Abstract
Purpose
To report unusual ocular manifestations of branchio-oculo-facial syndrome (BOFS) caused by a novel mutation in activating enhancer binding protein 2 alpha (TFAP2A).
Methods
Full ophthalmological evaluation and direct sequencing of TFAP2A.
Results
A 10-year-old girl with unusual ocular manifestations of BOFS such as elliptical shaped microcornea and a novel de novo TFAP2A mutation was identified.
Conclusions
This report expands the ocular phenotypic spectrum of BOFS and adds to the small number of reported TFAP2A mutations.
Introduction
In 1987, Fujimoto et al. [1] described an apparently novel syndrome that combines branchial sinus, ocular, and craniofacial anomalies and appropriately named it branchio-oculo-facial syndrome (BOFS OMIM 113620). Branchial sinus defects are usually in the form of cervical/infra-auricular skin defects. Craniofacial defects include pseudo-cleft/cleft lip, broad nasal bridge, and high forehead. Pseudo-cleft is a particularly helpful clinical feature that is highly specific to this syndrome. Ocular features are more diverse and include microphthalmia, lacrimal duct obstruction, and coloboma [2]. Since the original description, 81 cases have been reported, and while the phenotype was believed by many to be quite distinct, others have been more skeptical [3]. In particular, the overlap with branchio-oto-renal syndrome (BORS) has raised doubts that BOFS may in fact be an allelic disorder [3]. Both disorders are autosomal dominant and affect branchial structures, but the two developmental disorders, despite their extreme phenotypic variability, do have quite different predilection to certain organs, such as the kidney in the case of branchio-oto-renal syndrome and the eye in the case of BOFS. However, it was the identification of activating enhancer binding protein 2 alpha (TFAP2A) mutations in patients with BOFS that provided the unequivocal proof that BOFS is indeed a distinct clinical entity [4].
Since the original description of TFAP2A as the disease gene, very few subsequent reports have been published. In this study, we describe a girl with molecularly confirmed BOFS and who also has unusual ocular manifestations that we believe expand the ocular phenotype of this syndrome.
Methods
Human subjects
The patient was recruited with written informed consent (KFSHRC IRB #2070023) and had full ophthalmological and dysmorphological evaluations. The parents were also recruited with written informed consent to determine whether the mutation is familial or de novo. The patient and her parents were recruited at King Khalid University Hospital.
Mutation analysis
Genomic DNA was extracted from the patient and her parents (5 ml of blood in EDTA from each individual) and kept at 4 degrees until processed for DNA extraction the following day. On the patient’s sample, all TFAP2A coding exons as well as their flanking intronic sequences were PCR amplified on MyCycler (Bio-Rad, Hercules, CA) (primer sequences and PCR conditions are available upon request). On the parental samples, a targeted PCR amplification of the mutation-containing fragment was performed. Amplicons were purified then bidirectionally sequenced on an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA). Sequence analysis was performed using DNA Star package (Lasergene, Madison, WI).
Results
Clinical evaluation
The index is a 10-year-old girl who was referred to our clinical genetics service for evaluation because of her craniofacial dysmorphia. She was born at term to a 35-year-old gravida 8 para 7 mother following an uneventful pregnancy. Delivery was spontaneous, vaginal, and uncomplicated. At birth multiple anomalies were noted in the form of a high-arched palate and partial cutis aplasia in the right retroauricular region. Apparent microphthalmia prompted a full ophthalmological evaluation that revealed the additional presence of microcornea, iris and chorioretinal coloboma, inferiorly subluxed lenses with mild cataractous changes on the left, and nasolacrimal duct stenosis. High resolution karyotype was normal 46,XX and subtelomeric fluorescence in situ hybridization (FISH) was normal. Metabolic screening, echocardiogram, and kidney ultrasound were also normal. Magnetic resonance imaging of the eyes performed at the age of 4 years revealed an abnormal configuration of both eye globes, with evidence of a triangular shape of the lenses (which are displaced medially and inferiorly), small coloboma at the posterior aspect of both eye globes, normal symmetric appearance of both optic nerves, and normal extraocular muscles. She had mild speech delay, probably related to mild to moderate hearing loss, but is currently doing well in regular school where she is in an age-matched grade; her IQ was measured to be 85.
