Abstract
Objectives. We examined changes in socioeconomic status (SES) and Black to White inequalities in HIV/AIDS mortality in the United States before and after the introduction of highly active antiretroviral therapy (HAART).
Methods. Taking a fundamental cause perspective, we used negative binomial regression to analyze trends in county-level gender-, race-, and age-specific HIV/AIDS mortality rates among those aged 15 to 64 years during the period 1987–2005.
Results. Although HIV/AIDS mortality rates decreased once HAART became available, the declines were not uniformly distributed among population groups. The associations between SES and HIV/AIDS mortality and between race and HIV/AIDS mortality, although present in the pre-HAART period, were significantly greater in the peri- and post-HAART periods, with higher SES and White race associated with the greatest declines in mortality during the post-HAART period.
Conclusions. Our findings support the fundamental cause hypothesis, as the introduction of a life-extending treatment exacerbated inequalities in HIV/AIDS mortality by SES and by race. In addition to a strong focus on factors that improve overall population health, more effective public health interventions and policies would facilitate an equitable distribution of health-enhancing innovations.
As evidenced by the goals explicitly stated in Healthy People 2010, the sizeable and persistent health disparities in the United States are an area of tremendous concern.1 In 2005, all-cause age-adjusted mortality among persons aged 25 to 64 years with fewer than 12 years of education was 215% higher than it was among those in the same age group with 13 or more years of education.2 In 2005, all-cause age-adjusted mortality rates of Blacks in the United States exceeded those of Whites by 29%.2 In the context of these broader disparities, HIV/AIDS disproportionately affects disadvantaged individuals and racial minorities. In 2005, the age-adjusted HIV/AIDS mortality rate per 100 000 population was 782% greater among Blacks (19.4) than it was among Whites (2.2).3
As defined by Braveman, a health inequality or disparity is a
difference in which disadvantaged social groups—such as the poor, racial/ethnic minorities, women, or other groups who have persistently experienced social disadvantage or discrimination—systematically experience worse health or greater health risks than more advantaged social groups.4(p167)
Identifying health inequalities and understanding their root causes are essential prerequisites for eliminating disproportionate disease burdens and achieving health equity across disparate groups. Although the presence of health inequalities across socioeconomic status (SES) and racial/ethnic divisions in the United States is widely acknowledged, further research is needed to identify ways to prevent their occurrence and ameliorate them once entrenched.3
We examined the extent to which SES and racial inequalities in HIV/AIDS mortality in the United States have emerged over time and explore the fundamental cause hypothesis as a contributing explanation for such trends.5 The fundamental social causes theory was developed to explain why social conditions like SES are so reliably associated with mortality across time and place. The association was present in Mulhouse, France, in the early 1800s; Rhode Island in 1865; Chicago, Illinois, in the 1930s; and it occurs in Europe and the United States today.6–10 Given the vast differences in life expectancy, risk factors, diseases, and health care systems characterizing these places and times, the persistence of the SES–mortality association is remarkable. According to fundamental cause theory, mortality follows the SES gradient in a predictable pattern under dissimilar circumstances because SES embodies access to resources—knowledge, money, power, prestige, and beneficial social connections—that can be used in different places and at different times to confer a significant health advantage. The flexible utility of these resources enables the SES–mortality association to emerge in situations with disparate health conditions. For a more complete exposition of the conceptual approach, see Phelan et al.11 and Link and Phelan.5
Fundamental social cause theory asserts that as we learn more about how to prevent or treat diseases, the benefits of this new knowledge are not distributed equally throughout the population but are harnessed more securely by those who are less likely to be exposed to discrimination and who have greater access to knowledge, money, power, prestige, and beneficial social connections. This triggers the formation or exacerbation of health inequalities along typical social cleavages such as SES and race.5 Fundamental social cause theory makes a specific prediction about social inequalities in HIV/AIDS mortality before and after the development and dissemination of highly active antiretroviral therapy (HAART). SES-related resources and racial discrimination are likely to be important at multiple stages in the procurement of life-saving antiretroviral medications. Examples of mechanisms through which SES and race may affect HIV/AIDS mortality are knowing about treatment, living near a location where treatment is provided, having access to health care, receiving the correct diagnosis and optimal treatment upon consulting medical professionals, gaining support to follow through with treatment, and being encouraged by medical teams that interact with people of lower social status equitably and respectfully.
