Abstract
Low (≤35%) or absent expression of the complex ‘b’ pathway gangliosides GD1b, GT1b and GQ1b (CbG) correlates with an aggressive biological phenotype in human neuroblastoma tumors. To develop an in vitro model to probe mechanisms by which CbG may contribute to neuroblastoma behavior, we have comprehensively evaluated ganglioside expression in nine well-established human neuroblastoma cell lines, all derived from poor prognosis tumors. Total cellular ganglioside content ranged from 8 to 69 nmol/108 cells. High performance thin layer chromatography revealed that the simple disialoganglioside GD2 was prominent in eight of the cell lines (up to 60% of total gangliosides), whereas CbG were low (1–21%) in all nine cell lines. The structurally most complex ‘b’ pathway species, GQ1b, was not detected in any of the cell lines. The prominence of GD2 in neuroblastoma cell lines mirrors the high expression of GD2 that characterizes human neuroblastoma tumors, and the low CbG expression in the cell lines is analogous to that found in clinically and biologically unfavorable neuroblastoma tumors, thus establishing these neuroblastoma cell lines as valuable model systems for study of the role of CbG in the pathobiology of human neuroblastoma.
Keywords: Ganglioside, Neuroblastoma, Glycosphingolipid
Gangliosides, membrane-bound glycosphingolipid molecules that are frequently aberrantly expressed in tumors, have been implicated in the biology of various cellular processes, and linked to the behavior of many types of tumors [1], Extensive studies by ourselves and others have established the diagnostic and prognostic potential of specific ganglioside changes in neuroblastoma, a pediatric malignancy of neural crest origin [2–6].
Ganglioside molecules consist of a sialic acid-containing carbohydrate portion and a hydrophobic lipid backbone (ceramide), embedded in the outer leaflet of the cell membrane [7]. Ganglioside biosynthesis occurs as a set of stepwise glycosylations via two main pathways designated ‘a’ (GM2, GM1a, GD1a) and ‘b’ (GD3, GD2, GD1b, GT1b, GQ1b), from a common precursor (GM3) derived from lactosylceramide (Fig. 1) [8].
Fig. 1.
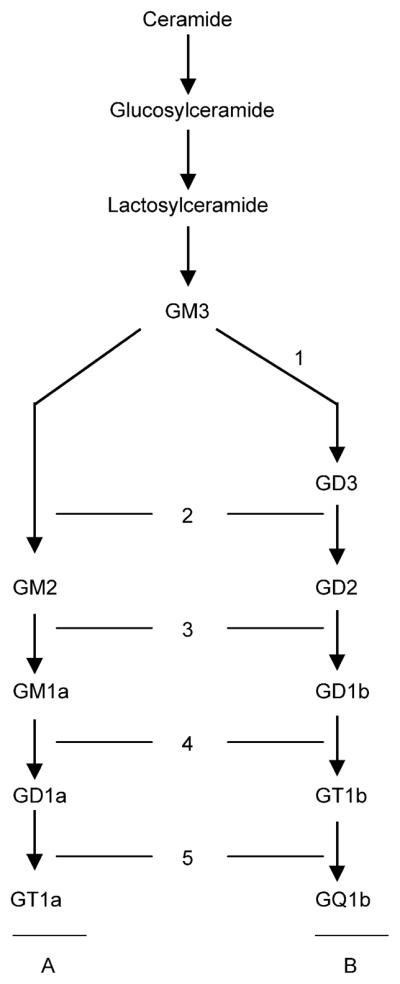
Schematic representation of the major pathways of ganglioside biosynthesis. The monosialoganglioside GM3, derived from lactosylceramide, is the common precursor for both ‘a’ and ‘b’ pathway gangliosides. Each ganglioside species consists of a ceramide backbone, and a carbohydrate chain containing one or more sialic acid residues. Parallel steps in both pathways are catalyzed by the same glycosyltransferases of the Golgi apparatus: (1) GD3 synthase (α-2,8-sialyltransferase), (2) GM2/GD2 synthase (β-1,4-GalNAc-transferase), (3) GD1b/GM1a synthase (β-1,3-galactosyl-transferase), (4) GT1b/GD1a synthase (α-2,3-sialyltransferase), (5) GQ1b/GT1a synthase (α-2,8-sialyltransferase). ‘a’ and ‘b’ pathway gangliosides downstream of GD1b/GM1a synthase (3) are designated complex ‘a’(CaG) and complex ‘b’(CbG) gangliosides, respectively.
