmsford, MA) with a mobile phase containing 0.1 M LiH2PO4, 0.85mM 1-octanesulfonic acid and 10% (v/v) methanol and detected by a 2-channel Coulochem II electrochemical detector (ESA, Inc. Chelmsford, MA). The concentration of dopamine is expressed as ng per mg protein. The protein concentrations of tissue homogenates were measured according to the Bio-Rad protein analysis protocol (Bio-Rad Laboratories, Hercules, CA) and Perkin Elmer Bio Assay Reader (Norwalk, CT).
HPLC analysis of MPP+
Mouse striatal tissues were sonicated and centrifuged in 0.1 M PCA and an aliquot of supernatant was injected onto a Brownlee aquapore × 03-224 cation exchange column (Rainin, Woburn, MA). Samples were eluted isocratically with 20 mM boric acid-sodium borate buffer, pH7.75, containing 3 mM tetrabutylammonium hydrogen sulfate, 0.25 mM 1-heptanesulfonic acid and 10% isopropanol. MPP+ levels were detected with a fluorescence detector set by excitation at 295 nm and emission at 375nm.
HPLC analysis for MDA
The determination of MDA by HPLC was carried out according to a reported method (Agarwal and Chase 2002). Briefly, fresh rat striata were homogenized in 40% ethanol solution. To a 50-μl aliquot of sample or MDA standard, 50 μl of 0.05% butylated hydroxytoluene (BHT), 400 μl of 0.44 M H3PO4, and 100 μl of 0.42 mM 2-thiobarbituric acid (TBA) were added and heated for 1 h at 100°C, followed by 250 μl n-butanol extraction of the MDA–TBA derivative. The HPLC mobile phase comprised of acetonitrile–buffer (20:80, v/v, buffer 50 mM KH2PO4, pH of 6.8 adjusted with KOH). The column was an ESA 150 × 3-mm C18 column with particle size of 3 μm (ESA, Inc.). The fluorescence detector was set at an excitation wavelength of 515 nm and emission wavelength of 553 nm. The concentration of MDA is expressed as nmol per mg protein.
HPLC analysis for dG and 8OH2dG
DNA extraction and HPLC analysis methods were modified from a previous report (Hofer et al. 2006). Briefly, rat striatal DNA was extracted using TRIzol reagent (Invitrogen, CA, USA) with the inclusion of 1mM deferoxamine mesylate (DFOM). DNA pellets in 80ul of H2O were hydrolyzed by adding 10μl of Nuclease P1 (0.4 U/μl in 300 mM sodium acetate, 0.2 mM ZnCl2, pH 5.3), and 5 μl of alkaline phosphatase (1 U/μl). The hydrolysate (100 μl) was mixed with 2 μl of 5M perchloric acid and centrifuged at 18,000 g for enzyme removal. The supernatant (50 μl) was isocratically eluted through a 4.6 × 250 mm C18 column (ESA, Inc Chelmsford, MA) with a mobile phase containing 20mM LiH2PO4, 4.0mM 1-octanesulfonic acid and 1% (v/v) methanol and detected first by a 2-channel Coulochem II electrochemical detector (ESA, Inc. Chelmsford, MA), set with potentials of Channel 1 at 165mV and Channel 2 at 300mV for 8OH2dG, and followed by a Waters 486 UV detector set with a wavelength at 260nm for dG. DNA oxidation was indicated by the concentration ratio of 8OH2dG × 103 vs. dG.
HPLC analysis for GSH and GSSG
Rat striatal tissues were homogenized in chilled 0.1 M perchloric acid and centrifuged. The supernatants were taken for HPLC as modified from a reported method (Melnyk et al. 1999). Breifly, 15ul supernatant was isocratically eluted through a 4.6 × 150 mm C18 column (ESA, Inc Chelmsford, MA) with a mobile phase containing 50mM LiH2PO4, 1.0mM 1-octanesulfonic acid and 1.5% (v/v) methanol and detected by a 2-channel Coulochem III electrochemical detector (ESA, Inc. Chelmsford, MA), set with guard cell potential 950mV, Channel 1 potential 500mM for GSH detection and Channel 2 potential 880mV for GSSG detection. Concentrations of GSH and GSSG are expressed as nmol per mg protein.
Immunohistochemistry and stereologic analysis
Tissues for histological analysis were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and then cryoprotected in 30% sucrose overnight at 4°C. A modified avidin-biotin-peroxidase technique was employed for immunohistochemistry. Briefly, 50 μm thick coronal brain sections were pretreated with 3% H2O2 in 0.1 M sodium phosphate-buffered saline (PBS) for 30 min. The sections were rinsed in PBS twice for 5 min each. The sections were incubated sequentially in (a) 1% bovine serum albumin (BSA)/0.2% Triton X-100 (Sigma, St. Louis, MO) for 30 minutes, (b) appropriate primary antibodies (rabbit anti-tyrosine hydroxylase (TH) affinity purified antibody (1:4,000; Chemicon, Temecula, CA), rabbit polyclonal anti-MDA (1:1,000; provided by Dr. C. Thomas, Hoechst Marion Roussel), mouse anti-α-synuclein (1:1,000; BD Transduction Laboratories, San Jose, CA), mouse anti-neuron-specific nuclear protein (NeuN) (1:1,000; Chemicon, Temecula, CA)) (diluted in PBS/0.5% BSA) for 18 hours, (c) appropriate biotinylated IgG (1:200, diluted in PBS/0.5% BSA, Vector Laboratories, Burlingame, CA) for 1 hour, and (d) avidin-biotin-peroxidase complex (1:200 in PBS; Vector Laboratories) for 1 hour. The immunoreaction was visualized using 3,3′-diaminobenzidine tetrahydrochloride dihydrate (DAB) with nickel intensification (Vector Laboratories) as the chromogen. All incubations and rinses were performed at room temperature with agitation using an orbital shaker. The sections were mounted onto gelatin-coated slides, dehydrated, cleared in xylene and coverslipped.