We examined SES and racial inequalities in HIV/AIDS mortality in light of major advances in the capacity to delay death, primarily because of the introduction of HAART following the approval of protease inhibitors by the US Food and Drug Administration in December 199512 and March 1996.13,14 Use of HAART has specifically been linked to declines in morbidity and mortality among persons with HIV/AIDS.15 The fundamental cause hypothesis suggests that these improvements, although they benefit all groups, will benefit persons of high SES and Whites more than they will persons of low SES and Blacks, thereby creating or exacerbating health inequalities over time.
To test the fundamental cause hypothesis, we analyzed trends in county-level HIV/AIDS-specific mortality rates among Black men, White men, Black women, and White women over a 20-year period before, during, and after the introduction of HAART from late 1995 through early 1996. We hypothesized that (1) the association between time and HIV/AIDS mortality would be nonlinear, rising before the introduction of HAART and dropping precipitously after its introduction; (2) the association between SES and HIV/AIDS mortality would be significantly greater during the peri- and post-HAART periods than during the pre-HAART period; and (3) the association between race and HIV/AIDS mortality would be significantly greater during the peri- and post-HAART periods than during the pre-HAART period.
METHODS
We relied on data from compressed mortality files, obtained from the National Center for Health Statistics, to calculate HIV/AIDS mortality rates.16 We determined age-, gender-, and race-specific death rates for US counties for the period 1987 through 2005. We calculated mortality rates by dividing the number of individuals who died from HIV/AIDS in county i and year j by the corresponding population in county i and year j. International Classification of Disease, Ninth Revision17 category codes 042–044 and International Classification of Disease, 10th Revision18 category codes B20–B24 were used to identify HIV/AIDS deaths during the study period. We included data from 99% of counties in our analyses, excluding a few counties with boundary changes during the study period, to create population-based estimates of HIV/AIDS mortality in the United States over time.
We constructed county-level measures of SES with data from the 1980, 1990, and 2000 decennial censuses using the National Historic Geographic Information System.19 We created a 5-item index incorporating key dimensions of SES (education, occupation, income, and poverty) that remained consistent in their definitions across 3 decennial censuses to capture the SES conditions of county i in year j. The 5 index variables were (1) the proportion of persons aged 25 years or older with fewer than 9 years of education, (2) the proportion of persons aged 25 years or older with at least 12 years of education, (3) the proportion of persons aged 16 years or older currently employed in a white collar occupation, (4) the proportion of families at or above the federally defined poverty level, and (5) the proportion of households with access to a telephone. We standardized each individual variable within year and then summed to create a composite SES index for county i and year j. We employed linear interpolation to calculate county- and year-specific values of the SES index for intercensal years. Factor analysis revealed a 5-variable, single-factor solution defining a highly reliable (Cronbach α = 0.89) index. To maximize ease of interpretation, we standardized the composite SES index across all counties and years. Because SES is measured as a continuous variable, our analyses indicate the effect of a 1 SD change in SES.
We also focused on race as an independent variable. To maximize definitional consistency in light of fluctuations in reporting race on death certificates both by states and over time, we analyzed HIV/AIDS deaths attributable to either Blacks or Whites within county i during year j.
We controlled for additional characteristics, including gender (0 = male, 1 = female), age category (15–24, 25–34, 35–44, 45–54, and 55–64 years), and degree of urbanicity, defined as the proportion of persons within county i who reside in urban rather than rural areas.
