Abstract
Objectives. We explored whether the United States, which does not regulate pharmaceutical prices, is responsible for the development of a disproportionate share of the new molecular entities (NMEs; a drug that does not contain an active moiety previously approved by the Food and Drug Administration) produced worldwide.
Methods. We collected data on NMEs approved between 1992 and 2004 and assigned each NME to an inventor country. We examined the relation between the proportion of total NMEs developed in each country and the proportion of total prescription drug spending and gross domestic product (GDP) of each country represented.
Results. The United States accounted for 42% of prescription drug spending and 40% of the total GDP among innovator countries and was responsible for the development of 43.7% of the NMEs. The United Kingdom, Switzerland, and a few other countries innovated proportionally more than their contribution to GDP or prescription drug spending, whereas Japan, South Korea, and a few other countries innovated less.
Conclusions. Higher prescription drug spending in the United States does not disproportionately privilege domestic innovation, and many countries with drug price regulation were significant contributors to pharmaceutical innovation.
In contrast with most other countries, the United States does not employ a form of drug price regulation to control spending on pharmaceuticals,1 mainly because of concern that regulatory controls drive down profits and discourage the flow of capital to support the development of new molecular entities (NMEs).2 Industry and government officials in the United States have targeted other countries for their implementation of national policies surrounding drug price regulation. For example, the Pharmaceutical Manufacturers Association of America has claimed that foreign governments are free riding on US innovation and are not paying for their fair share of drug development costs.3,4 In addition, US government officials have stated that the United States is now covering most of the costs of developing a new drug.3 The concern that regulatory controls in other countries may affect global pharmaceutical innovation has also affected US trade negotiations and domestic policy.5 The US government has placed pressure on other countries to modify their current price regulation of pharmaceuticals or formulary structure.5 In 2003, the US Congress inserted a ban on government negotiation of drug prices in the Medicare Modernization Act of 2003, presumably because of concerns over the impact of drug price regulation on innovation.6,7 Several influential commentators have also voiced concern over the potential negative impact of US health care reform on pharmaceutical innovation.8 On the other hand, patient advocates and researchers are concerned about the potential increase in costs, reduced access to medications, and threats to public health that a lack of drug price regulation in the United States or abroad may entail.9,10
The statements of US government officials and industry representatives imply that the US market is paying for the development of most new drugs. There is ample evidence that domestic profits in several countries that have price or profit control cover research and development expenditures.11 For example, in Canada, domestic sales on average are about 10 times the research and development costs. In the United Kingdom, the pharmaceutical industry invests more of its revenues from domestic sales in research and development than do companies in the United States.11 Statements by US government officials and industry representatives also imply that the United States is becoming a dominant source of innovation because of its lack of drug price regulation. From a purely theoretical standpoint, these statements are troubling because they imply a country-specific source of innovation. The industry is private, however, not government owned, and operates in a worldwide market.3 It is also doubtful that pure price considerations would affect where a drug was developed, and more strategic considerations such as the availability of drug-specific research resources and infrastructure in a particular country may be a more important consideration.
Given the impact of these statements on domestic policy and trade relations, we sought to examine innovation from a country-specific vantage point and bring data to this discussion. We explored whether the United States, which does not employ a form of drug price regulation, is responsible for the development of a disproportionate share of NMEs, and also examined whether other countries, which do employ some form of price or profit control, contribute disproportionately less.
METHODS
We collected data on all 373 NMEs from the Food and Drug Administration (FDA) Web site (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ucm121136) that were approved as human therapeutic medications between 1992 and 2004. NMEs are drugs that contain an active moiety that has never been approved for use by the FDA. We identified 1992 as the start date for data collection because the public data on newly approved drugs are available on the FDA Web site starting in 1992. We excluded 27 diagnostic drugs or contrast dyes, which may be more accurately classified as medical devices, leaving 346 human therapeutic NMEs for analysis.