For stereological cell counts, three sets of sections were prepared with each set consisting of 7-8 sections, 100 μm apart. One set of sections was stained first with TH as described above and then Nissl (cresyl violet). The other two sets were processed for α-synuclein and MDA immunohistochemistry as described above. The numbers of Nisslstained, TH- or MDA-immunoreactive neurons in the substantia nigra pars compacta (SNpc) were counted using the optical fractionator method in the Stereo Investigator (v 4.35) software program (Microbrightfield, Burlington, VT).
For quantification of α-synuclein immunoreactive cells in SNpc, the staining of SNpc neurons of control animals was used as baseline level and neurons with staining intensity higher than that were considered positive for α-synuclein. Since in our study the α-synuclein positive neurons were rare in the SNpc of MPTP-treated animals, we did not use the stereological method of quantification. The stereological approach requires a minimal number of “countable” neurons. In cases where the numbers of neurons are too small, the random paradigm used in stereology is unreliable because the rare positive neurons may be missed, resulting in false results. Following the technique used by Vila et al. (Vila et al. 2000), we quantitated the α-synuclein positive neurons in terms of the number of intensely stained cells per section. For each animal (n = 5 per group), seven sections (150 μm apart) encompassing the entire SNpc were analyzed by examining the entire SNpc bilaterally. The number of neurons in each section was added to provide a measure of the total number of SNpc α-synuclein-positive neurons and then divided by the number of counted sections (7) for each animal. Data for each group is expressed as mean of counts from 5 animals.
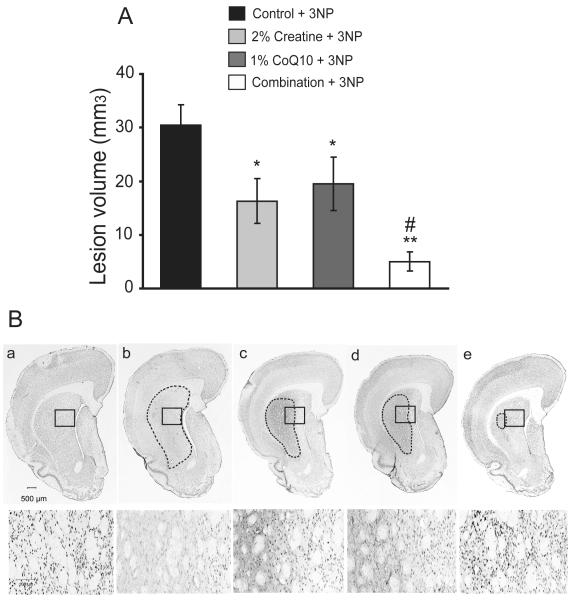
For rat 3-NP study, one set of serial sections (210 μm apart) immunostained using a mouse antibody against the neuronal marker NeuN were measured for areas of 3-NP-induced NeuN loss to determine the lesion volume using the cavalieri principle as estimator in the same stereology software program. The investigators who did the counting for the image analysis were not blinded. A demarcation line was drawn between the normal tissue and the lesion area where an identifiable border is shown by the loss of NeuN positive neurons as shown in the high magnification photomicrograph in Fig 3B.
Fig. 3. Additive neuroprotective effects of creatine with CoQ10 in reducing striatal damage caused by 3-nitropropionic toxicity.
Lewis rats pretreated with either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of two for one week were subcutaneously delivered with 3-NP (50 mg/kg/day) or PBS by implanted osmotic pumps for 7 days. A. The measurement of lesion volume in coronal brain slices of 3-NP treated controls shows obvious striatal damage caused by 3-NP toxicity, which was significantly reduced by the treatment of creatine, CoQ10 or the combination diet. The combination exerted an additive protective effect in reducing the volume of 3-NP-induced striatal lesion. Data represent mean ± SEM. *p < 0.05, when compared to 3-NP with control diet; # p < 0.05 compared to 3-NP group with either creatine or CoQ10 diet, by ANOVA. n= 15 rats per group. B. Representative photomicrographs of NeuN-immunostained sections through the coronal section of striatum and cortex of rats as described above show a marked lesion area in striatum of 3-NP treated rats with control diet contrasted to the intact PBS treated controls. The treatment with creatine, CoQ10 or the combination diet significantly reduced the 3-NP-induced lesion area in the striatum, which was the smallest in the combination diet treatment section (upper panel). High magnifications in lower panel show the loss of the striatal NeuN-positive neurons caused by 3-NP toxicity and the preservation exerted by the treatment of creatine, CoQ10 or the combination diet. In figure: a, control diet alone; b, control diet with 3-NP; c, 2% creatine diet with 3-NP; d, 1% CoQ10 with 3-NP; e, the combination diet with 3-NP.
Statistical analysis
Data represent mean ± standard error of means (SEM) from groups of animals. One way analysis of variance (ANOVA) was used for data analysis. When F values implied significance at a level p < 0.05, Student-Newman-Keul’s multiple comparison tests was applied to determine where the differences among groups arose. All statistical analysis was performed using the Graphpad Instat software (GraphPad, San Diego, CA).