Physical examination at 10 years of age revealed a small body build, with height at 127.5 cm (between the 5th and 10th centiles), weight at 20.4 kg (<5%), and head circumference at 49.6 cm (just above the 2nd percentile). In addition to the previously documented ocular anomalies, she has pseudocleft of the upper lip, oligodontia, mixed hearing loss, and abnormal orientation of the nasal spine, which appears flushed with the forehead (Figure 1). There is partial cutis aplasia in the right retroauricular region, but the neck appears normal otherwise. The rest of the physical examination was essentially normal. Specifically, there is no evidence of chest, spine, nail, or hair abnormalities. A repeat ophthalmological examination revealed the following:
Figure 1.
Clinical photographs of the patient. Facial appearance with the typical pseudocleft of the upper lip is shown in A. Microcornea, inferonasal coloboma, and cataract are shown in B and C. Lateral facial profile is shown in D.
1- Visual acuity: Searching and wandering eye movement. She did not fix or follow light or objects. Cycloplegic refraction through the phakic part is −1.50 for the right eye and −1.50 for the left eye.
2- Intraocular pressure: Right eye 17 mmHg and left eye 25 mmHg.
3- Slit-lamp examination: Microphthalmia bilaterally, clear but elliptical cornea bilaterally, anterior chamber is deep and quiet, iris shows a large coloboma bilaterally inferiorly with lens opacity. In addition, the lens appeared to be slightly subluxated inferiorly with zonules on both sides showing superiorly.
4- Fundus examination: Showed a large chorioretinal coloboma in both eyes. B-scan ultrasound confirmed a chorioretinal coloboma close to the disc in both eyes, but no retinal detachment or any other pathology was appreciated.
Mutation analysis
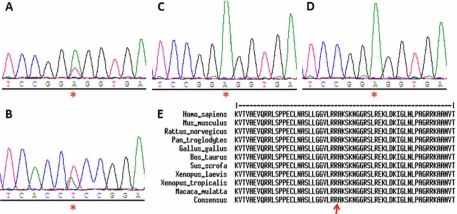
A novel heterozygous TFAP2A missense mutation in exon 4, c.763A>T, was detected that results in replacement of basic arginine by nonpolar tryptophan (p.Arg255Trp; Figure 2). This mutation was confirmed on the reverse direction and on repeat PCR on the patient but not on her parents (paternity was verified), thus confirming the de novo nature of this mutation (Figure 2). The mutation affects a highly conserved amino acid residue (Figure 3) and was not found in a panel of 106 Saudi normal controls (212 chromosomes), further supporting its pathogenic nature.
Figure 2.
A novel mutation in TFAP2A. Sequence chromatogram of the patient is shown in A and B (forward and reverse) and that of her parents in C and D (forward only) with the c.763A>T mutation indicated by a red asterisk. Protein alignment across species in E shows very strong conservation of the R255 residue which is indicated by the red arrow.
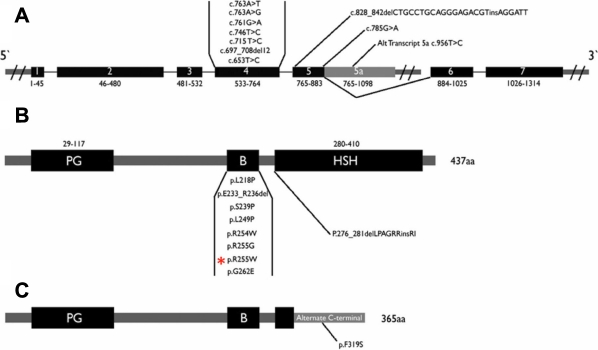
Figure 3.