We identified the earliest patent filed for each drug by using the FDA's Orange Book.12 We used the patent numbers to look up the patent inventors and assignees through the US Patent and Trademark Office's Web site (http://www.uspto.gov/patents/process/search). Each patent lists the inventors, their country locations, and the patent assignees (the company the patent was assigned to). This information was used to assign each NME to a country on the basis of inventor location. In addition, on the basis of the patent information, the patent was assigned to an inventor company. If the inventors listed on a patent were from multiple countries, we assigned only the first country listed as the source country. As a sensitivity test, we also examined how the distribution of inventor countries would change if we assigned the second country listed on the patent as the source country.
Some NMEs are unpatented and are marketed with a 5-year exclusivity granted under the Hatch-Waxman Act.13 The development of these drugs cannot be assigned to a specific country because the vast majority of companies that market drugs are multinationals and have international subsidiaries. It is also not clear which country to assign an NME without a patent, and we therefore excluded these NMEs without patents (n = 58) from the analysis.
Company Classification
We collected information on the company assignee for each NME. We then determined the headquarters location for that company. The headquarters location was determined by the information listed on its Securities and Exchange Commission (SEC) annual filing for companies publicly traded in the United States.14 Facility information was available in SEC annual filings listed under the Properties and Subsidiaries sections. If there was no SEC information, then headquarters location was determined by the company's Web site. If no Web site was available, then historical press releases were used. Companies were further classified by the headquarters location of the patent assignee and also whether they were domestic, foreign, or multinational. A company was defined as domestic if it had facilities only in the United States, and a company with facilities only outside the United States was classified as foreign. A company was defined as multinational if it had facilities in 2 or more countries. A foreign multinational had facilities only in foreign countries and a domestic–foreign multinational had facilities in both the United States and in other countries. In all cases, we attempted to assess the company at the most recent point in time mainly because of the allocation problem presented by numerous mergers.
Country-Specific Data
Because country-specific comparisons of new drug development without a measure to account for size and wealth are relatively meaningless, we collected data on gross domestic product (GDP) for 2000, midway between the dates drug collection began and ended for the 20 countries that developed at least 1 NME. Similarly, we collected data on prescription drug spending in the same time period from the Organization for Economic Cooperation and Development (OECD). Prescription drug spending for the United Kingdom and Belgium was not available from the OECD in 2000 or 2001. Published reports indicated that prescription drug spending in Belgium was 11.3% of annual health care spending in 2004.15 Using OECD data on health spending, we estimated Belgium's prescription drug spending as 11.3% of annual health care spending in 2000. Similarly, published reports indicated that prescription drug spending in the United Kingdom was 15.9% of annual health care spending in 1997.16 We estimated prescription drug spending in the United Kingdom as 15.9% of annual health care spending in 2000. Data on prescription drug spending for Israel were collected from published reports in the literature.17 We collected data on the GDP of all countries by using the International Macroeconomic dataset available at the US Department of Agriculture.18 One drug was developed in the former Czechoslovakia. The innovation of this drug was assigned to the Czech Republic, and spending data from this country was used in the analysis.19
Analysis
We classified drugs by the country of the inventor and the country of the company listed on the patent. We examined the relationship between the proportion of total NMEs developed in each innovator country and the proportion of total prescription drug spending and GDP each country represented. Analyses were conducted with Stata version 9.2 (StataCorp, LP, College Station, TX).
RESULTS
We identified 346 human therapeutic NMEs. Fifty-eight (16.7%) drugs did not have patents in force at the time of approval; therefore, we could not examine the patents for these drugs and they were excluded from the analysis.
Pharmaceutical Innovation by Country
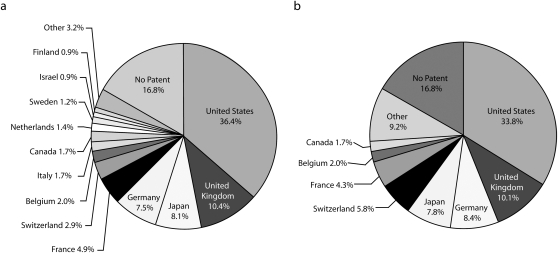
Thirty-six percent of all NMEs were developed in the United States (Figure 1). The United Kingdom was the next largest source of NME development (10.4%). Examination of drugs with patents (n = 288) revealed that 126 (43.7%) of the NMEs had their earliest patent filed by inventors in the United States. Of the 288 drugs with patents in force at the time of FDA approval, 28 (10%) had more than 1 country listed as the home country of the patent holder. The distribution of inventor countries did not appreciably change if we assigned the second country listed on the patent as the inventor (data not shown).