Results
Additive neuroprotective effects of creatine with CoQ10 against MPTP-induced nigrostriatal dopaminergic neurodegeneration
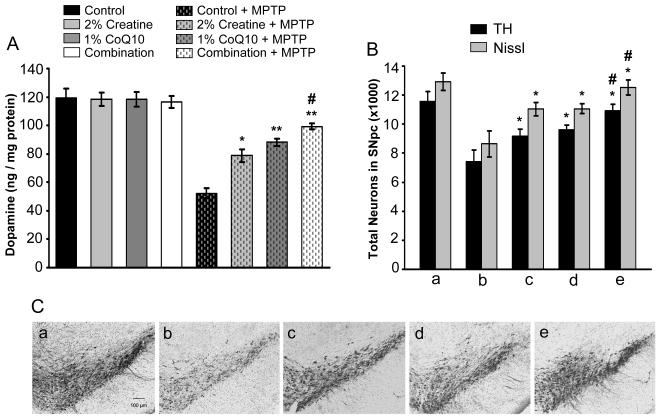
We investigated whether the combination of creatine and CoQ10 exert additive neuroprotective effects in a chronic osmotic minipump MPTP mouse model of PD. The chronic MPTP model better mimics the neuropathological features of PD in humans (Andreassen et al. 2001; Fornai et al. 2005). Mice were fed with either an unsupplemented diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of these two for one week before being implanted subcutaneously with osmotic minipumps filled with MPTP 170mg/ml in PBS that delivered MPTP at a dose of 40mg/kg body weight daily for 28 day. After 28 days, there was a significant reduction (56%) in total striatal dopamine in the mice treated with an unsupplemented control diet, whereas the MPTP-induced striatal dopamine depletion was significantly attenuated in the mice treated with 2% creatine (33%), 1% CoQ10 (26%) or the combination of creatine and CoQ10 (16%). The combination diet exerted a significant additive neuroprotective effect in attenuating the MPTP-induced dopamine reduction when compared to either the 2% creatine or 1% CoQ10 supplemented diet treatment alone (ANOVA p<0.05) (Figure 1A). Immunohistochemical analysis of dopaminergic neurons of the SNpc showed a profound loss of TH-immunoreactivity after 28 days of chronic MPTP toxicity as compared to PBS controls. All three supplemented Diets significantly protected against the loss of TH-immunoreactive dopaminergic neurons in the SNpc, in which the combination diet produced a significant additive effect in its protection of TH-immunoreactive dopaminergic neurons (ANOVA, p<0.05) (Figure 1B and C). The loss of TH-positive neurons caused by MPTP and improvement by administration of the supplemented diets was confirmed with Nissl staining (Nissl positive cells) for counting the loss of total neurons in the SNpc (Figure 1B).
Fig. 1. Additive neuroprotective effects of creatine with CoQ10 in attenuating MPTP-induced nigrostriatal dopaminergic neurodegeneration.
Mice were fed with either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of two for one week before implanted subcutaneously with osmotic minipumps that delivered MPTP at a dose of 40mg/kg daily for 28 day. A. Striatal levels of dopamine analyzed by HPLC show chronic MPTP administration resulted in a significant depletion of striatal dopamine, and treatment with creatine, CoQ10 or their combination significantly attenuated MPTP-induced depletion of striatal dopamine, in which the combination treatment showed an additive protection effect. B. Stereologic cell counts of total (Nissl-positive) and TH-immunopositive dopaminergic neurons in the SNpc showed a significant cell loss following MPTP administration which was significantly attenuated by creatine, CoQ10 or their combination treatment. The combination exerted significantly better protection than either the creatine or the CoQ10. n = 15 mice per group. Data represent means ± SEM, * P < 0.05 ** P < 0.01 when compared with the control diets with MPTP group; #p< 0.05 compared to either creatine or CoQ10 diet with MPTP group, by ANOVA. C. Representative photomicrographs of TH-immunostained sections through the SNpc of mice show a significant reduction in TH-positive neurons of SNpc by chronic MPTP, and treatment of creatine, CoQ10 or their combination significantly blocked MPTP-induced loss of TH-positive neurons. In figure: a, control diet alone; b, control diet with MPTP; c, 2% creatine diet with MPTP; d, 1% CoQ10 with MPTP; e, the combination diet with MPTP.
The neuroprotective effects provided by the three supplemented diets following MPTP-neurotoxicity were not due to impairment of the metabolism of MPTP, since the striatal tissue levels of its metabolite MPP+ (1-methyl-4-phenyl-pyridinium ion) showed no differences between the control diet (11.6 ± 0.79 ng/mg protein, n = 12) and the combination diet treated mice (10.9 ± 0.70 ng/mg protein, n = 12), when measured after 28 days of chronic treatment with MPTP (40 mg/kg/day for 28 days).
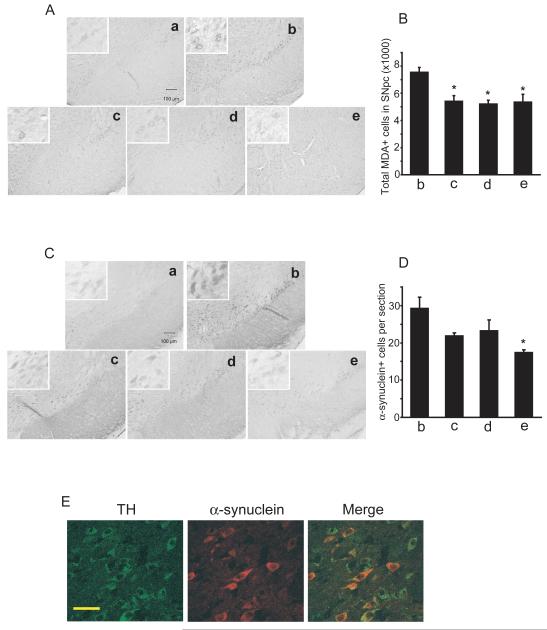
Creatine, CoQ10 and their combination block MPTP-induced lipid peroxidation and α-synuclein accumulation
In order to determine if neuroprotective effects of creatine, CoQ10 and the combination of the both compounds can block MPTP-induced oxidative damage and increase in α-synuclein accumulation, we examined MDA, which is a marker for lipid peroxidation, and α-synuclein immunostaining in SNpc. Chronic administration of MPTP resulted in a significant induction of oxidative damage as measured by MDA immunostaining (Fig. 2A and B), and caused a marked increase in α-synuclein accumulation in SNpc neurons (Fig. 2C and D). The α-synuclein accumulation is colocalized in TH-immunoreactive neurons in the SNpc (Fig. 2E). Treatment with creatine, CoQ10 or their combination significantly blocked MPTP-induced mesencephalic MDA formation (Fig. A and B), and reduced pathological accumulation of α-synuclein with a significant reduction reached by combination treatment (Fig. 2C and D). These data show that the chronic MPTP toxicity produces oxidative stress and pathologic α-synuclein accumulation in the SNpc, which are reduced via the neuroprotective effects exerted by the treatment of creatine, CoQ10 or the combination of the two.
Fig. 2. Creatine, CoQ10 and their combination block chronic MPTP-induced lipid peroxidation and α-synuclein accumulation in SNpc dopaminergic neurons.