Human neuroblastoma consists of distinct disease categories, with unfavorable disease marked by early dissemination, older age at diagnosis and biological anomalies such as MYCN amplification, loss of heterozygosity (LOH) for chromosome 1p, aneuploid karyotype, and expression of the receptor for nerve growth factor, TrkA [9]. Previous studies have defined the ganglioside carbohydrate species that are expressed and shed by neuroblastoma cells, and strongly support a link between expression of certain ganglioside molecules and the behavior of this tumor [2–6,10–12]. Firstly, overexpression of GD2 distinguishes neuroblastoma from benign neural tumors [10]. Secondly, high total ‘b’ pathway ganglioside levels (GD3, GD2, GD1b, GT1b, GQ1b) characterize ganglioside expression in infant neuroblastoma compared to disease in older children [5]. Thirdly, low CbG expression (GD1b, GT1b, and most dramatically, GQ1b) characterizes progressive versus non-progressive neuroblastoma tumors and completely accounts for the lower overall ‘b’ pathway ganglioside expression in unfavorable tumors. Differences in cellular CbG content correlate with patient outcome, and expression of ≥35% CbG in neuroblastoma tissue strongly predicts favorable outcome. Among patients expressing multiple tumor markers that suggest a favorable prognosis (MYCN not amplified and aneuploid DNA), event-free survival is lower when tumors exhibit low CbG content (<35%). This permits further stratification of patients classified as ‘good prognosis’ by established markers and identifies patients at very low risk of relapse or death [6]. Studies of others, in astrocytoma and medulloblastoma, have also described an association between a progressive loss of CbG, higher histological grades, and lower survival [13–15].
Since a number of biological properties that could conceivably alter tumor host interactions and cell survival have been attributed to gangliosides [16–18], we speculate that ganglioside changes in neuroblastoma may play an important functional role in the progression of this tumor. To identify an in vitro system that would permit study of complex ‘b’ pathway ganglioside synthesis and its biological consequences in neuroblastoma, we have analyzed ganglioside expression in nine human neuroblastoma cell lines (LAN-1, LAN-5, IMR-32, SK-N-SH, SHSY-5Y, SMS-KCN, SMS-KCNR, SMS-KAN, and SMS-KANR), characterizing their total ganglioside content and ganglioside class, as a means to establish the basis for the use of these cell lines as in vitro model systems to study the role of neuroblastoma tumor cell gangliosides in the pathogenesis of this tumor.
1. Materials and methods
1.1. Cell culture
Ganglioside expression was studied in human neuroblastoma cell lines LAN-1, LAN-5, IMR-32, SHSY-5Y, SK-N-SH, SMS-KCN, SMS-KCNR, SMS-KAN and SMS-KANR. LAN-1 and LAN-5 were maintained in Waymouth’s MB 752/1 medium (GIBCO, Grand Island, NY, USA), SMS-KCN, SMS-KCNR, SMS-KAN and SMS-KANR in RPMI 1640 medium (GIBCO, Grand Island, NY, USA), IMR-32 and SK-N-SH in MEM medium (BioWhittaker, Walkersville, MD), and SHSY-5Y in DMEM medium (BioWhittaker, Walkersville, MD). Medium was supplemented with 2 mM L-glutamine and 10% heat-inactivated fetal calf serum (GIBCO, Grand Island, NY, USA), respectively. Cells were grown in adherent monolayer cultures using 75-cm2 and 175-cm2 cell culture flasks (Becton Dickinson, Franklin Lakes, NJ, USA) at 37 °C in a 5% CO2 atmosphere in a humidified incubator. Viable cells were counted using trypan blue dye exclusion and the cellular protein content was estimated by the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) using a bovine serum albumin standard.
1.2. Ganglioside purification and quantification
Methods for extraction and purification of gangliosides were previously described [19]. Briefly, total lipid extracts of cell pellets were obtained by extracting the lyophilized starting material twice with chloroform/methanol (1:1 v/v) at 4 °C with stirring. Gangliosides were isolated by partitioning the dried total lipid extract in diisopropyl ether/1-butanol/17 mM aqueous NaCl (6:4:5 v/v). Gangliosides, in the lower aqueous phase, were further purified by Sephadex G-50 gel filtration. HPTLC analysis of gangliosides [7] was performed using 10×10- or 10×20-cm precoated silica gel HPTLC plates (Merck, Darmstadt, Germany). The plates were developed in chloroform/methanol/0.25% CaCl2·H2-O (60:40:9 by volume) and gangliosides were stained with resorcinol.