FIGURE 1.
Source of pharmaceutical innovation classified by location of the company headquarters by (a) country of the inventor and (b) patent assignees: 1992–2004.
Note. Data based on drugs approved between 1992 and 2004. The sample size was N = 346. Other includes Denmark, Spain, Norway, Austria, Korea, Czechoslovakia, and Australia. Percentages do not add up to 100% because of rounding,
Innovation as a Function of Company Location
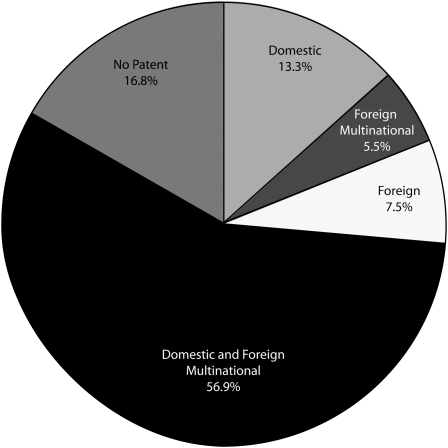
Overall, 171 companies were listed as the assignees for 288 NMEs with patents. Examination of the patents revealed that 33% of the assignees had company headquarters located in the United States, and 10% had company headquarters in the United Kingdom (Figure 1). However, further examination showed that most companies were multinationals with facilities located in 2 or more countries (Figure 2).
FIGURE 2.
Pharmaceutical patent assignees, by type of company: 1992–2004.
Note. Data based on drugs approved between 1992 and 2004. The sample size was N = 346.
Relations With Gross Domestic Product and Prescription Drug Spending
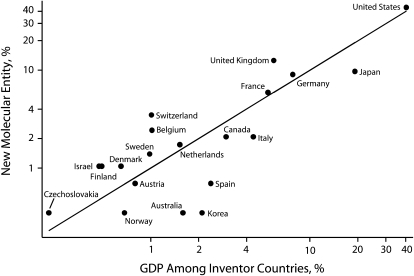
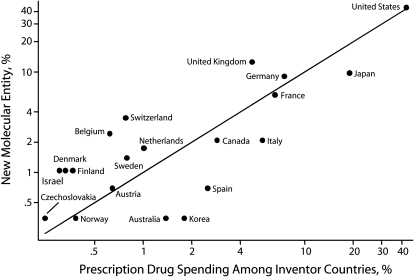
The relationship between the proportion of each country's GDP to the total GDP among all countries and the proportion of NME development is shown in Figure 3. Similarly, the relationship between the proportion of each country's prescription drug spending and their respective proportion of NME development is depicted in Figure 4. In both figures, countries above the 45 degree line innovate more in relation to their prescription drug spending and GDP, and countries below the line innovate less. As shown in Figures 3 and 4, the United States accounted for roughly 42% of prescription drug spending and 40% of the GDP among NME innovator countries and was responsible for the development of 43.7% of the NMEs. The US contribution to global discovery of NMEs was roughly proportional to its contribution to global wealth and prescription drug spending. The United Kingdom was responsible for the development of 12.5% of the NMEs and accounted for 4.7% of prescription drug spending and 5.9% of the GDP among innovator countries. In contrast, Japan was responsible for the development of 9.7% of the NMEs and accounted for 18.9% of prescription drug spending and 19.1% of the GDP among innovator countries. Similarly, Switzerland, Belgium, and a few other countries innovated proportionally more than their contribution to the GDP or prescription drug spending and Spain, Korea, Australia, and Italy innovated proportionally less.
FIGURE 3.
Pharmaceutical innovation as a function of gross domestic product (N = 288): 2000.
