Preface
The roles of nuclear factor of activated T cells (NFAT) transcription factors have been extensively studied in the immune system. However, ubiquitous expression of NFAT isoforms in mammalian tissues has been recently observed, as well as an emerging role for these transcription factors in human cancer. Various NFAT isoforms are functional in tumor cells and multiple compartments in the tumor microenvironment including fibroblasts, endothelial cells and infiltrating immune cells. How do NFAT isoforms regulate the complex interplay between these compartments during carcinoma progression? The answers lie with the multiple functions attributed to NFAT including cell growth, survival, invasion and angiogenesis. In addition to sorting out the complex role of NFAT in cancer we face the challenge of targeting this pathway therapeutically.
Introduction
The nuclear factor of activated T cells (NFAT) signaling axis is a vertebrate-specific pathway important for a variety of cellular functions. NFAT proteins are best characterized as transcription factors that induce genes important in cellular processes ranging from development and activation of lymphocytes to differentiation of cardiac muscle cells 1, 2. In the canonical pathway first elucidated in immune cells, NFAT is activated as a result of calcium flux released from endoplasmic reticulum stores and from the extracellular environment through the activation of store-operated channels in the plasma membrane. In the basal state, NFAT is hyperphosphorylated in the cytoplasm. Subsequent to cell stimulation and calcium release, NFAT is dephosphorylated by the phosphatase calcineurin and translocates to the nucleus where it cooperates with other factors and co-activators to promote de novo gene transcription.
The foundation of the NFAT field is based on the original discovery that it is an inducible nuclear factor bound to the IL-2 promoter during the activation of T-cells 3. The importance of NFAT signaling is also highlighted by the fact that immunosuppressants such as cyclosporin A (CsA) and FK506, which specifically inactivate the canonical NFAT pathway, are widely used in the clinic to prevent organ transplant rejection. Since their discovery two decades ago, it has become increasingly clear that NFAT transcription factors are not only expressed in immune cells, but are found in all cells and tissues, including epithelial cells. In this context, a number of recent key findings have pointed to important roles for NFAT in modulating phenotypes associated with malignancy and tumor progression. NFAT isoforms are overexpressed in human solid tumors and hematologic malignancies 4, 5 and appear to have roles in cancer cell autonomous functions such as invasive migration, differentiation and survival of cells in the tumor and its microenvironment. NFAT also seems to play a key role in tumor angiogenesis 6. Understanding the roles played by NFAT in tumor progression is predicted to provide insight into development of effective therapeutics targeting the NFAT pathway in cancer progression and metastasis.
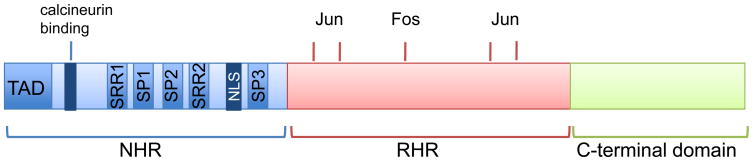
Primary structure of the NFAT family
In humans the NFAT family comprises five distinct gene products that are named as follows: NFAT1 (also known as NFATc2 and NFATp); NFAT2 (also known as NFATc1 and NFATc); NFAT3 (also known as NFATc4); NFAT4 (also known as NFATc3 and NFATx) and NFAT5 (also known as TonEBP and OREBP) (TABLE 1). As the name implies, NFAT proteins were originally identified and characterized in immune cells, however it is now established that all isoforms are ubiquitously expressed and most cell types express at least one isoform. In addition, each isoform has alternative splice variants that differ in the amino and carboxyl termini 7–9. The calcium-regulated isoforms NFAT1-4 share two conserved domains (Fig. 1): the Rel homology region (RHR) so called because of its structural similarity to the DNA binding domain of Rel family transcription factors (also known as the nuclear factor-κB (NF-κB) family) 10; and the more moderately conserved NFAT homology region (NHR). NFAT5 has a distinct domain structure and only retains the RHR region of homology to the calcium-regulated isoforms 11. NFAT5 does not possess a calcineurin-binding site, and thus is calcium and calcineurin-insensitive 11, 12. The NHR domain contains the NFAT transactivation region that binds promoter elements and thus initiates gene transcription. The NHR also contains numerous serine residues that are phosphorylated by distinct protein kinases in resting cells and, as discussed below, reversible phosphorylation of NFAT modulates nuclear and cytoplasmic shuttling and in turn transcriptional activity.
Table I.
Expression pattern of NFAT family members in cells that comprise the tumor and tumor microenvironment.
| NFAT Protein | Other Names | Expression in immune cells | Expression in tumor cells | Expression in endothelial cells | Expression in CAFs |
|---|---|---|---|---|---|
| NFAT1 | NFATc2 NFATp |
Yes | Yes | Yes | n.d. |
| NFAT2 | NFATc1 NFATc |
Yes | Yes | Yes, in LECs | n.d. |
| NFAT3 | NFATc4 | No | Yes | Yes | n.d. |
| NFAT4 | NFATc3 NFATx |
Yes | Yes | Yes | n.d. |
| NFAT5 | TonEBP OREBP |
Yes | Yes | n.d. | n.d. |
CAFs, carcinoma-associated fibroblasts; NFAT, nuclear factor of activated T cells; TonEBP, tonicity-responsive enhancer-binding protein; LEC, lymphatic endothelial cell; nd., not determined.