Absolute cellular ganglioside content was determined by HPTLC densitometry using known concentrations of human brain gangliosides as the standard. Individual gangliosides were identified using a human brain ganglioside standard. To categorize individual ganglioside species, we used a previously established classification scheme based on biosynthetic pathways [6]. Structurally complex molecules downstream of GD1b/GM1a synthase (β-1,3-galactosyltransferase) were designated complex ‘a’ gangliosides (CaG: GM1a, GD1a and GT1a) and complex ‘b’ gangliosides (CbG: GD1b, GT1b and GQ1b) (Fig. 1).
2. Results
The neuroblastoma cell lines studied were derived from tumors of six children with disseminated disease and have been previously described. IMR-32 [20–24], SMS-KCN [22,25], and SMS-KAN [9,22,25,26] were established from an untreated primary tumor at the time of first diagnosis. In contrast, LAN-1 [22,24,27, 28], LAN-5 [29–31], SK-N-SH [20,22–24,32,33], SMS-KCNR [22,25], and SMS-KCNR [22,25] were derived from the bone marrow of patients whose disease had progressed despite prior treatment. Two sets of paired cell lines, SMS-KCN and SMS-KCNR, and SMS-KAN and SMS-KANR were each established from a single patient, from the primary tumor at diagnosis and the bone marrow at relapse, respectively [25]. The cell line SHSY-5Y [22,28,34] is a subclone of SK-N-SH [20]. Table 1 summarizes the clinical background for each of the uniformly fatal tumors that originally gave rise to the cell lines.
Table 1.
Patient and tumor tissue characteristics
| Cell line | Organ of origin | Initiation of culture | Age | Sex | Treatmenta | Reference |
|---|---|---|---|---|---|---|
| LAN-1 | Bone marrow | 5 months post dxb | 2 years | Male | CT | [27] |
| LAN-5 | Bone marrow | No published data available | [29] | |||
| IMR-32 | Primary (abdomen) | First dx | 13 months | Male | None | [21] |
| SHSY-5Y | Bone marrow | Subclone of SK-N-SH | [34] | |||
| SK-N-SH | Bone marrow | 7 months post dx | 4 years | Female | CT, RT | [33] |
| SMS-KCNc | Primary (adrenal) | First dx | 11 months | Male | None | [25] |
| SMS-KCNRc | Bone marrow | 2 months post dx | 11 months | Male | CT | [25] |
| SMS-KANd | Primary (pelvic) | First dx | 3 years | Female | None | [25] |
| SMS-KANRd | Bone marrow | 8 months post dx | 3 years | Female | CT, RT | [25] |
Prior to initiation of cell culture. CT, chemotherapy; RT, radiation therapy.
dx, diagnosis.
SMS-KCN and SMS-KCNR were derived from the same patient at the times of diagnosis and progression of disease, respectively.
SMS-KAN and SMS-KANR were derived from the same patient at the times of diagnosis and progression of disease, respectively.
Biological features such as MYCN amplification, karyotype and chromosome 1p LOH provide a measure of the inherent malignant potential of neuroblastoma cells (Table 2) [9]. All cell lines studied, except for SK-N-SH and SHSY-5Y, were characterized by unfavorable MYCN [23,25,28,30,32] and 1p status [20,24,25]. Similarly, all neuroblastoma cell lines studied have been associated with an unfavorable karyotype [24,25]. IMR-32, SK-N-SH and SHSY-5Y are near diploid with 1–3 extra chromosomes. SMS-KCN, SMS-KCNR, SMS-KAN and SMS-KANR are diploid, and LAN-1 cells are near tetraploid. Overall, the poor biological marker status of neuroblastoma cell lines is consistent with the unfavorable clinical features that characterized the tumors from which they arose. From a morphological perspective, neuroblastoma cell lines can be classified as neuroblastic (N-type) and substrate-adherent (S-type) according to their appearance in vitro [22]. Neuroblastic cells have a small, round cell body that may have neuritic processes, while S-type cells present with epithelial or fibroblast-like morphology. All the cell lines studied uniformly exhibited N-type morphology except for SMS-KCN, which consisted of both N- and S-type cells.
Table 2.
Morphology and molecular characteristics of the human neuroblastoma cell lines studied
| Cell line | Morphology | MYCNa | Karyotypeb | LOH 1pc | References |
|---|---|---|---|---|---|
| LAN-1 | N | Amp | 87 | + | [22,24,27,28] |
| LAN-5 | Nd | Amp | [29–31] | ||
| IMR-32 | Ne | Amp (15–20) | 49 | + | [20–24] |
| SK-N-SH | N | Not amp (1–2) | 47 | − | [20,22–24,32,33] |
| SHSY-5Y | N | Not amp (1–2) | 47 | [22,28,34] | |
| SMS-KCN | N/S | Amp | 46 | + | [22,25] |
| SMS-KCNR | N | Amp | 46 | + | [22,25] |
| SMS-KAN | N | Amp (150) | 46 | + | [9,22,25,26] |
| SMS-KANR | N | Amp | 46 | + | [22,25] |
Amplification of the proto-oncogene MYCN (copy number).