Summary of previously reported TFAP2A mutations. A schematic of the TFAP2A gene is shown in A along with the genomic location of previously reported mutations. B and C show two different isoforms of the protein encoded by TFAP2A (AP2) along with the location of previously reported mutations at the protein level. The mutation in the current study is indicated by red asterisk. PG: proline/glutamine rich domain, B: basic DNA binding domain, HSH: helix span helix domain.
Discussion
Ocular manifestations of BOFS are variable, as shown in Table 1, with microphthalmia, coloboma, and nasolacrimal duct stenosis being the most common. To our knowledge, the appearance of an elliptical-shaped cornea has never been reported in association with BOFS. Other unusual eye findings in our patient include lens subluxation and microcornea, which have only been observed in three BOFS patients to date (Table 1) [2-21].
Table 1. The reported ocular manifestations of BOFS.
| |
Reference |
|
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ocular feature | [2] | [7] | [6] | [5] | [8] | [3]* | [9] | [10] | [11] | [14] | [12] | [13] | [15] | [16] | [18] | [19] | [4]** | [21] | [20] | [17] | This study | Total | % |
| Elliptical shaped cornea |
0\15 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0\5 |
0\5 |
1\1 |
1\54 |
2 |
| Microphthalmia |
14\42 |
1\2 |
0\2 |
1\2 |
1\2 |
0\2 |
0\1 |
1\1 |
0\1 |
0\1 |
1 |
0\3 |
0\1 |
0\1 |
0\1 |
1\1 |
3\5 |
0\1 |
2\5 |
4\5 |
1\1 |
30\81 |
37 |
| Anophthalmia |
4\42 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
4\81 |
4.9 |
| Small PF |
9\42 |
0\2 |
0\2 |
0\2 |
1\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
1\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
11\81 |
14 |
| NLDS |
29\39 |
2\2 |
0\2 |
2\2 |
2\2 |
2\2 |
1\1 |
0\1 |
0\1 |
1\1 |
1\1 |
0\3 |
1\1 |
1\1 |
0\1 |
0\1 |
2\5 |
0\1 |
5\5 |
1\5 |
1\1 |
51\78 |
65 |
| Coloboma |
16\35 |
1\2 |
1\2 |
0\2 |
0\2 |
1\2 |
1\1 |
0\1 |
0\1 |
0\1 |
1\1 |
0\3 |
0\1 |
0\1 |
0\1 |
1\1 |
3\5 |
0\1 |
5\5 |
1\5 |
1\1 |
32\74 |
43 |
| Sublaxation |
0\15 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
1\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
1\5 |
1\1 |
3\54 |
6 |
| Catract |
8\33 |
0\2 |
0\2 |
0\2 |
0\2 |
1\2 |
0\1 |
0\1 |
0\1 |
0\1 |
1\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
2\5 |
0/5 |
1\1 |
13\72 |
18 |
| Myopia |
9\28 |
0\2 |
1\2 |
0\2 |
0\2 |
1\2 |
1\1 |
0\1 |
0\1 |
0\1 |
0\1 |
2\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
1\1 |
15\67 |
22 |
| Strabismus |
11\36 |
0\2 |
0\2 |
0\2 |
1\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
3\5 |
0/5 |
0\1 |
15\75 |
20 |
| Ptosis |
10\36 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
1\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
11\75 |
15 |
| Hypertolirsm |
3\15 |
0\2 |
0\2 |
0\2 |
1\2 |
0\2 |
0\1 |
1\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
5\54 |
9 |
| Telecanthus |
0\15 |
1\2 |
1\2 |
0\2 |
2\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
3\3 |
0\1 |
0\1 |
1\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
8\81 |
10 |
| Microcrnea |
1\15 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
1/5 |
1\1 |
3\54 |
6 |
| Dermoid cyst |
1\15 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
1\54 |
2 |
| Eyelid cyst |
1\15 |
0\2 |
0\2 |
0\2 |
0\2 |
0\2 |
0\1 |
0\1 |
0\1 |
0\1 |
0\1 |
0\3 |
0\1 |
0\1 |
0\1 |
0\1 |
0\5 |
0\1 |
0/5 |
0/5 |
0\1 |
1\54 |
2 |
| Primary aphakia | 0\15 | 0\2 | 0\2 | 0\2 | 0\2 | 0\2 | 0\1 | 0\1 | 0\1 | 0\1 | 0\1 | 0\3 | 0\1 | 0\1 | 0\1 | 0\1 | 0\5 | 0\1 | 0\5 | 2\5 | 0\1 | 1\54 | 2 |
Note that the ratios reflect patients who had the ocular feature to total patients examined. Abbreviations: PF, palpebral fissure ; NLDS, nasolacrimal duct stenosis. The asterisk indicates that seven patients were reported in this study but only two were new. The double asterisk indicates that six patients were reported in this study including one previously reported by Lin et al. [2].