Individual sociodemographic strata identified by county of residence, race of decedent, gender of decedent, and age at time of death were the units of analysis in this study. Because we sought to analyze count data, we could have relied on Poisson or negative binomial regression strategies. Because of small numbers of cases and the likelihood of zero deaths in some strata, overdispersion, a condition where the variance is greater than the mean, presented a problem for the Poisson approach. Under such circumstances, estimates are inefficient and SEs are biased downward.20,21 Therefore, we used the negative binomial model in analyses, as it includes an additional parameter that accounts for the possibility that the conditional variance of y will exceed its conditional mean.22 We assumed that the number of deaths followed a negative binomial distribution:
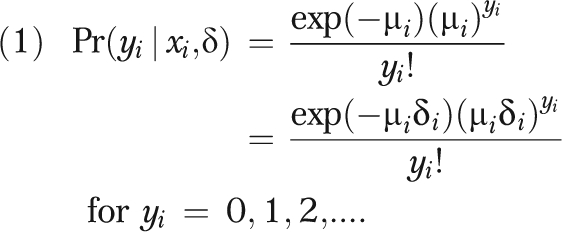 |
where y equals a random variable representing number of times the even occurred during the time period, μ equals a random variable capturing mean birth rate and unobserved heterogeneity (ϵ), and δ equals exponential function e (ϵi).
The death rate (μ) depends on a vector of sociodemographic characteristics (x) shared by individuals within each cell, such that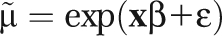 . This strategy allowed us to control SES in the assessment of race effects and race in the assessment of SES effects. We used the maximum likelihood method of estimation to determine the value of the coefficients (βB). To account for heteroskedasticity and the nonindependence of error terms, we calculated robust SEs using the Huber–White correction method and clustered them by county.
. This strategy allowed us to control SES in the assessment of race effects and race in the assessment of SES effects. We used the maximum likelihood method of estimation to determine the value of the coefficients (βB). To account for heteroskedasticity and the nonindependence of error terms, we calculated robust SEs using the Huber–White correction method and clustered them by county.
To test the hypotheses, we identified 3 periods: the pre-HAART period (1987 through 1994), the peri-HAART period (1995 through 1998), and the post-HAART period (1999 through 2005). We used interaction terms to estimate fluctuations in HIV/AIDS mortality across distinct subsets of our study population. Because sensitivity analyses, in which either the composite SES measure was lagged by 1, 2, 3, or 5 years or the study population was limited to states with a sizeable Black population, did not qualitatively change our findings, we present results using the unlagged SES index and include almost all countries.
We conducted all analyses using Stata SE version 10 (StataCorp, College Station, TX) at the county level to examine how HIV/AIDS mortality changed over time by SES and by race and whether these temporal patternings were consistent with predictions under the fundamental cause hypothesis. Aggregate measures of mortality, as opposed to individual probabilities of death, provided several analytic strengths to the current study. First, because HIV/AIDS mortality is concentrated within certain geographic locations, a nationally representative sample of individuals would not have provided a sizeable population at risk. Second, the investigation of aggregate mortality patterns has a fruitful history in such disciplines as epidemiology, demography, sociology, and economics. Much has been and can be gleaned from studies conducted on an aggregate level, provided mechanisms are not assumed to operate similarly on an individual level. Bias in estimation becomes a problem primarily when aggregate measures of predictors, such as SES, are assumed to be a proxy for individual-level characteristics.23,24 Third, counties are small enough geographic units to ensure that a sizeable proportion of residents encounter similar demographic conditions and health risks, yet are large enough that an adequate number of HIV/AIDS deaths are available for detailed analysis. Lastly, unlike census tracts or block groups, county boundaries do not change sizably over time, ensuring that geographic units remain consistent throughout the study period, a concern in longitudinal analyses.