Modal number of chromosomes.
Chromosome 1p LOH.
Neuroblastic appearance in culture, but no published data available.
Uniformly neuroblastic appearance in culture, while literature reports both N-type and S-type cells [12].
Numerous biological and clinical factors (histology, clinical stage, MYCN amplification, TrkA expression, chromosome 1p LOH, ploidy) can be used to predict outcome and assign treatment in neuroblastoma. However, even the use of combinations of prognostic factors cannot predict outcome with certainty. We have recently found that using tumor ganglioside expression, patients assigned a risk category based upon standard factors can be further substratified on the basis of tumor complex ‘b’ pathway ganglioside (CbG) expression [6]. Therefore, this work was focused on the investigation of total content and composition of the ganglioside complement in neuroblastoma cell lines, with particular focus on the expression of the CbG.
While there is some degree of variation between the ganglioside patterns of different neuroblastoma cell lines, quantitative dissection of relative ganglioside expression reveals several important similarities (Table 3). The ‘b’ pathway disialoganglioside GD2 is a prominent ganglioside component (18–60%) in eight out of nine cell lines studied, mirroring the typically high expression of GD2 that characterizes neuroblastoma tumors [10]. Further, complex ‘b’ pathway gangliosides (CbG) account for less than 35% of total gangliosides (1–22%) in all nine cell lines, with no detectable amounts of the most complex molecule, GQ1b, found in any of the cell lines studied.
Table 3.
Ganglioside class composition of human neuroblastoma cell lines
| % of total gangliosidesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM3 | GM2 | GM1a | GD1a | GD3 | GD2 | GD1b | GT1b | GQ1b | CbGb | |
| LAN-1 | 2±1 | 44±12 | 1±0 | 0±0 | 20±4 | 32±7 | 2±1 | 2±1 | 0±0 | 3±2 |
| LAN-5 | 0±0 | 35±8 | 2±1 | 2±0 | 8±3 | 44±5 | 2±1 | 8±2 | 0±0 | 10±3 |
| IMR-32 | 0±0 | 17±3 | 9±2 | 3±1 | 17±1 | 42±5 | 5±1 | 7±1 | 0±0 | 12±2 |
| SK-N-SH | 4±3 | 38±12 | 13±4 | 35±15 | 3±1 | 6±2 | 0±0 | 1±1 | 0±0 | 1±1 |
| SHSY-5Y | 0±0 | 25±8 | 4±2 | 44±6 | 5±3 | 18±6 | 1±1 | 3±2 | 0±0 | 4±3 |
| SMS-KCN | 1±0 | 34±5 | 2±1 | 17±5 | 1±0 | 26±4 | 2±1 | 19±3 | 0±0 | 21±4 |
| SMS-KCNR | 0±0 | 22±4 | 1±0 | 3±0 | 7±2 | 60±6 | 2±1 | 7±3 | 0±0 | 8±3 |
| SMS-KAN | 0±0 | 21±2 | 6±2 | 24±4 | 2±1 | 31±3 | 3±0 | 13±2 | 0±0 | 15±2 |
| SMS-KANR | 0±0 | 24±2 | 6±1 | 36±5 | 1±0 | 22±1 | 1±1 | 9±1 | 0±0 | 10±1 |
Values represent mean ± SEM of three separate experiments for each cell line, except for LAN-5 (n=5), LAN-1 (n=4), and IMR-32 (n=2).
CbG=GD1b+GT1b+GQ1b.
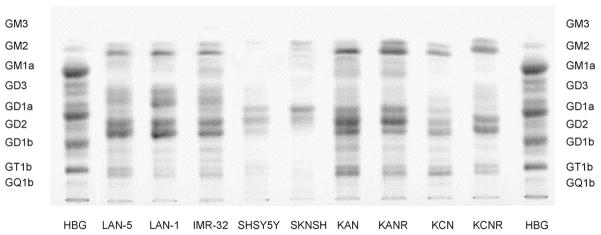
Fig. 2 illustrates differences in ganglioside pattern among the nine cell lines. The predominance of GD2 is evident in all cell lines except SHSY-5Y and SK-N-SH. The relative lack of GD1b and the complete absence of GQ1b are easily discernible in all nine cell lines. The content of GT1b appears to be more variable among the cell lines, with the highest content in the SMS-KAN and SMS-KCN pairs. In each cell line, however, the total content of CbG is well below 35%, the clinical demarcation between good and poor prognosis tumors [6]. Interestingly, considering the two paired cell lines included in this study, SMS-KCN/SMS-KCNR and SMS-KAN/SMS-KANR, Fig. 2 also highlights that the cell lines of the pairs that were established at the time of progression are characterized by lower CbG levels than are those established from the initial diagnostic specimens. This offers the tantalizing suggestion that tumor ganglioside expression may change in vivo, and provides further support for the concept that reduced CbG expression is a feature of a more aggressive tumor phenotype. Parenthetically, complex ‘a’ gangliosides (CaG), synthesized by the same enzymes responsible for CbG synthesis, are more variable (1–48%) than CbG. We conclude that low CbG expression in neuroblastoma cell lines is concordant with the unfavorable malignant phenotype of these cells, as evidenced by their unfavorable clinical background and molecular marker status (Tables 1 and 2).