The identification of TFAP2A as the disease gene in BOFS provided long sought answers to the ocular phenotype of this syndrome. TFAP2A is a retinoic acid responsive gene that encodes activating enhancer-binding protein 2 alpha (AP-2α), a member of the AP-2 family of transcription factors that regulate gene expression during embryogenesis of the eye, ear, face, body wall, limbs, and neural tube [4]. The role of AP-2α in eye development has been established in several studies, perhaps the most compelling of which is the demonstration that Tfap2a knockout mouse embryos exhibit grossly abnormal ocular development in the form of anophthalmia, aphakia, absent cornea, coloboma, lens stalk, lack of ciliary body and iris formation, absent eyelids, and ectopic neural retina that replaces part of the retinal pigmented epithelium [22,23].
Mechanistic insight into some of these anomalies comes from a more complicated set of data. For instance, targeted deletion of Tfap2a in the murine lens placode reduced cadherin 1 (Cdh1) expression and increased epidermal growth factor receptor (Egfr) and alpha smooth muscle actin (α-SMA) expression, indicating abnormal lens epithelial mesenchymal transformation, which may explain the occurrence of cataract [24]. In another study [25] double heterozygous mice for paired box gene 6 (Pax6) and Tfap2a showed a more severe ocular phenotype than single heterozygotes in the form of persistent lens stalk or lens protrusion into the cornea, which suggests cooperation between the two transcription factors in lens development. On the other hand, insight into the mechanism of the observed posterior segment defects comes from a zebrafish study that examined how partial abrogation of Tfap2a affects the expressivity of ocular phenotypes. Knockdown of either bone morphogenetic protein 4 (Bmp4) or transcription factor 7-like 1a (Tcf7l1a), which encode a Bmp ligand and a transcriptional effector of Wnt signaling, respectively, do not cause an ocular phenotype. However, partial knockdown of Tfap2a in either of these two mutants led to abnormal ocular development in the form of coloboma, anophthalmia, and microphthalmia, thus establishing a genetic interaction between Tfap2a and these two genes [17].
In the first TFAP2A mutation report by Milunsky et al. [4], a total of five mutations were described, one of which was a large genomic deletion and the remaining four were missense mutations mainly in exon 4. Three subsequent papers described a total of six mutations: one indel, one deletion, one large genomic deletion, and three missense [4,17,20,21] (Figure 3). Mutation analysis of TFAP2A in our patient showed a de novo novel mutation in exon 4, a mutation hotspot that encodes the basic region of the DNA-binding domain [4]. Four of the five TFAP2A mutations in the original paper by Milunsky et al. [4] were also de novo, and this mutation affects the same residue (R255) described in that paper but results in a different substitution. Both our mutation (R255W) and that by Milunsky et al. [4] (R255G) replace the positively charged arginine with nonpolar amino acids in the DNA-binding domain, which is likely to adversely affect the capacity of TFAP2A to bind DNA. Although no functional validation was performed, the fact that the TFAP2A mutational spectrum is not exclusive to missense mutations is highly suggestive of haploinsufficiency as the most likely mechanism. However, the finding that gain of function N-ethyl-N-nitrosourea-induced mutation in mouse results in a highly similar ocular phenotype to that observed in the Tfap2a knockout mouse leaves open the possibility that some missense mutations may in fact mediate their effect in human BOFS patients by increasing TFAP2A transcriptional activity [26].