RESULTS
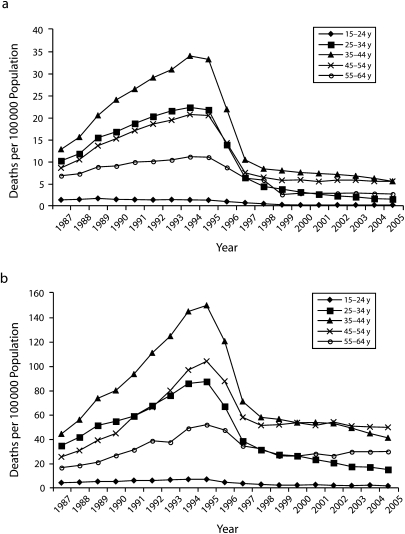
Figure 1 illustrates changes in HIV/AIDS mortality during 1987–2005 for Whites and Blacks aged 15 to 64 years. Consistent with our first hypothesis, there is a strong curvilinear relationship between HIV/AIDS mortality rate and time. For both Whites and Blacks of all age groups, HIV/AIDS death rates increased from 1987 to 1995, declined precipitously beginning in 1996, consistent with the introduction of HAART, and started to plateau around 1998. Negative binomial regression models predicting HIV/AIDS mortality using both a year and year-squared term provide additional support for this curvilinear association (results not shown). Figure 1 also demonstrates that the death rates were substantially higher among Blacks than they were among Whites. Moreover, the decline in mortality rate following the introduction of HAART was more precipitous among Whites than it was among Blacks (Figure 1).
FIGURE 1.
HIV/AIDS deaths per 100 000 population among (a) Whites aged 15 to 64 years and (b) Blacks aged 15 to 64 years, by age group and year: United States, 1987–2005.
Table 1 presents incidence rate ratios (IRRs) and 95% confidence intervals (CIs) from 4 negative binomial regression models predicting HIV/AIDS mortality rates during 1987–2005. Results from model 1 demonstrate that there were significantly fewer HIV/AIDS deaths in the peri- and post-HAART periods than there were in the pre-HAART period, after adjusting for gender, age, and urbanicity. Compared with the pre-HAART period, mortality rates in the peri- and post-HAART periods decreased by 7% and 61%, respectively. Furthermore, both SES and race appear to be significant independent predictors of HIV/AIDS mortality. A 1 SD increase in SES was associated with a 19% reduction in HIV/AIDS deaths, and Blacks exhibited mortality rates over 5 times those of Whites. Thus, according to model 1, the net effects of disadvantaged social statuses, such as lower SES and being Black, placed an individual at a greater risk of dying from HIV/AIDS throughout the study period.
TABLE 1.
Incident Rate Ratios From Negative Binomial Regression Models Predicting HIV Mortality in an Examination of HIV/AIDS Mortality Inequalities: United States, 1987–2005
| Variable | Model 1, IRR (95% CI) | Model 2, IRR (95% CI) | Model 3, IRR (95% CI) | Model 4, IRR (95% CI) |
| County | 0.99 (0.99, 0.99) | 0.99 (0.99, 0.99) | 0.99 (0.99, 0.99) | 0.99 (0.99, 0.99) |
| Peri-HAART period (1995–1998) | 0.93 (0.91, 0.95) | 0.99 (0.96, 1.01) | 0.83 (0.81, 0.85) | 0.87 (0.85, 0.90) |
| Post-HAART period (1999–2005) | 0.39 (0.37, 0.41) | 0.43 (0.41, 0.46) | 0.28 (0.27, 0.29) | 0.31 (0.30, 0.33) |
| SES index | 0.81 (0.74, 0.88) | 0.88 (0.81, 0.96) | 0.80 (0.74, 0.88) | 0.87 (0.80, 0.95) |
| Black | 5.23 (4.88, 5.62) | 5.25 (4.89, 5.63) | 3.77 (3.49, 4.06) | 3.80 (3.52, 4.10) |
| Female | 0.25 (0.24, 0.26) | 0.25 (0.24, 0.26) | 0.25 (0.24, 0.26) | 0.25 (0.24, 0.26) |
| Age category, y | ||||
| 25–34 | 9.94 (9.55, 10.35) | 9.96 (9.57, 10.38) | 9.91 (9.52, 10.33) | 9.93 (9.53, 10.35) |
| 35–44 | 15.37 (14.63, 16.16) | 15.42 (14.67, 16.21) | 15.38 (14.63, 16.17) | 15.42 (14.67, 16.22) |
| 45–54 | 11.65 (11.02, 12.32) | 11.69 (11.06, 12.36) | 11.61 (10.98, 12.27) | 11.64 (11.01, 12.31) |
| 55–64 | 7.81 (7.44, 8.20) | 7.83 (7.45, 8.22) | 7.77 (7.40, 8.17) | 7.79 (7.42, 8.19) |