Note. GDP = gross domestic product; NME = new molecular entity. Axes are on a log scale. The United States almost falls on the 45 degree line where contribution to GDP and NME development is roughly proportional. Countries above the line develop a higher percentage of drugs compared with their percentage contribution to GDP. For example, the United States accounted for 40% of the GDP among NME innovator countries and was responsible for the development of 43.7% of the NMEs. The UK contributed proportionally more NMEs than its national income would indicate, and Australia and Japan proportionally less.
FIGURE 4.
Pharmaceutical innovation (development of NMEs) as a function of prescription drug spending (N = 288): 2000.
Note. NME = new molecular entity. Axes are on a log scale. Countries above the line develop a higher percentage of drugs compared with their percentage contribution to prescription drug spending.
DISCUSSION
Pharmaceutical innovation is an international enterprise. Although the United States is an important contributor to pharmaceutical innovation, we found that more than 20 countries contributed to the development of the 288 NMEs with patents at the time of approval. More than 171 companies were involved in the development of these NMEs, and the vast majority of companies were multinationals with facilities located in more than 2 countries. We also found that the United Kingdom, Switzerland, Belgium, and a few other countries innovated proportionally more than their contribution to the global GDP or prescription drug spending, whereas Japan, Spain, Australia, and Italy innovated less.
In contrast with the United States, all other countries investigated had instituted at least 1 form of drug pricing regulation.1 Critics of drug price regulation argue that free market pricing strategies and higher prices in the United States are instrumental to innovation.20,21 One might therefore expect the United States to be the most innovative given that it is the only country with a predominantly unregulated pharmaceutical market. However, US pharmaceutical innovation appeared to be roughly proportional to its national wealth and prescription drug spending. Our data suggest that the United States is important but not disproportionate in its contribution to pharmaceutical innovation. Interestingly, some countries with direct price control, profit control, or reference drug pricing appeared to innovate proportionally more than their contribution to the global GDP or prescription drug spending.
There are 3 general types of price regulation strategies that are implemented in OECD countries: (1) direct control of prices, (2) reference pricing and generic substitution, and (3) profit control (an indirect form of price control in which a country limits the profits generated by a company within its territory).20,22 The United Kingdom, Spain, and South Korea1 use profit control to lower drug costs.23 Canada uses a mixture of measures to control drug prices in different provinces.24 Denmark, Germany, the Netherlands, Italy, Norway, Spain have all implemented a form of reference drug pricing.1 Belgium, Switzerland, Sweden, Italy, Austria, and Finland set the manufacturer price, the reimbursement price, or both.1 Although many researchers20,21,25 have speculated that reference drug pricing in the United States would have dire consequences for innovation, our data suggest that the pharmaceutical innovation of countries with reference drug pricing is more or less what one would expect given their prescription drug spending or even the general size of their economies.
Many countries with significant price regulation were important innovators of pharmaceuticals; therefore, our data suggest that country-specific pricing policies probably do not affect country-specific innovation. For example, although prices in the United Kingdom are much less than are prices in the United States, the industry continues to be very profitable and innovative.11 In Canada, income from domestic sales of brand name companies is, on average, about 10 times greater than is research and development costs, even in the face of prices that are approximately 40% lower than in the United States.11 In addition, companies in the United Kingdom invest proportionately more revenue from domestic sales into research and development activity than do their US counterparts.11 Despite the above average profitability of US-based companies,26 the higher prices paid by US consumers are not rewarded by more than expected domestic innovation. US consumers pay disproportionately higher prices for brand name drugs,6 but the United States is not disproportionately innovative.