In summary, we add one novel mutation to the allelically heterogeneous disorder of BOFS and describe unusual eye findings that expand the ocular phenotype of this disorder.
Acknowledgments
We are thankful to our patient and her family for their support. This study was funded in part by a grant from KACST to F.S.A. (08-MED497–20).
AUTHOR QUERIES
Please provide sufficient details of the protocols used so that readers understand what was done.
References
- 1.Fujimoto A, Lipson M, Lacro RV, Shinno NW, Boelter WD, Jones KL, Wilson MG. New autosomal dominant branchio-oculo-facial syndrome. Am J Med Genet. 1987;27:943–51. doi: 10.1002/ajmg.1320270422. [DOI] [PubMed] [Google Scholar]
- 2.Lin AE, Gorlin RJ, Lurie IW, Brunner HG, van der Burgt I, Naumchik IV, Rumyantseva NV, Stengel-Rutkowski S, Rosenbaum K, Meinecke P, Muller D. Further delineation of the branchio-oculo-facial syndrome. Am J Med Genet. 1995;56:42–59. doi: 10.1002/ajmg.1320560112. [DOI] [PubMed] [Google Scholar]
- 3.Lin AE, Semina EV, Daack-Hirsch S, Roeder ER, Curry CJ, Rosenbaum K, Weaver DD, Murray JC. Exclusion of the branchio-oto-renal syndrome locus (EYA1) from patients with branchio-oculo-facial syndrome. Am J Med Genet. 2000;91:387–90. doi: 10.1002/(sici)1096-8628(20000424)91:5<387::aid-ajmg13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, Lin AE. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet. 2008;82:1171–7. doi: 10.1016/j.ajhg.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennaceur S, Buisson T, Bertolus C, Couly G. Branchio-oculo-facial syndrome with cleft lip and bilateral dermal thymus. Cleft Palate Craniofac J. 1998;35:454–9. doi: 10.1597/1545-1569_1998_035_0454_bofswc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Megarbane A, Hawat N, Chedid P, Bleik J, Delezoide AL. Branchio-oculo-facial syndrome associated with a white forelock. Clin Dysmorphol. 1998;7:221–3. doi: 10.1097/00019605-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Su CS, O'Hagen SB, Sullivan TJ. Ocular anomalies in the branchio-oculo-facial syndrome. Aust N Z J Ophthalmol. 1998;26:43–6. doi: 10.1046/j.1440-1606.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 8.Raveh E, Papsin BC, Forte V. Branchio-oculo-facial syndrome. Int J Pediatr Otorhinolaryngol. 2000;53:149–56. doi: 10.1016/s0165-5876(00)00310-4. [DOI] [PubMed] [Google Scholar]
- 9.McGaughran J. Another case of preaxial polydactyly and white forelock in branchio-oculo-facial syndrome. Clin Dysmorphol. 2001;10:67–8. doi: 10.1097/00019605-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor S, Kapur N. Branchio-oculo-facial syndrome with valvular pulmonic stenosis. Indian Pediatr. 2004;41:1180–1. [PubMed] [Google Scholar]
- 11.Demirci H, Shields CL, Shields JA. New ophthalmic manifestations of branchio-oculo-facial syndrome. Am J Ophthalmol. 2005;139:362–4. doi: 10.1016/j.ajo.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 12.El Darouti MA, Marzouk SA, Azzam OA, Nada HR, Sobhi RM, El Nabarawi I. Branchio-oculo-facial syndrome with bilateral linear scars of the neck. Int J Dermatol. 2005;44:674–6. doi: 10.1111/j.1365-4632.2005.01284.x. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni ML, Deshmukh S, Kumar A, Kulkarni PM. Branchio-oculo-facial syndrome. Indian J Pediatr. 2005;72:701–3. doi: 10.1007/BF02724081. [DOI] [PubMed] [Google Scholar]