A. Photomicrographs of MDA-immunostained sections through the SNpc of mice pretreated with control, 2% creatine, 1% CoQ10 or the combination of two for 7 days followed with a chronic administration of MPTP 40mg/kg daily for 28 days, show an increase in MDA staining in SNpc cells of mice with MPTP and control diet. Creatine, CoQ10 or their combination treatment resulted in a marked reduction of MPTP-induced MDA immunostaining. High magnification inserts show the MDA staining in neurons. B. Using sections from control mice with control diet as the baseline background, stereological analysis showed the total number of MDA-positive neurons in SNpc of MPTP-treated mice was significantly reduced by the treatment of creatine, CoQ10 or their combination. C. Photomicrographs of α-synuclein stained sections through the SNpc of mice described above show a significant increase of α-synuclein accumulation in SNpc of MPTP treated animals on control diet. Creatine, CoQ10 or their combination treatment resulted in a significant reduction of MPTP-induced α-synuclein accumulation in SNpc neurons. High magnification inserts show the α-synuclein staining in neurons. D. Quantification of α-synuclein labeled cells was performed by determining the average number of intensely labeled neurons per section, and only labeled neurons in MPTP-treated groups with staining intensity higher than that of controls were counted. Treatment with creatine, CoQ10 or their combination reduced α-synuclein positive cells in SNpc, with a significant reduction reached by combination treatment. In figure: a, control diet alone; b, control diet with MPTP; c, 2% creatine diet with MPTP; d, 1% CoQ10 with MPTP; e, the combination diet with MPTP. n= 5 mice in each group. Scale bar = 100μm. Data represent means ± SEM, * P < 0.05. E. Confocal photomicrographs of sections through the SNpc of mice on chronic MPTP stained with anti-TH antibody (green) and anti-α-synuclein antibody (red) show the colocalization of the α-synuclein in the TH-positive neurons (yellow in merge), using a Zeiss LSM510 confocal microscope. Scale bar = 100μm.
Additive neuroprotective effects of creatine with CoQ10 in blocking 3-NP-induced striatal toxicity
3-NP administration in rodents is known to cause striatal degeneration which replicates the pathological features of HD (Beal et al. 1993). Lewis rats were pretreated with either an unsupplemented diet, or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of both for one week before 3-NP (50mg/kg/day for 7 days) or PBS was delivered by subcutaneous implantation of mini-osmotic pumps. Animals were maintained on the same diets throughout the administration of 3-NP. Quantitative assessment after 7 days of 3-NP intoxication showed that 3-NP caused obvious damage with a mean lesion volume of 30.5 ± 3.7 mm3 in the striatum of rats treated with control diet. Lesion volumes were significantly reduced by the administration of a 2% creatine diet by 47% (16.3 ± 4.2 mm3, p<0.05), 1% CoQ10 diet by 38% (19.5 ± 4.9 mm3, p<0.05) or the diet combined with both by 83% (5.0 ± 1.8 mm3, p<0.01) (Fig. 3A). The combination diet exerted a significant additive neuroprotective effect in blocking 3-NP-induced striatal lesions when compared to either the 2% creatine or 1% CoQ10 supplemented diet alone (p<0.05). Analysis of striatal neurons by NeuN immunohistochemistry after 3-NP administration showed a profound loss of striatal neurons in the non-supplemented rats. The administration of 2% creatine, 1% CoQ10 or the combination diet significantly attenuated the 3-NP-induced loss of striatal NeuN stained neurons (Fig. 3B).
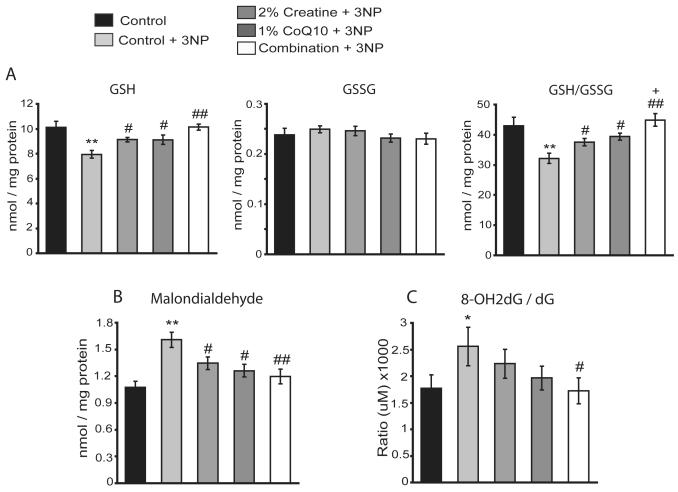
Creatine, CoQ10 or their combination blocks 3-NP-induced impairment of striatal glutathione homeostasis and reduces brain oxidative damages
Striatal degeneration caused by 3-NP administration in rodents induces oxidative damage, especially alterations in glutathione homeostasis (Klivenyi et al. 2000). Administration of 3-NP by mini-osmotic pump (50 mg/kg/day for 7 days) resulted in a significant reduction of reduced glutathione (GSH) and the ratio of reduced to oxidized glutathione (GSH/GSSG) in the striatum tissue of rats treated with control diet, implying a consumption of the intrinsic antioxidative glutathione reserve by 3-NP-induced oxidative stress. Treatment with 2% creatine, 1% CoQ10 or their combination significantly preserved striatal GSH levels and the ratio of GSH/GSSG against the 3-NP-induced depletion (Fig. 4A). The combination diet treatment exerted a significant additive effect in preserving the ratio of GSH/GSSG when compared to either 2% creatine or 1% CoQ10 diet treatment (Fig. 4A) (p<0.05).
Fig. 4. Creatine, CoQ10 or their combination blocks 3-NP-induced impairment of striatal glutathione homeostasis and reduces oxidative damage.