| Urbanicity | 6.42 (4.72, 8.74) | 6.25 (4.61, 8.47) | 6.56 (4.81, 8.95) | 6.41 (4.72, 8.71) |
| Peri-HAART period × SES | 0.91 (0.89, 0.93) | 0.92 (0.90, 0.94) | ||
| Post-HAART period × SES | 0.80 (0.77, 0.83) | 0.82 (0.79, 0.85) | ||
| Peri-HAART period × Black | 1.42 (1.36, 1.47) | 1.40 (1.35, 1.46) | ||
| Post-HAART period × Black | 2.21 (2.09, 2.35) | 2.16 (2.04, 2.28) | ||
Note. CI = confidence intervals; HAART = highly active antiretroviral therapy; IRR = incidence rate ratio; SES = socioeconomic status. All models adjusted for gender, age, and urbanicity.
In model 2, we tested our second hypothesis by adding 2 interaction terms (peri-HAART period × SES and post-HAART period × SES) to model 1. Both interactions test the extent to which the SES–HIV/AIDS mortality association significantly differed in the period following the widespread introduction of HAART versus the period before it. Results from model 2 indicate that a 1 SD increase in SES was associated with a 12% reduction in HIV/AIDS deaths during the pre-HAART period compared with 20% and 29% reductions during the peri- and post-HAART periods, respectively. Because the IRRs for both interaction terms are significantly below 1.00, our findings are consistent with our second hypothesis, indicating that the SES–HIV/AIDS mortality association became stronger following the introduction of the innovative treatment HAART.
Model 3 includes interaction terms (peri-HAART period × Black and post-HAART period × Black) to test our third hypothesis concerning race and HIV/AIDS mortality in relation to the introduction of HAART. As model 3 shows, being Black was associated with HIV/AIDS mortality, and the significant interaction terms indicate this strong association became even stronger after HAART was the standard of care. Model 3 demonstrates that compared with early epidemic years, once HAART became an established treatment for HIV/AIDS, Black race was associated with significantly greater risks of mortality than was White race.
Model 4 includes multiplicative terms for period and SES index and for period and race. When race and SES interactions are simultaneously entered into the regression analysis, IRRs for all 4 interaction terms remain substantially unchanged and statistically significant (P < .05). Thus, our findings remain robust across different model specifications, suggesting that the relationships between both SES and HIV/AIDS mortality and race and HIV/AIDS mortality vary over time.
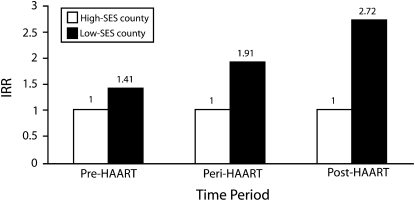
To provide a clear depiction of our key findings, we employed results from model 4 to generate Figure 2 and Figure 3. Figure 2 presents adjusted IRRs comparing HIV/AIDS mortality in a low-SES county at the 95th percentile of SES to a high-SES county at the 5th percentile in the pre-, peri-, and post-HAART periods. During the pre-HAART period, the risk of HIV/AIDS mortality was 1.4 times greater in a low-SES county than it was in a high-SES county. However, in the peri- and post-HAART periods, HIV/AIDS death rates in a county at the 95th percentile of SES were 1.9 and 2.7 times greater than were those in a county at the 5th percentile of SES, respectively. These results are consistent with our second hypothesis, as the HIV/AIDS mortality advantage associated with living in a high- versus low-SES county was significantly greater during the peri- and post-HAART periods than it was during the pre-HAART period.