- 14.Ozturk O, Tokmak A, Demirci L, Silan F, Guclu E. Branchio-oculo-facial syndrome with the atresia of external ear. Int J Pediatr Otorhinolaryngol. 2005;69:1575–8. doi: 10.1016/j.ijporl.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Verret DJ, Murray AD, Hobar PC. Branchio-oculo-facial syndrome with ectodermal parathyroid tissue. Otolaryngol Head Neck Surg. 2005;133:983–4. doi: 10.1016/j.otohns.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa H, Sasaki S, Uchiyama E, Kawashima K, Yamamoto J. Bilateral dermal thymus of neck in branchio-oculo-facial syndrome. J Plast Reconstr Aesthet Surg. 2006;59:1385–7. doi: 10.1016/j.bjps.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Gestri G, Osborne RJ, Wyatt AW, Gerrelli D, Gribble S, Stewart H, Fryer A, Bunyan JD, Prescott K, Collin JR, Fitzgerald T, Robinson D, Carter NP, Wilson SW, Ragge NK. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009;126:791–803. doi: 10.1007/s00439-009-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa RF, Zen PR, Graziadio C, Paskulin GA. Branchio-oculo-facial syndrome (BOFS) and congenital heart defects. Arq Bras Cardiol. 2009;92:e6–8. doi: 10.1590/s0066-782x2009000200015. [DOI] [PubMed] [Google Scholar]
- 19.Skarzynski H, Podskarbi-Fayette R. Treatment of otorhinolaryngological manifestations of three rare genetic syndromes: Branchio-Oculo-Facial (BOF), Ectrodactyly Ectodermal dysplasia Clefting (EEC) and focal dermal hypoplasia (Goltz syndrome). Int J Pediatr Otorhinolaryngol. 2009;73:143–51. doi: 10.1016/j.ijporl.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Stoetzel C, Riehm S, Bennouna Greene V, Pelletier V, Vigneron J, Leheup B, Marion V, Helle S, Danse JM, Thibault C, Moulinier L, Veillon F, Dollfus H. Confirmation of TFAP2A gene involvement in branchio-oculo-facial syndrome (BOFS) and report of temporal bone anomalies. Am J Med Genet A. 2009;149A:2141–6. doi: 10.1002/ajmg.a.33015. [DOI] [PubMed] [Google Scholar]
- 21.Tekin M, Sirmaci A, Yuksel-Konuk B, Fitoz S, Sennaroglu L. A complex TFAP2A allele is associated with branchio-oculo-facial syndrome and inner ear malformation in a deaf child. Am J Med Genet A. 2009;149A:427–30. doi: 10.1002/ajmg.a.32619. [DOI] [PubMed] [Google Scholar]
- 22.West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- 23.Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2{alpha} knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet. 2010;19:1791–804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontoriero GF, Deschamps P, Ashery-Padan R, Wong R, Yang Y, Zavadil J, Cvekl A, Sullivan S, Williams T, West-Mays JA. Cell autonomous roles for AP-2alpha in lens vesicle separation and maintenance of the lens epithelial cell phenotype. Dev Dyn. 2008;237:602–17. doi: 10.1002/dvdy.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makhani LF, Williams T, West-Mays JA. Genetic analysis indicates that transcription factors AP-2alpha and Pax6 cooperate in the normal patterning and morphogenesis of the lens. Mol Vis. 2007;13:1215–25. [PubMed] [Google Scholar]
- 26.Ahituv N, Erven A, Fuchs H, Guy K, Ashery-Padan R, Williams T, de Angelis MH, Avraham KB, Steel KP. An ENU-induced mutation in AP-2alpha leads to middle ear and ocular defects in Doarad mice. Mamm Genome. 2004;15:424–32. doi: 10.1007/s00335-004-2334-z. [DOI] [PubMed] [Google Scholar]