Figure 1. Primary structure of NFAT.
Schematic structure of NFAT. The domains depicted are retained in NFAT isoforms 1–4, but in NFAT5 the NHR region is truncated and lacks the calcineurin-binding site. The structure shows the NFAT-homology region (NHR) region that comprises the amino-terminal transactivation domain (TAD), the calcineurin-binding site, the nuclear localization sequence (NLS) and the serine-rich regions SRR1, SRR2, and SRR3, as well as the SP1 and SP2 motifs (Ser-Pro rich) that are targeted by maintenance and export kinases. The Rel-homology region (RHR) comprises the DNA binding motif and points of contact with transcriptional binding partners such as Fos and Jun. Note that NFAT1-4 differ in the size of the carboxyl-terminal domain, and alternative splice variants of NFAT1 (isoforms A, B, C), NFAT2 (isoforms A, B, C) and NFAT4 (isoforms 1, 2, 3, 4) also exist that differ in the length of the carboxyl-terminus.
This review will focus on the NFAT family members that are expressed in cells that comprise the tumor and its microenvironment. In this context, NFAT1-5 mRNA and protein have been detected in multiple cell types in human solid tumors and cells derived from these tumors, including epithelial cells, endothelial cells of the tumor vasculature and infiltrating immune cells (TABLE 1). Although expression of NFAT isoforms, particularly NFAT3, has been detected in various fibroblast cell lines 13, to date there is no information regarding the endogenous expression of NFAT family members in fibroblasts within the tumor stroma, specifically carcinoma-associated fibroblasts or myofibroblasts.
NFAT transcription factors interact with DNA targets in a versatile manner. NFAT proteins bind DNA as homo- or heterodimers, and more commonly the DNA binding domain of NFAT can cooperate with DNA binding domains from other transcription factors to elicit high-affinity binding 14. The best documented example is the unrelated transcription factor AP-1 (activator protein-1, comprised of Fos-Jun complexes) which forms a quaternary complex with NFAT and DNA, and is the primary transcriptional partner for NFAT required for T cell activation 10, 15. In addition to AP-1, NFAT cooperates with numerous other transcriptional factors implicated in cell activation and differentiation, including (but not limited to) GATA-4, EGR, MEF2, and with particular importance in cancer, FOXP (Forkhead Box P) 16. Several recent articles provide a comprehensive review of the mechanisms of gene transcription by NFAT and its binding partners 2, 17.
Mechanisms of NFAT activation by calcium flux
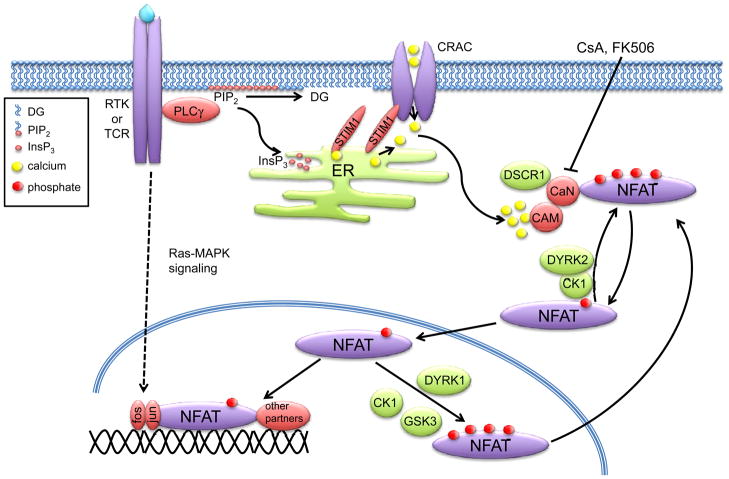
The activation of NFAT by calcium signaling arguably represents one of the best-characterized mechanisms of signal relay initiated by cell surface receptors. In this context, it is important to note that the mechanisms of NFAT activation have largely been deduced from studies in immune cells. However, the same mechanisms of NFAT activation and function are recapitulated in cells that comprise the tumor and its microenvironment, particularly endothelial cells. A key rate-limiting event in NFAT activation is a rise in intracellular calcium (which also affects other cancer-associated pathways (BOX 1)) initiated by cell-surface receptors that stimulate activation of phospholipase type C (PLC) enzymes such as PLCγ (Fig. 2). Efficient NFAT activation also requires a sustained calcium signal, and this is achieved by the opening of calcium-release activated calcium channels (CRAC) at the plasma membrane that occurs in response to PLC-initiated emptying of calcium from the endoplasmic reticulum (ER). In turn, calcium release is sensed by the high-affinity ER calcium sensor stromal interaction molecule 1 (STIM1) 18, 19, leading to a conformational change in the CRAC channel protein Orai1 20–23, opening of the channel and an influx of extracellular calcium 24. Subsequent to sustained flux through ER and store-operated calcium channels, calcium binds to calmodulin, which in turn binds to and activates the serine/threonine phosphatase calcineurin. Activation of calcineurin is rate-limiting for NFAT activation in all cells.