Our study had several limitations that deserve comment. Fifty-eight drugs were excluded from the analyses involving patents because they could not be assigned to any particular country. For these drugs, we determined the FDA applicant and investigated whether we could substitute the country where the FDA applicant resided as the innovator country. We searched the Securities and Exchange Committee filings and found that the FDA applicant for most of these NMEs was a company with international subsidiaries and was not exclusive to 1 country. Only 7 of the 58 drugs without patents could be exclusively assigned to 1 country with certainty. Our inability to conclusively assign each of these 58 drugs to a particular country further illustrates the global nature of pharmaceutical innovation. Furthermore, we did not examine the country-specific innovation of biological drugs. The United States has a large biotech industry and may have developed a disproportionate number of biological drugs. In addition, we assigned the innovation of a drug to the country for which the first patent was filed and the first company to which the patent was assigned. This is a limited view of innovation, because the development of an NME could take up to a decade and involve numerous countries or companies. However, the filing of the first patent is the first traceable step in innovation. In addition, NMEs may be approved in 1 country and not in others.
Furthermore, we examined innovation by using drugs approved in the United States. If we had had chosen another country, the drug sample and the breakdown in the origin of drugs may have differed. However, the claims regarding US dominance in innovation are made in the United States; therefore, the data collection and analysis was structured to examine these specific claims. Finally, we made no effort to examine country-specific innovation from the vantage point of therapeutic value; however, there is no standard method of assessing therapeutic value across such a wide variety of drug classes developed over a decade apart. Such an analysis was beyond the scope of this article.
US government officials have stated that other countries are not shouldering their fair share of research and development costs by paying lower prices.3 The pharmaceutical market, with the varying strength of its players through patent monopolies or government purchasing power, is hardly a perfect market. Perhaps other countries are paying an appropriate price and the prices in the United States are too high because the government does not leverage any power to purchase drugs. The financial success of the pharmaceutical industry compared with many other industries26 provides ample evidence that concerns regarding the future financial health of this industry and its ability to invest in drug development if the United States were to exert purchasing power are overstated. The relative success of the pharmaceutical industry in each country may be more related to the country-specific investments in human capital, education, technology, information infrastructure, and strategic choices. For example, per capita pharmaceutical research and development spending of the United Kingdom, Switzerland, and Sweden from 1995 to 2000 exceeded that of the United States.27 Some countries choose to invest in pharmaceutical research and development over other types of industries. Future research could examine the types of incentives and policies that promote country-specific investments that provide a superior environment for pharmaceutical research and development.
Pharmaceutical innovation is an international enterprise. Higher prescription drug spending in the United States does not disproportionately privilege domestic innovation. Conversely, many countries with national health systems and drug pricing regulation were significant contributors to pharmaceutical innovation.
Acknowledgments
This project was not supported by any direct funds. S. Keyhani is funded by a VA HSRD Career Development Award.
Human Participant Protection
No protocol approval was necessary because the writing of this article did not involve human research participants.
References
- 1.Kyle M. Pharmaceutical price controls and entry strategy. Rev Econ Stat 2007;89(1):88–99 [Google Scholar]
- 2.Foreign Government Pharmaceutical Price and Access Controls Washington, DC: Pharmaceutical Research and Manufacturers of America (prepared for the Dept of Commerce); 2004 [Google Scholar]
- 3.Hopkins Tanne J. FDA chief wants other rich countries to share drug development costs. BMJ 2003;327(7419):830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PhRMA Statement regarding benefits of US innovation [news release]. Available at: http://www.phrma.org/news_room/press_releases/phrma_statement_regarding_benefits_of_u.s._innovation. Accessed September 7, 2009
- 5.International Trade and Pharmaceuticals Subcommittee on Health Care and Subcommittee on International Trade, Committee on Finance, United States Senate, April 27, 2004 (testimony of William K. Hubbard, Associate Commissioner for Policy and Planning, Food and Drug Administration). Washington, DC: US Department of Health and Human Services; 2004 [Google Scholar]
- 6.Anderson GF, Shea DG, Hussey PS, Keyhani S, Zephyrin L. Doughnut holes and price controls. Health Aff (Millwood) 2004;(suppl web exclusives):W4-396–404 [DOI] [PubMed] [Google Scholar]
- 7.Iglehart JK. The new Medicare prescription-drug benefit—a pure power play. N Engl J Med 2004;350(8):826–833 [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb S. How Obama would stifle drug innovation. Wall Street Journal October 18, 2008 [Google Scholar]
- 9.Henry D, Lexchin J. The pharmaceutical industry as a medicines provider. Lancet 2002;360(9345):1590–1595 [DOI] [PubMed] [Google Scholar]
- 10.Soumerai SB, Pierre-Jacques M, Zhang F, et al. Cost-related medication nonadherence among elderly and disabled Medicare beneficiaries: a national survey 1 year before the Medicare drug benefit. Arch Intern Med 2006;166(17):1829–1835 [DOI] [PubMed] [Google Scholar]
- 11.Light DW, Lexchin J. Foreign free riders and the high price of US medicines. BMJ 2005;331(7522):958–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Electronic Orange Book Washington, DC: Food and Drug Administration; July 2008. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed May 17, 2008 [Google Scholar]
- 13.Barton JH, Emanuel EJ. The patents-based pharmaceutical development process: rationale, problems, and potential reforms. JAMA 2005;294(16):2075–2082 [DOI] [PubMed] [Google Scholar]
- 14.EDGAR Company Search. Available at: http://www.sec.gov/edgar/searchedgar/companysearch.html. Accessed March 20, 2009.