Fig. 2.
Ganglioside expression in nine human NB cell lines (LAN-1, LAN-5, IMR-32, SK-N-SH, SHSY-5Y, SMS-KCN, SMS-KCNR, SMS-KAN, SMS-KANR). Human brain gangliosides (HBG) were employed as a standard. Most tumor ganglioside bands appear as doublets due to ceramide structural heterogeneity. The lower GD1a band and the upper GD2 band overlap.
To complement our findings on cellular ganglioside composition, we also determined total ganglioside content by HPTLC densitometry, which allowed calculation of absolute levels of individual gangliosides (Table 4). Providing an internal control for the validity of our findings, the total ganglioside content of LAN-1, LAN-5 and SHSY-5Y cells that we report here are consistent with previously published data [35,36]. We observed that total cellular ganglioside content differs widely between the neuroblastoma cell lines studied (8–69 nmol/108 cells). The striking finding of our analysis is that, in eight of the nine cell lines, the level of CbG was less than 6 nmol/108 cells (range 0.25–5.7), and even in the ninth cell line, SMS-KCN, CbG content was only 14.5 nmol/108 cells.
Table 4.
Absolute ganglioside content of human neuroblastoma cell lines
| Cell line | Total | GD2a | CbGa | CaGa |
|---|---|---|---|---|
| LAN-1 | 30b | 9.6 | 1.2 | 0.3 |
| LAN-5 | 57 | 25.1 | 5.7 | 2.3 |
| IMR-32 | 23 | 9.7 | 2.8 | 2.8 |
| SK-N-SH | 25 | 1.5 | 0.3 | 12.0 |
| SHSY-5Y | 8 | 1.4 | 0.3 | 3.8 |
| SMS-KCN | 69 | 17.9 | 14.5 | 13.1 |
| SMS-KCNR | 15 | 9.0 | 1.2 | 0.6 |
| SMS-KAN | 32 | 9.9 | 4.8 | 9.6 |
| SMS-KANR | 31 | 6.8 | 3.1 | 13.0 |
Absolute cellular content of GD2, CbG and CaG was calculated from total cellular ganglioside content, and data on relative ganglioside composition in Table 3.
nmol gangliosides/108 cells.
This study demonstrates that complex ‘b’ pathway gangliosides (CbG) in nine well-established neuroblastoma cell lines account for less than 15% of total cellular gangliosides, with no detectable amounts of the most complex molecule, GQ1b, expressed in any of the cell lines studied. We conclude that low CbG expression in human neuroblastoma cell lines is analogous to that observed in clinically and biologically unfavorable neuroblastoma tumors [6].
3. Discussion
Gangliosides represent a diverse group of molecules, consisting of a carbohydrate chain attached to a lipid backbone (ceramide) in the cell membrane [7]. Marked structural differences in tumor tissue compared to normal tissue include both the carbohydrate and the lipid portion. Because of our previous findings establishing the prognostic value of certain carbohydrate species in neuroblastoma [5,6] we focused this study on carbohydrate structural heterogeneity of neuroblastoma cell line gangliosides.
Tumor gangliosides are generally believed to favor tumor progression, and co-incubation of tumor cells with exogenous gangliosides has been shown to increase tumorigenicity in vivo [37]. However, the biological effects of tumor-derived gangliosides may differ based upon structural complexity. Published studies suggest that complex ganglioside species, compared to structurally simpler gangliosides, may be involved in modulating certain cellular functions that could potentially ameliorate the phenotype of a tumor cell carrying those molecules. For example, proliferation of, and IL-8 production by human metastatic melanoma cells is inhibited by GD1b, GT1b and GQ1b [16]. Neuronal differentiation in vitro can be induced by exposure of neuroblastoma cells to exogenous gangliosides, most potently GT1b and GQ1b [38,39]. A shift from Th-2 to Th-1 cytokine production in PHA stimulated T-cells can be caused by CbG [17]. Increased expression of GD1b and GT1b in rat PC 12 pheochromocytoma cells transfected with GD3 synthase occurs in parallel with TrkA dimerization [18], and the CaG molecule GM1a augments the effects of nerve growth factor in these cells by enhancing activation of its high-affinity receptor, TrkA [40,41]. Taking these results together, we hypothesize that CbG may function not only as tumor markers, but may also serve a functional role in tumor progression.