FIGURE 2.
Incidentrate ratios from negative binomial regression analysis comparing HIV/AIDS mortality among low-SES and high-SES counties during the pre-, peri-, and post-HAART periods: United States, 1987–2005.
Note. HAART = highly active antiretroviral therapy; IRR = incident rate ratio; SES = socioeconomic status. For each period, the results from the model were adjusted for age, gender, and urbanicity. Low-SES counties were at the 95th percentile and high-SES counties were at the 5th percentile (reference group).
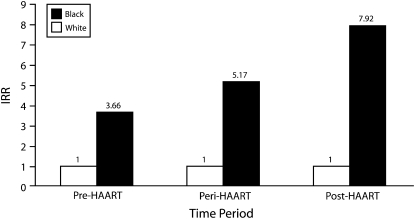
FIGURE 3.
Incidentrate ratios from negative binomial regression analysis comparing HIV/AIDS mortality among Blacks and Whites during the pre-, peri-, and post-HAART periods: United States, 1987–2005.
Note. HAART = highly active antiretroviral therapy; IRR = incident rate ratio. For each period, the results from the model were adjusted for age, gender, and urbanicity. Whites were the reference group.
Figure 3 presents adjusted IRRs comparing HIV/AIDS mortality among Blacks and Whites in the pre-, peri-, and post-HAART periods. During the pre-HAART period, death rates among Blacks were 3.7 times greater than were those among Whites. However, in the peri- and post-HAART periods, death rates among Blacks were over 5 times and almost 8 times greater than were those among Whites, respectively. These findings support our third hypothesis that once HAART is introduced, the risk of HIV/AIDS mortality experienced by Blacks increases relative to that experienced by Whites.
DISCUSSION
Although HIV/AIDS mortality rates decreased among all age and racial groups studied once HAART became available in 1996, IRRs comparing Blacks with Whites and people from low-SES counties to people from high-SES counties actually increased substantially. The SES–HIV/AIDS mortality association, although present in the pre-HAART period, was greater in the peri-HAART period and greater still in the post-HAART period, even when race and other factors were controlled. Similarly, compared with the pre-HAART period, the association between race and mortality became stronger in the peri-HART period and even stronger in the post-HAART period with SES and other factors controlled.
These findings are consistent with fundamental cause theory, which holds that when innovations render a disease more treatable, the benefits of such developments are not evenly distributed. Rather, those less likely to face prejudice and discrimination and those with greater access to resources such as knowledge, money, power, prestige, and beneficial social connections will disproportionately profit from the new treatment. It follows that this disparate capacity to harness the advantages of an innovation can lead to the creation or intensification of health inequalities along established lines of social disadvantage such as SES and race. This is precisely the pattern our results support. During the pre-HAART period, higher SES and White race were each associated with some advantages in HIV/AIDS mortality. However, once HAART, a life-prolonging medical development, became available, we saw significant exacerbations of inequalities in HIV/AIDS mortality by both SES and race.
We focused on health inequalities by comparing the effect of HAART for people from low- versus high-SES counties and for Blacks versus Whites. Another way to examine changes in inequalities over the 3 periods is to note that absolute gaps in HIV/AIDS morality rates between these groups grew much smaller. As HIV/AIDS death rates declined substantially for all groups, the absolute gaps in mortality rates between low- and high-SES counties and between Blacks and Whites also necessarily became smaller. Although this is a positive development, we are concerned about relative rates, which grew much larger between the pre- and post-HAART periods (Figure 1 and 3), because we think policy and practice should address inequalities by ensuring everyone benefits equally from treatments like HAART. Furthermore, if similar patterns are replicated for other lifesaving discoveries, the cumulative effect of the maldistribution of such benefits will leave our society with enduring, perhaps even growing, health inequalities.