The striatum and cortex tissues collected from the Lewis rats described in methods were used for analysis of glutathione metabolites and oxidative damage markers. A. 3-NP administration resulted in a statistically significant reduction of striatal levels of GSH and the ratio of GSH/GSSG, whereas levels of GSSG were not significantly affected by 3-NP treatment measured by HPLC. Creatine, CoQ10 or the combination diet treatment significantly blocked 3-NP induced reductions in striatal levels of GSH and the ratio GSH/GSSG without impacting the GSSG levels. The combination diet exerted an additive effect in preserving striatal GSH/GSSG ratio. B. HPLC analysis of MDA levels in striatum revealed a statistically significant increase in MDA levels in 3-NP intoxicated rats treated with control diet. Creatine, CoQ10 or the combination diet treatment significantly reduced 3-NP-induced MDA formation in the striatum with the combination exerting a more significant reduction in striatal MDA. C. HPLC analysis of the ratio of 8OH2dG over dG, a marker of DNA oxidation, showed a significant increase in oxidative DNA damage in the cerebral cortex following 3-NP treatment, which is attenuated by treatment of creatine, CoQ10 or the combination diet, with the combination treatment reaching a statistically significant attenuation. Data represent mean ± SEM. *p< 0.05, ** p< 0.01 compared to control alone; #p< 0.05, ##p< 0.01 compared to 3-NP with control diet; + p< 0.05 compared to 3-NP with either creatine or CoQ10 diet, by ANOVA. n= 15 rats per group.
Immunohistochemical analysis of the striatum slices after 3-NP toxicity showed a marked increase in MDA immunostaining, and treatment with 2% creatine, 1% CoQ10 or their combination showed a reduction in 3-NP-induced MDA staining in the striatum (data not shown). Quantitative measurement of striatal MDA by HPLC revealed a significant increase in the amount of MDA in the striatum of rats fed with control diet following 3-NP toxicity. The 3-NP-induced elevation of MDA levels was significantly attenuated by treatment with 2% creatine, 1% CoQ10 or their combination, in which the combination diet exerted a more significant reduction in the striatal MDA level (p<0.01) (Fig. 4B). Furthermore, administration of 3-NP resulted in significant increase in DNA oxidation as measured by HPLC the ratio of 8OH2dG / dG, a marker for assessing DNA oxidative damage, in the cerebral cortex of rats, which was attenuated by the treatment with 2% creatine, 1% CoQ10 or their combination, with the reduction by the combination treatment reaching statistical significance (p<0.05) (Fig. 4C).
Additive effects of creatine with CoQ10 on improving motor performance and extending the survival of R6/2 HD mice
We investigated whether the combination of creatine and CoQ10 exert additive protective effects in a transgenic animal model of HD. The R6/2 transgenic mice, which express exon 1 of the human expanded CAG repeat-containing Huntingtin gene and display similar clinical and pathological features to those found in human HD, represent a reasonable model with which to identify candidate therapies for testing in humans (Beal and Ferrante 2004). Oral administration of either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of these two was started at 21 days of age and continued through death.
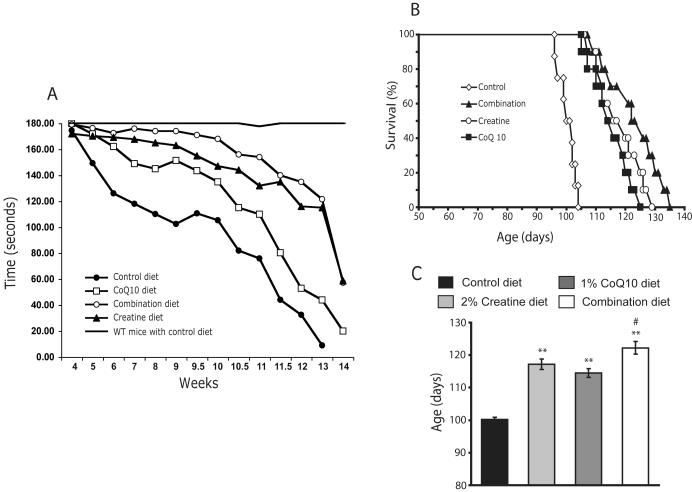
An analysis of motor performance on the rotarod revealed significant differences between R6/2 mice and wild-type littermates (Fig. 5A). Separate administration of CoQ10 and creatine in R6/2 HD mice significantly improved rotarod performance starting at 5 weeks through 13 weeks of age, in comparison to untreated R6/2 HD mice (CoQ10-treated R6/2 mice: F(49,821) 15.16, P<0.01; creatine-treated R6/2 mice: F(49,821) 13.72, P<0.005). The greatest improvement in rotarod occurred using the combined administration of creatine and CoQ10 (creatine/CoQ10-treated R6/2 mice: F(49,821) 11.12, P<0.001) (Fig. 5A).
Fig. 5. Additive effect of creatine and CoQ10 on improving motor performance and extending survival of R6/2 HD mice.
Oral administration of either an unsupplemented control diet or a diet supplemented with 2% creatine or 1% CoQ10 or a combination of these two was started in R6/2 HD mice at 21 days of age old. Motor performance of R6/2 HD mice and wild-type (WT) littermates was evaluated by recording twice weekly the time (up to 3 min) that they remained on a rotarod turning at 16 rpm (A). Significant differences were seen between R6/2 mice and WT littermates. Administration of CoQ10 or creatine in R6/2 HD mice significantly improved rotarod performance starting at 5 weeks through 13 weeks of age, in comparison to R6/2 HD mice treated with control diet, and the greatest improvement in rotarod occurred using the combined administration of creatine and CoQ10 (see results section for statistic values). Kaplan-Meier probability of survival analysis (B) and the column graph (C) show that creatine, CoQ10 or the combination diet significantly extended R6/2 HD mice survival compared to the R6/2 mice treated with control diet. The combination diet produced an additive effect in extending the survival. **p< 0.01 compared to control diet group; #p< 0.05 compared to either creatine or CoQ10 diet group. n= 20 mice per group.
Administration of the three supplemented diets resulted in significant improvements in the survival of R6/2 HD mice compared with the survival of mice fed the unsupplemented control diet (Fig. 5B and C). The mean survival of mice on supplemented diets increased from 100.1 ± 0.7 days to 117.2 ± 1.6 days using 2% creatine (p < 0.01), to 114.5 ± 1.3 days using 1% CoQ10 (p < 0.01), and 122.3 ± 1.9 days using the combination of creatine and CoQ10 (p < 0.001), and an additive neuroprotective effect was seen in the combination diet treated group when compared with either the creatine or the CoQ10 treated group (p < 0.05) (Fig. 5C). Survival extensions were 17.1% by creatine, 14.4% by CoQ10, and 22.3% by the creatine/CoQ10 combination.