The establishment of in vitro and in vivo human neuroblastoma tumor models is crucial to the study of the role of complex gangliosides in tumor progression, and their mechanism of action. Specifically, the pharmacologic or genetic modulation of cellular ganglioside content would make possible the study of the action of specific gangliosides and ganglioside subclasses (i.e. CbG). Several approaches can be considered. For example, retinoic acid treatment of human neuroblastoma cell lines reveals cellular differentiation, enhancement of total ganglioside expression [36], and enhanced expression of CbG [42]. Retinoic acid not only causes cellular differentiation in vitro, but is also used in the maintenance therapy of neuroblastoma. However, the potential role of gangliosides, particularly complex gangliosides, in mediating the cellular effects of retinoic acid have, to date, not been considered, and these cell lines we have characterized here may be excellent models for such studies. Second, abrogation of cellular ganglioside synthesis with the inhibitor of glucosylceramide synthase, D-l-threo-1-phenyl-2-palmitoylamino-3-pyrrolidino-1-propanol-HCL (PPPP), has been shown in P19 murine embryonal carcinoma, DAOY human medulloblastoma, and PC12 rat pheochromocytoma cells, to result in abrogation of cellular ganglioside synthesis without cellular toxicity [43–45]. Use of this inhibitor to deplete the cell lines we have characterized here of total gangliosides would permit ‘add-back’ of individual complex gangliosides in vitro to assess their specific effects on cell processes known to be important to tumor cell pathogenesis (i.e. proliferation, differentiation, adhesion). Finally, while numerous studies have resulted in successful transfection of the genes for ganglioside synthases upstream of the complex gangliosides, to date, there have been no published studies of the successful transfection of genes for the enzymes responsible for the synthesis of complex gangliosides (GD1b synthase, GT1b synthase, GQ1b synthase). Given the overall low expression of complex gangliosides in the nine cell lines studied here, these cell lines would seem to be ideal targets for transfection of these specific ganglioside synthases. Cells overexpressing complex gangliosides could then be employed in both in vitro and in vivo studies of neuroblastoma.
Acknowledgments
We thank Drs. Robert Seeger, Patrick Reynolds, and M. Susan D’Orisio, for kindly providing the human neuroblastoma cell lines used in these studies.
Abbreviations
- CaG
complex ‘a’ pathway gangliosides
- CbG
complex ‘b’ pathway gangliosides
- HBG
human brain gangliosides
- HPTLC
high-performance thin layer chromatography
- LOH
loss of heterozygosity
Footnotes
Supported by National Institutes of Health Grants CA-90362 (K.K.), CA-42361 (S.L.) and by The Children’s Cancer Foundation, Baltimore, MD. Dr Hettmer was the recipient of a fellowship by the Dr Mildred Scheel Stiftung/Deutsche Krebshilfe.