- 15.Peterson CL, Burton R : Service CR, CRS Report for Congress: US Health Care Spending: Comparison with other OECD Countries Washington, DC: US Congress; 2007. Available at: http://assets.opencrs.com/rpts/RL34175_20070917.pdf. Accessed October 20, 2009 [Google Scholar]
- 16.Maynard A, Bloor K. Dilemmas in regulation of the market for pharmaceuticals. Health Aff (Millwood) 2003;22(3):31–41 [DOI] [PubMed] [Google Scholar]
- 17.Sax P. Spending on medicines in Israel in an international context. Isr Med Assoc J 2005;7(5):286–291 [PubMed] [Google Scholar]
- 18.International Macroeconomic Dataset, United States Department of Agriculture Washington, DC: Economic Research Service; 2008 [Google Scholar]
- 19.Reinhardt UE, Hussey PS, Anderson GFUS. Health care spending in an international context. Health Aff (Millwood) 2004;23(3):10–25 [DOI] [PubMed] [Google Scholar]
- 20.Danzon PM, Ketcham JD. Reference pricing of pharmaceuticals for Medicare: evidence from Germany, The Netherlands, and New Zealand. Front Health Policy Res 2004;7:1–54 [DOI] [PubMed] [Google Scholar]
- 21.López GP. Review of the Literature on Reference Pricing Barcelona, Spain: Department of Economics and Business, Universitat Pompeu Fabra; 1999. Working Papers, Research Center on Health and Economics no. 362. Available at: http://ideas.repec.org/p/upf/upfses/362.html. Accessed October 17, 2009 [Google Scholar]
- 22.Ess SM, Schneeweiss S, Szucs TD. European healthcare policies for controlling drug expenditure. Pharmacoeconomics 2003;21(2):89–103 [DOI] [PubMed] [Google Scholar]
- 23.Bloor K, Maynard A, Freemantle N. Lessons from international experience in controlling pharmaceutical expenditure III: regulating industry. BMJ 1996;313(7048):33–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaserud M, Dahlgren AT, Kosters JP, Oxman AD, Ramsay C, Sturm H. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev 2006; (2):CD005979 [DOI] [PubMed] [Google Scholar]
- 25.Vernon JA. Examining the link between price regulation and pharmaceutical R&D investment. Health Econ 2005;14(1):1–16 [DOI] [PubMed] [Google Scholar]
- 26.Profitability Among Pharmaceutical Manufacturers Compared to Other Industries 1995-2004, In: Trends and Indicators in the Changing Health Care Marketplace The Kaiser Family Foundation. Available at: http://www.kff.org/insurance/7031/ti2004-1-21.cfm. Accessed June 20, 2009
- 27. A Comparison of Pharmaceutical Research and Development Spending in Canada and Select Countries. Patented Medicine Prices Review Board; December 2002. PMPRB Study Series S-0217. Available at: http://www.pmprb-cepmb.gc.ca/CMFiles/ss-0217e14HCB-492003-5262.pdf. Accessed September 7, 2009.