Our study joins a rich history of investigations of social determinants of infectious disease mortality dating at least as far back as Virchow's 1840s typhus studies.25 To our knowledge, ours is the first study to test the fundamental cause hypothesis through an in-depth examination of changes over time in mortality owing to an infectious disease. HIV/AIDS in the United States provides an ideal case to explore the interplay of fundamental causes in the mortality disparities trajectory because we can precisely identify when protease inhibitors were approved by the US Food and Drug Administration and because HAART was found to be extremely effective at delaying HIV/AIDS deaths.
There are a number of limitations to this study. First, identification of HIV/AIDS deaths may pose challenges because the definition of the disease and accuracy of diagnosis evolved over time. However, the most substantial of these changes occurred early in the epidemic26,27 and are unlikely to have affected our findings concerning the transition from the pre- to post-HAART periods. Additionally, there is evidence of underdiagnosis and underreporting of HIV/AIDS as a cause of death among disadvantaged populations, particularly among Blacks.28,29 If underreporting of HIV/AIDS deaths occurred among minorities or persons within lower-SES counties, our results may underestimate true levels of mortality among these groups. Because such bias is likely to be consistent over the study period and across multiple demographic measures used to calculate mortality rates, it is unlikely that our findings have been systematically distorted. Another potential limitation is related to the transition between the ninth and 10th revisions of the International Classification of Disease codes that occurred during the study period. However, such a transition would contribute to biased results only if counties differentially instituted changes in reporting policies, which is unlikely given the uniformity of death certificate reporting. Finally, we were not able to incorporate data at both the individual and aggregate level. However, given that this is the first study to examine how the introduction of HAART affected mortality inequalities from a fundamental cause perspective and county-specific conditions are likely to affect access to resources, there is a good rationale for using aggregate data to test our hypotheses. In future studies, it will be important to see if our findings are replicated with individual-level data.
These results demonstrate that inequalities in HIV/AIDS mortality rates by SES and race were greater in the peri- and post-HAART periods than they were in the pre-HAART period. The findings are consistent with fundamental cause theory, as inequalities worsened once a life-extending treatment became available. Therefore, public health interventions and policies are needed to facilitate a more equitable distribution of health-enhancing innovations. Such policies can build on existing programs such as Medicaid and Ryan White programs, without which inequalities in HIV/AIDS mortality are likely to have been worse. It is also important to determine what puts groups and individuals at risk for being exposed to conditions that have deleterious effects on health and to target interventions so that those with fewer resources are more likely to know of, have access to, and benefit from life-extending treatments. Pursing structural and contextualized interventions to prevent and address inequalities in HIV/AIDS mortality is essential for achieving health equity in the United States.
Acknowledgments
The authors thank the Robert Wood Johnson Foundation Health and Society Scholars program for its financial support. The authors also thank the Centers for Disease Control and Prevention for its financial support of this article through Public Health Dissertation Research funding (grant 1R36SH000004-01).
Note. The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of Centers for Disease Control and Prevention.
Human Participant Protection
The Columbia University institutional review board declared this study exempt.