References
- 1.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 2.Schengrund CL, Repman MA, Shochat SJ. Ganglioside composition of human neuroblastomas. Correlation with prognosis. A Pediatric Oncology Group Study. Cancer. 1985;56:2640–2646. doi: 10.1002/1097-0142(19851201)56:11<2640::aid-cncr2820561118>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Schengrund CL, Shochat SJ. Gangliosides in neuroblastomas. Neurochem Pathol. 1988;8:189–202. doi: 10.1007/BF03160146. [DOI] [PubMed] [Google Scholar]
- 4.Shochat SJ, Corbelletta NL, Repman MA, Schengrund CL. A biochemical analysis of thoracic neuroblastomas: a Pediatric Oncology Group study. J Pediatr Surg. 1987;22:660–664. doi: 10.1016/s0022-3468(87)80122-7. [DOI] [PubMed] [Google Scholar]
- 5.Kaucic K, Etue N, LaFleur B, Woods W, Ladisch S. Neuroblastomas of infancy exhibit a characteristic ganglioside pattern. Cancer. 2001;91:785–793. doi: 10.1002/1097-0142(20010215)91:4<785::aid-cncr1065>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Hettmer S, Malott C, Woods W, Ladisch S, Kaucic K. Biological stratification of human neuroblastoma by complex ‘B’ pathway ganglioside expression. Cancer Res. 2003;63:7270–7276. [PubMed] [Google Scholar]
- 7.Ledeen RW, Yu RK. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- 8.van Echten G, Sandhoff K. Ganglioside metabolism. enzymology, topology, and regulation. J Biol Chem. 1993;268:5341–5344. [PubMed] [Google Scholar]
- 9.Brodeur GM. Molecular basis for heterogeneity in human neuroblastomas. Eur J Cancer. 1995;31A:505–510. doi: 10.1016/0959-8049(95)00040-p. [DOI] [PubMed] [Google Scholar]
- 10.Wu ZL, Schwartz E, Seeger R, Ladisch S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986;46:440–443. [PubMed] [Google Scholar]
- 11.Yogeeswaran G, Murray RK, Pearson ML, Sanwal BD, McMorris FA, Ruddle FH. Glycosphingolipids of clonal lines of mouse neuroblastoma and neuroblastoma X L cell hybrids. J Biol Chem. 1973;248:1231–1239. [PubMed] [Google Scholar]
- 12.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–1567. [PubMed] [Google Scholar]
- 13.Sung CC, Pearl DK, Coons SW, Scheithauer BW, Johnson PC, Zheng M, et al. Correlation of ganglioside patterns of primary brain tumors with survival. Cancer. 1995;75:851–859. doi: 10.1002/1097-0142(19950201)75:3<851::aid-cncr2820750317>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Yates AJ, Comas T, Scheithauer BW, Burger PC, Pearl DK. Glycolipid markers of astrocytomas and oligodendrogliomas. J Neuropathol Exp Neurol. 1999;58:1250–1262. doi: 10.1097/00005072-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Yates AJ, Franklin TK, McKinney P, Collins R, Comas T, Boesel CP, et al. Gangliosides and neutral glycolipids in ependymal, neuronal and primitive neuroectodermal tumors. J Mol Neurosci. 1999;12:111–121. doi: 10.1007/BF02736925. [DOI] [PubMed] [Google Scholar]
- 16.Kanda N, Nakai K, Watanabe S. Gangliosides GD1b, GT1b, and GQ1b suppress the growth of human melanoma by inhibiting interleukin-8 production: the inhibition of adenylate cyclase. J Invest Dermatol. 2001;117:284–293. doi: 10.1046/j.0022-202x.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanda N, Watanabe S. Gangliosides GD1b, GT1b, and GQ1b enhance IL-2 and IFN-gamma production and suppress IL-4 and IL-5 production in phytohemagglutinin-stimulated human T cells. J Immunol. 2001;166:72–80. doi: 10.4049/jimmunol.166.1.72. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto S, Mutoh T, Hasegawa T, Miyazaki H, Okada M, Goto G, et al. GD3 synthase gene expression in PC12 cells results in the continuous activation of TrkA and ERK1/2 and enhanced proliferation. J Biol Chem. 2000;275:5832–5838. doi: 10.1074/jbc.275.8.5832. [DOI] [PubMed] [Google Scholar]
- 19.Ladisch S, Gillard B. A solvent partition method for microscale ganglioside purification. Anal Biochem. 1985;146:220–231. doi: 10.1016/0003-2697(85)90419-1. [DOI] [PubMed] [Google Scholar]
- 20.Brodeur GM, Sekhon G, Goldstein MN. Chromosomal aberrations in human neuroblastomas. Cancer. 1977;40:2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Tumilowicz JJ, Nichols WW, Cholon JJ, Greene AE. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970;30:2110–2118. [PubMed] [Google Scholar]
- 22.Rettig WJ, Spengler BA, Chesa PG, Old LJ, Biedler JL. Coordinate changes in neuronal phenotype and surface antigen expression in human neuroblastoma cell variants. Cancer Res. 1987;47:1383–1389. [PubMed] [Google Scholar]
- 23.Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305:245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 24.Brodeur GM, Green AA, Hayes FA, Williams KJ, Williams DL, Tsiatis AA. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981;41:4678–4686. [PubMed] [Google Scholar]