Discussion
Both CoQ10 and creatine are promising agents for neuroprotection. Coenzyme Q which is also known as ubiquinone is composed of redox active quinoid moieties as well as a hydrophobic tail. The predominant form of Coenzyme Q in humans is CoQ10, containing 10 isoprenoid units in the tail. CoQ10 protects against oxidative damage produced by either H2O2 or paraquat in human neuroblastoma cells (McCarthy et al. 2004; Somayajulu et al. 2005), and against death of dopaminergic neurons produced by rotenone (Moon et al. 2005). CoQ10 enrichment decreases oxidative damage in human lymphocytes (Tomasetti et al. 1999). CoQ10 also prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging activity by blocking the mitochondrial permeability transition (Papucci et al. 2003). CoQ10 supplementation preserves the endogenous antioxidant glutathione level in tissues from a liver disease animal model (Othman et al. 2008).
We have demonstrated that CoQ10 administration increased brain mitochondrial CoQ10 concentrations (Matthews et al. 1998a). In animal studies, CoQ10 attenuates striatal lesions produced by malonate or 3-NP (Beal et al. 1994; Matthews et al. 1998a). It attenuates malonate-induced depletions of ATP and increases in lactate concentrations (Beal et al. 1994). CoQ10 also improves survival in transgenic mouse models of amyotrophic lateral sclerosis (ALS) and HD (Matthews et al. 1998a; Ferrante et al. 2002). Both CoQ10 and reduced CoQ10 protect against MPTP neurotoxicity and in a chronic MPTP model, CoQ10 reduced α-synuclein aggregates (Cleren et al. 2008). CoQ10 decreases brain oxidative stress, Aβ42 levels, β-amyloid deposition and improves memory in a transgenic mouse model of Alzheimer’s disease (AD) (Kipiani et al. 2009), and it decreased β-amyloid levels in cerebral cortex of mice with a presenilin-1 mutation (Yang et al. 2008).
Creatine exists as both free creatine and phosphocreatine (PCr). PCr functions as a temporal energy buffer in which ADP is rephosphorylated to adenosine triphosphate (ATP) during periods of high energy demand (PCr + ADP + H+ ↔ Cr + ATP). This phosphoryl group transfer is catalyzed by creatine kinase (CK). The creatine / PCr system functions as a spatial energy buffer between the cytosol and mitochondria, using a unique mitochondrial CK isoform. The mitochondrial CK isoform exists in the intermembrane space of the mitochondria where it can convert from an octameric to a dimeric form (Dolder et al. 2003). The octameric form facilitates the functional coupling between porin on the outer mitochondrial membrane, and the adenine nucleotide translocator in the inner mitochondrial membrane. Together, they form components of the mitochondrial permeability transition (MPT) pore, whose opening (which promotes apoptosis) is inhibited when mitochondrial CK is in the octameric form (Dolder et al. 2003). The octameric form is converted into the dimeric form in the presence of free radicals such as peroxynitrite, thereby promoting opening of the pore and apoptosis. Both creatine and PCr can inhibit peroxynitrite-mediated inactivation and dimerization of mitochondrial CK, thereby blocking opening of the MPT pore (Dolder et al. 2003). Creatine administration however, still exerts neuroprotective effects in the absence of mitochondrial CK (Klivenyi et al. 2004b).
Creatine supplementation attenuates the accumulation of oxidative stress in animal models. Creatine per se would not be expected to function as an acceptor of an unpaired electron, but likely functions as an antioxidant through an enhancement of energy transduction and ADP recycling (indirect antioxidant function) (Meyer et al. 2006). The antioxidant function of creatine would also attenuate the inactivation of mitochondrial CK and opening of the MPT pore (Tarnopolsky and Beal 2001). Preservation of the reduced glutathione level by creatine supplementation was observed in a cardiac stress animal model (Rakpongsiri and Sawangkoon 2008).
Creatine exerts neuroprotective effects both in vitro as well as in vivo. It protects against both glutamate and β-amyloid toxicity in rat hippocampal neurons (Brewer and Wallimann 2000), and against 3-NP and glutamate neurotoxicity in rat hippocampal and striatal neurons (Brustovetsky et al. 2001). Creatine protects against both NMDA and ibotenic acid striatal excitotoxic lesions in vivo (Malcon et al. 2000; Pena-Altamira et al. 2005). We showed that creatine protects against striatal lesions produced by both malonate and 3-NP (Matthews et al. 1998b). It also protects against traumatic brain injury and spinal cord injury (Sullivan et al. 2000; Hausmann et al. 2002). Creatine administration also is effective in models of cerebral ischemia (Zhu et al. 2004; Prass et al. 2007). Creatine produces dose-dependent protection against MPTP toxicity (Matthews et al. 1999). Creatine is protective in the wobbler mouse model of motor neuron disease (Ikeda et al. 2000). Creatine improves survival, behavior and neuropathologic sequelae in transgenic mouse models of amyotrophic lateral sclerosis and HD (Klivenyi et al. 1999; Ferrante et al. 2000; Andreassen et al. 2001).
In animal studies, combinations of either CoQ10 or creatine with other agents exert additive neuroprotective effects. CoQ10 produces additive effects when administered with the NMDA antagonist remacemide in a transgenic mouse model of HD (Ferrante et al. 2002). Creatine in combination with cyclooxygenase 2 inhibitors exerts additive neuroprotective effects against MPTP neurotoxicity, and in a transgenic mouse model of ALS (Klivenyi et al. 2003; Klivenyi et al. 2004a). Creatine exerts additive neuroprotective effects with minocycline in a mouse model of ALS (Zhang et al. 2003).