References
- 1.Healthy People 2010: Understanding and Improving Health Washington, DC: US Department of Health and Human Services; 2000. Available at: http://web.health.gov/healthypeople/document. Accessed July 1, 2009 [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu JQ, et al. Deaths: final data for 2005. Natl Vital Stat Rep 2008;56(10):1–120 [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality National Healthcare Disparities Report Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; December 2006. AHRQ Pub. No. 07-0012 [Google Scholar]
- 4.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health 2006;27:167–194 [DOI] [PubMed] [Google Scholar]
- 5.Link BG, Phelan JC. Social conditions as fundamental causes of disease. J Health Soc Behav 1995;Spec No:80–94 [PubMed] [Google Scholar]
- 6.Villerme L. Tableau d' etat physique et moral des ouvries Vol 2 Paris: Jules Renouard et Cie; 1840 [Google Scholar]
- 7.Antonovsky A. Social class, life expectancy, and overall mortality. Milbank Mem Fund Q 1967;45(2):31–73 [PubMed] [Google Scholar]
- 8.Sorlie PD, Backlund M, Keller JB. US mortality by economic, demographic, and social characteristics: the National Longitudinal Mortality Study. Am J Public Health 1995;85(7):949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lantz PM, House JS, Lepowski JM, et al. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA 1998;279(21):1703–1708 [DOI] [PubMed] [Google Scholar]
- 10.Kunst AE, Groenhof F, Mackenbach JB, et al. Occupational class and cause-specific mortality in middle-aged men in 11 European countries: comparison of population-based studies. EU Working Group on Socioeconomic Inequalities in Health. BMJ 1998;316(7145):1636–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelan JC, Link BG, Diez-Roux A, et al. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav 2004;45(3):265–285 [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration FDA Approves First Protease Inhibitor Drug for Treatment of HIV Silver Spring, MD: Food and Drug Administration; December 7, 1995. P95-10 [Google Scholar]
- 13.Food and Drug Administration FDA Approves Second Protease Inhibitor to Treat HIV Silver Spring, MD: Food and Drug Administration; March 1, 1996. P96-4 [Google Scholar]
- 14.Food and Drug Administration FDA Grants Accelerated Approval to Third Protease Inhibitor to Treat HIV Silver Spring, MD: Food and Drug Administration; March 14, 1996. P96-5 [Google Scholar]
- 15.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853–860 [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics Compressed mortality file. Available at: http://www.cdc.gov/nchs/products/elec_prods/subject/mcompres.htm. Published 2008. Accessed August 30, 2009
- 17.International Classification of Diseases, Ninth Revision Geneva, Switzerland: World Health Organization; 1980 [Google Scholar]
- 18.International Classification of Diseases, 10th Revision Geneva, Switzerland: World Health Organization; 1990. Available at: http://www.who.int/classifications/icd/en. Accessed August 30, 2009 [Google Scholar]
- 19.National Geographic Information System, Minnesota Population Center, University of Minnesota. NHGIS project description Web site. Available at: http://www.nhgis.org/project-information. Accessed August 30, 2009
- 20.Cameron AC, Trivedi PK. Econometric models based on count data: comparisons and applications of some estimators and tests. J Appl Econ 1986;1(1):29–53 [Google Scholar]
- 21.Gourieroux C, Monfort A, Trognon A. Pseudo maximum likelihood methods: applications to Poisson models. Econometrica 1984;52(3):701–720 [Google Scholar]
- 22.Long JS. Regression Models for Categorical and Limited Dependent Variables. Advanced Quantitative Techniques in the Social Sciences Thousand Oaks, CA: Sage Publications; 1997 [Google Scholar]
- 23.Geronimus AT. Invited commentary: using area-based socioeconomic measures—think conceptually, act cautiously. Am J Epidemiol 2006;164(9):835–840 [DOI] [PubMed] [Google Scholar]
- 24.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol 1998;148(5):475–486 [DOI] [PubMed] [Google Scholar]
- 25.Virchow RC. Report on the typhus epidemic in Upper Silesia. 1848. Am J Public Health 2006;96(12):2102–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep 1987;36(suppl 1):1S–15S [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep 1992;41(RR-17):1–19 [PubMed] [Google Scholar]
- 28.Lindan CP, Hearst N, Singleton JA, et al. Underreporting of minority AIDS deaths in San Francisco Bay area, 1985–86. Public Health Rep 1990;105(4):400–404 [PMC free article] [PubMed] [Google Scholar]
- 29.Scheer S, McQuitty M, Denning P, et al. Undiagnosed and unreported AIDS deaths: results from the San Francisco Medical Examiner. J Acquir Immune Defic Syndr 2001;27(5):467–471 [DOI] [PubMed] [Google Scholar]