- 25.Reynolds CP, Biedler JL, Spengler BA, Reynolds DA, Ross RA, Frenkel EP, et al. Characterization of human neuroblastoma cell lines established before and after therapy. J Natl Cancer Inst. 1986;76:375–387. [PubMed] [Google Scholar]
- 26.Schneider SS, Zehnbauer BA, Vogelstein B, Brodeur GM. Yeast artificial chromosome (YAC) vector cloning of the MYCN amplified domain in neuroblastomas. Prog Clin Biol Res. 1991;366:71–76. [PubMed] [Google Scholar]
- 27.Seeger RC, Rayner SA, Banerjee A, Chung H, Laug WE, Neustein HB, et al. Morphology, growth, chromosomal pattern and fibrinolytic activity of two new human neuroblastoma cell lines. Cancer Res. 1977;37:1364–1371. [PubMed] [Google Scholar]
- 28.Janet T, Ludecke G, Otten U, Unsicker K. Heterogeneity of human neuroblastoma cell lines in their proliferative responses to basic FGF, NGF, and EGF: correlation with expression of growth factors and growth factor receptors. J Neurosci Res. 1995;40:707–715. doi: 10.1002/jnr.490400602. [DOI] [PubMed] [Google Scholar]
- 29.Abemayor E, Chang B, Sidell N. Effects of retinoic acid on the in vivo growth of human neuroblastoma cells. Cancer Lett. 1990;55:1–5. doi: 10.1016/0304-3835(90)90057-5. [DOI] [PubMed] [Google Scholar]
- 30.Nishi Y, Akiyama K, Korf BR. Characterization of N-myc amplification in a human neuroblastoma cell line by clones isolated following the phenol emulsion reassociation technique and by hexagonal field gel electrophoresis. Mamm Genome. 1992;2:11–20. doi: 10.1007/BF00570436. [DOI] [PubMed] [Google Scholar]
- 31.Sidell N, Lucas CA, Kreutzberg GW. Regulation of acetylcholinesterase activity by retinoic acid in a human neuroblastoma cell line. Exp Cell Res. 1984;155:305–309. doi: 10.1016/0014-4827(84)90795-x. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro DN, Valentine MB, Rowe ST, Sinclair AE, Sublett JE, Roberts WM, et al. Detection of N-myc gene amplification by fluorescence in situ hybridization. Diagnostic utility for neuroblastoma. Am J Pathol. 1993;142:1339–1346. [PMC free article] [PubMed] [Google Scholar]
- 33.Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- 34.Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- 35.Rebhan M, Vacun G, Bayreuther K, Rosner H. Altered ganglioside expression by SH-SY5Y cells upon retinoic acid-induced neuronal differentiation. Neuroreport. 1994;5:941–944. doi: 10.1097/00001756-199404000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Ladisch S. Alteration of neuroblastoma ganglioside metabolism by retinoic acid. J Neurochem. 1992;59:2297–2303. doi: 10.1111/j.1471-4159.1992.tb10123.x. [DOI] [PubMed] [Google Scholar]
- 37.Ladisch S, Kitada S, Hays EF. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest. 1987;79:1879–1882. doi: 10.1172/JCI113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leskawa KC, Hogan EL. Quantitation of the in vitro neuroblastoma response to exogenous, purified gangliosides. J Neurosci Res. 1985;13:539–550. doi: 10.1002/jnr.490130409. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji S, Arita M, Nagai Y. GQ1b, a bioactive ganglioside that exhibits novel nerve growth factor (NGF)-like activities in the two neuroblastoma cell lines. J Biochem (Tokyo) 1983;94:303–306. doi: 10.1093/oxfordjournals.jbchem.a134344. [DOI] [PubMed] [Google Scholar]
- 40.Farooqui T, Franklin T, Pearl DK, Yates AJ. Ganglioside GM1 enhances induction by nerve growth factor of a putative dimer of TrkA. J Neurochem. 1997;68:2348–2355. doi: 10.1046/j.1471-4159.1997.68062348.x. [DOI] [PubMed] [Google Scholar]
- 41.Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hettmer S, Ladisch S, Kaucic K. Alterations in neuroblastoma ganglioside synthesis by induction of GD1b synthase by retinoic acid. Pediatr Res. 2002;51:233a. doi: 10.1038/sj.bjc.6601914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liour SS, Yu RK. Differential effects of three inhibitors of glycosphingolipid biosynthesis on neuronal differentiation of embryonal carcinoma stem cells. Neurochem Res. 2002;27:1507–1512. doi: 10.1023/a:1021652506370. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Kong Y, Ladisch S. Nerve growth factor-induced neurite formation in PC12 cells is independent of endogenous cellular gangliosides. Glycobiology. 1998;8:597–603. doi: 10.1093/glycob/8.6.597. [DOI] [PubMed] [Google Scholar]
- 45.Olshefski R, Ladisch S. Synthesis, shedding, and intercellular transfer of human medulloblastoma gangliosides: abrogation by a new inhibitor of glucosylceramide synthase. J Neurochem. 1998;70:467–472. doi: 10.1046/j.1471-4159.1998.70020467.x. [DOI] [PubMed] [Google Scholar]