There are however, no reports examining additive neuroprotective effects of CoQ10 with creatine. In the present study, we found that CoQ10 in combination with creatine exerts additive neuroprotective effects against both MPTP and 3-NP neurotoxicity, and in a transgenic mouse model of HD. The combination therapy reduced lipid peroxidation and pathologic α-synuclein accumulation in SNpc neurons, and loss of dopaminergic neurons, which occurs in the chronic MPTP model of PD. In the 3-NP model of HD, the combination therapy significantly blocked 3-NP-induced striatal lesion volume by 83%, and reduced 3-NP induced lipid oxidation and DNA oxidation. The combination therapy shows an additive effect in preserving the endogenous antioxidant GSH level in striatum of 3-NP HD rats, and maintaining the GSH/GSSG ratio, which is an important marker for cell oxidative status, at a normal level. In addition, the combination therapy exerts additive neuroprotective effects in improving motor performance and extending the survival of the R6/2 transgenic mouse model of HD. Our data, therefore, provide evidence that the combination of creatine and CoQ10 improves neuroprotective efficacy as compared to either compound alone. This may reflect varying mechanisms of action. CoQ10 has effects both as an antioxidant and it may improve function of the electron transport chain. It is difficult to determine which effect is most important in vivo since they are interrelated. Creatine administration produces an increase in PCr, which acts as a buffer against energetic stresses. We suspect that the predominant effects of CoQ10 are as an antioxidant, whereas the predominant effects of creatine are as an energy metabolism, which would then be responsible for additive neuroprotective effects.
Our combination diet contains 1% CoQ10 (v/v), which equals a daily dose of 1,600-2,000mg/kg body weight in 25-30g mice (around 5 g diet per day). In our previous study, 0.8% CoQ10 diet (1,600 mg/kg/day in a 25 g mouse) showed the best neuroprotective effect in both acute and chronic MPTP models of PD (Cleren et al. 2008). In our present study, protective effects of CoQ10 in this dose range were significantly enhanced by its combination with 2% creatine, which we have shown in our earlier study to exert significant additive neuroprotective affects when combined with cyclooxygenase 2 inhibitors in MPTP treated mice (Klivenyi et al. 2003). Humans have a different CoQ10 bioabsorption and metabolism from rodents, and peak plasma concentrations are higher in humans. In a tolerance study, in humans CoQ10 was well tolerated and safe at doses as high as 3,000 mg/day, but the plasma CoQ10 level reaches a plateau at the dose of 2,400 mg/day (Ferrante et al. 2005).
Clinical trials of both CoQ10 and creatine show promise. CoQ10 produced modest effects in improving the total functional scale of HD patients (Huntington-Study-Group 2001), and a further phase III trial using a higher dose has been initiated (CARE-HD). A phase II clinical trial in PD showed dose-dependent efficacy, with the best effect being a 44% slowing of the Unified Parkinson’s Disease Rating Scale (Shults et al. 2002). CoQ10 was not futile in a 1-year trial in PD (NINDS NET-PD Investigators 2006). No symptomatic effects of CoQ10 were observed in a 3-month trial (Storch et al. 2007). A phase III trial of CoQ10 in early stage PD patients (QE3) has been initiated. Clinical trials of CoQ10 has also shown beneficial effects in two other neurodegenerative diseases, Friedreich’s ataxia (Hart et al. 2005) and progressive supranuclear palsy (Stamelou et al. 2008).
Creatine has been assessed in both PD and HD. Creatine in early PD could not be rejected as futile, and a Phase III trial has started (NINDS NET-PD Investigators 2006); (Bloom 2007). In HD patients, creatine was well tolerated and decreased a plasma marker of DNA oxidative damage (Hersch et al. 2006). In a small 1-year trial of 41 HD patients no toxicity or benefits were observed (Verbessem et al. 2003). In an open-label dose escalation study using doses up to 40g/day for 18 months, 30 g/day was thought to be the optimal dose. A phase III trial is planned for creatine in 650 early stage HD patients (CREST-E).
The results of the present studies are, therefore, of potential importance in the design of future clinical trials in PD, HD and other neurodegenerative diseases. If both CoQ10 and creatine show efficacy in PD and HD clinical trials, then future studies of the two compounds in combination may be warranted. A combination of the two compounds would also be a promising approach for treating presymptomatic individuals, since both compounds are natural products and are well tolerated with few side effects.
Acknowledgements
Support for the current study was provided by the Department of Defense, NINDS grants NS39258, NS045242 and NS045806, UOI. We thank Dr. Bobby Thomas and Dr. Rebecca Banerjee for assistance with confocal analysis, Beverly J. Lorenzo for technical assistance and Greta Strong for assistance with the preparation of this manuscript.
Abbreviations
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MDA
malondialdehyde
- GSH
glutathione
- GSSG
oxidized form of GSH
- dG
deoxyguanosine
- 8OH2dG
8-hydroxy-2-deoxyguanosine
- CK
creatine kinase
- PCr
phosphocreatine
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- 3-NP
3-nitropropionic acid
- PD
Parkinson’s disease
- HD
Huntington’s disease
- MPT
mitochondrial permeability transition
References
- Agarwal R, Chase SD. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;775:121–126. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Beal MF, Shults CW. Effects of Coenzyme Q10 in Huntington’s disease and early Parkinson’s disease. Biofactors. 2003;18:153–161. doi: 10.1002/biof.5520180218. [DOI] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ. Experimental therapeutics in transgenic mouse models of Huntington’s disease. Nat Rev Neurosci. 2004;5:373–384. doi: 10.1038/nrn1386. [DOI] [PubMed] [Google Scholar]
- Beal MF, Henshaw DR, Jenkins BG, Rosen BR, Schulz JB. Coenzyme Q10 and nicotinamide block striatal lesions produced by the mitochondrial toxin malonate. Ann Neurol. 1994;36:882–888. doi: 10.1002/ana.410360613. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MZ. NIH announces phase III clinical trial of creatine for Parkinson’s disease. Consult Pharm. 2007;22:378. [PubMed] [Google Scholar]
- Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem. 2001;76:425–434. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- Burklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The Creatine Kinase/Creatine Connection to Alzheimer’s Disease: CK-Inactivation, APP-CK Complexes and Focal Creatine Deposits. J Biomed Biotechnol. 2006;2006:35936. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, Fantasia M, Taft J, Beal MF, Traynor B, Newhall K, Donofrio P, Caress J, Ashburn C, Freiberg B, O’Neill C, Paladenech C, Walker T, Pestronk A, Abrams B, Florence J, Renna R, Schierbecker J, Malkus B, Cudkowicz M. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsmark-Andree P, Lee CP, Dallner G, Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, Turner C, Blamire AM, Manners D, Styles P, Schapira AH, Cooper JM. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol. 2005;62:621–626. doi: 10.1001/archneur.62.4.621. [DOI] [PubMed] [Google Scholar]
- Hausmann ON, Fouad K, Wallimann T, Schwab ME. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord. 2002;40:449–456. doi: 10.1038/sj.sc.3101330. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ferrante RJ. Translating therapies for Huntington’s disease from genetic animal models to clinical trials. NeuroRx. 2004;1:298–306. doi: 10.1602/neurorx.1.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem. 2006;387:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- Huntington-Study-Group A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Iwasaki Y, Kinoshita M. Oral administration of creatine monohydrate retards progression of motor neuron disease in the wobbler mouse. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:207–212. doi: 10.1080/14660820050515205. [DOI] [PubMed] [Google Scholar]
- Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- Kipiani K, Dumont M, Yu F, Wille E, Calingasan NY, Beal MF, Gouras GK, Lin MT. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2009 doi: 10.3233/JAD-2011-110209. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Mol Neurosci. 2003;21:191–198. doi: 10.1385/jmn:21:3:191. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Kiaei M, Gardian G, Calingasan NY, Beal MF. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004a;88:576–582. doi: 10.1046/j.1471-4159.2003.02160.x. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Calingasan NY, Starkov A, Stavrovskaya IG, Kristal BS, Yang L, Wieringa B, Beal MF. Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiol Dis. 2004b;15:610–617. doi: 10.1016/j.nbd.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Malcon C, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine administration against NMDA and malonate toxicity. Brain Res. 2000;860:195–198. doi: 10.1016/s0006-8993(00)02038-2. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998a;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci. 1998b;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Meyer LE, Machado LB, Santiago AP, da-Silva WS, De Felice FG, Holub O, Oliveira MF, Galina A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem. 2006;281:37361–37371. doi: 10.1074/jbc.M604123200. [DOI] [PubMed] [Google Scholar]
- Moon Y, Lee KH, Park JH, Geum D, Kim K. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. J Neurochem. 2005;93:1199–1208. doi: 10.1111/j.1471-4159.2005.03112.x. [DOI] [PubMed] [Google Scholar]
- NINDS NET-PD Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- Noack H, Kube U, Augustin W. Relations between tocopherol depletion and coenzyme Q during lipid peroxidation in rat liver mitochondria. Free Radic Res. 1994;20:375–386. doi: 10.3109/10715769409145637. [DOI] [PubMed] [Google Scholar]
- Othman AA, Shoheib ZS, Abdel-Aleem GA, Shareef MM. Experimental schistosomal hepatitis: protective effect of coenzyme-Q10 against the state of oxidative stress. Exp Parasitol. 2008;120:147–155. doi: 10.1016/j.exppara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Ouary S, Bizat N, Altairac S, Menetrat H, Mittoux V, Conde F, Hantraye P, Brouillet E. Major strain differences in response to chronic systemic administration of the mitochondrial toxin 3-nitropropionic acid in rats: implications for neuroprotection studies. Neuroscience. 2000;97:521–530. doi: 10.1016/s0306-4522(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- Pena-Altamira E, Crochemore C, Virgili M, Contestabile A. Neurochemical correlates of differential neuroprotection by long-term dietary creatine supplementation. Brain Res. 2005;1058:183–188. doi: 10.1016/j.brainres.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stockler-Ipsiroglu G, Wallimann T, Priller J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J Cereb Blood Flow Metab. 2007;27:452–459. doi: 10.1038/sj.jcbfm.9600351. [DOI] [PubMed] [Google Scholar]
- Rakpongsiri K, Sawangkoon S. Protective effect of creatine supplementation and estrogen replacement on cardiac reserve function and antioxidant reservation against oxidative stress in exercise-trained ovariectomized hamsters. Int Heart J. 2008;49:343–354. doi: 10.1536/ihj.49.343. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Smith KM, Matson S, Matson WR, Cormier K, Del Signore SJ, Hagerty SW, Stack EC, Ryu H, Ferrante RJ. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim Biophys Acta. 2006;1762:616–626. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol Dis. 2005;18:618–627. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Stamelou M, Reuss A, Pilatus U, Magerkurth J, Niklowitz P, Eggert KM, Krisp A, Menke T, Schade-Brittinger C, Oertel WH, Hoglinger GU. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord. 2008;23:942–949. doi: 10.1002/mds.22023. [DOI] [PubMed] [Google Scholar]
- Storch A, Jost WH, Vieregge P, Spiegel J, Greulich W, Durner J, Muller T, Kupsch A, Henningsen H, Oertel WH, Fuchs G, Kuhn W, Niklowitz P, Koch R, Herting B, Reichmann H. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–574. [PubMed] [Google Scholar]
- Tomasetti M, Littarru GP, Stocker R, Alleva R. Coenzyme Q10 enrichment decreases oxidative DNA damage in human lymphocytes. Free Radic Biol Med. 1999;27:1027–1032. doi: 10.1016/s0891-5849(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R. Creatine supplementation in Huntington’s disease: a placebo-controlled pilot trial. Neurology. 2003;61:925–930. doi: 10.1212/01.wnl.0000090629.40891.4b. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Chen J, Ley JJ, Becker DA, Beal MF. A novel azulenyl nitrone antioxidant protects against MPTP and 3-nitropropionic acid neurotoxicities. Exp Neurol. 2005;191:86–93. doi: 10.1016/j.expneurol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Narayanan M, Friedlander RM. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol. 2003;53:267–270. doi: 10.1002/ana.10476. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, Beal MF, Brown RH, Jr., Kristal BS, Ferrante RJ, Friedlander RM. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci. 2004;24:5909–5912. doi: 10.1523/JNEUROSCI.1278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]