Box 1. Calcium signaling in cancer.
Given the importance of calcium signaling in the activation of NFAT in immune cells, any role for NFAT in cancer progression will also be affected by calcium flux. Consistent with this, calcium signaling impacts tumor cell proliferation and invasive migration. In migrating immune cells that infiltrate the tumor microenvironment, calcium signaling controls cell polarity, cytoskeletal remodeling and directionality 94. In macrophages, intracellular calcium exhibits a back-to-front concentration gradient with the lowest concentrations at the front of the migrating cell. This gradient appears to be reversed from what is expected since leading edge lamella have numerous signaling components that require high calcium levels for signal relay 95. High calcium microdomains known as flickers are most active at the leading edge thus facilitating the turning of migrating cells96. This may explain the paradox mentioned above: calcium flickers in the context of low calcium background would permit the correct spatio-temporal activation of calcium-regulated signaling required to orchestrate cell migration 96. Knockdown of the store-operated calcium CRAC channels Orai1 and STIM1 in breast cancer cells attenuates migration in vitro and reduces tumor metastasis in mice, also supporting a role for calcium influx in cell migration97.
Calcium signaling also controls key aspects of cell death by apoptosis, necrosis or autophagy. Disruption of calcium signaling affects mitochondrial integrity, whereby overload of calcium in mitochondria promotes apoptosis. In contrast, changes in calcium flux can also promote survival of cancer cells, whereby phosphorylation of InsP3 receptors by the PI 3-K/Akt pathway results in reduced calcium release98.
The specific contribution of NFAT as the effector of calcium in the context of the cell migration phenotypes described above remains to be established. Regardless, alterations in calcium flux in the tumor microenvironment affect multiple cellular responses, and these are likely exploited by tumor cells, which commonly exhibit changes in calcium flux.
Figure 2. Calcium signaling and activation of NFAT.
Receptor tyrosine kinases (RTKs) and immunoreceptors such as the T cell receptor (TCR) activate phospholipase Cγ (PLCγ), which hydrolyses phosphatidylinositol-4,5-bisphosphate (PIP2) releasing inositol-1,4,5-triphosphate (InsP3) and diacylglycerol (DG). InsP3 and loss of calcium binding on STIM1 (stromal interaction molecule 1) induces calcium release from the endoplasmic reticulum (ER). Calcium-release-activated calcium channels (CRACs), including Orai1, are then opened, allowing a sustained influx of extracellular calcium. Calmodulin (CAM) binds calcium and in turn the phosphatase calcineurin (CaN). Binding of calcium to the calcineurin regulatory B subunit (CaNB) exposes the calmodulin-binding site on the catalytic A subunit (CaNA). An autoinhibitory sequence in calcineurin is then released from the catalytic pocket, and the phosphatase can dephosphorylate cytoplasmic nuclear factor of activated T cells (NFAT). Inactive NFAT is basally hyperphosphorylated; dephosphorylation promotes nuclear translocation and gene transcription. NFAT cooperates with multiple other transcription factors, including the activator protein 1 (AP-1) complex (Fos-Jun dimers). RTK and TCR activation also stimulates signaling through the MAPK pathway leading to AP-1 activation. The NFAT activation cycle is maintained through complex mechanisms of maintenance kinases that retain cytoplasmic hyperphosphorylated NFAT, such as casein kinase-1 (CK1) and dual-specificity tyrosine-phosphorylation regulated kinase 2 (DYRK2), as well as nuclear export kinases such as CK1, DYRK1 and GSK-3 (glycogen synthase kinase-3). These kinases are counteracted by negative regulators of calcineurin, such as DSCR1 (Down syndrome candidate region 1). Pharmacological antagonists of calcineurin, such as FK506 and cyclosporin A (CsA) are potent inhibitors of NFAT dephosphorylation and nuclear accumulation.
In the basal state, NFAT is localized in an inactive conformation in the cytoplasm. Over 20 distinct phosphorylation sites have been identified in NFAT1, 18 of which are located in the regulatory region 25. These sites are found within multiple distinct serine-rich sequences; the serine-rich region (SRR1) and the SPXX (Ser-Pro, where X denotes any amino acid) motifs SP1, SP2 and SP3 (Fig. 1). Dephosphorylation of the SP motifs in NFAT by calcineurin exposes a nuclear localization sequence and masks a nuclear export sequence, thus promoting nuclear import leading to transcriptional activation. Calcineurin also maintains NFAT in a dephosphorylated state in the nucleus 26. Nuclear export of NFAT is a critical mechanism as it leads to termination of transcriptional activity and gene transcription. Cytoplasmic accumulation of NFAT is achieved by multiple redundant mechanisms, including inhibition of calcineurin activity, which can retain cytoplasmic NFAT or promote nuclear export of NFAT. In addition, nuclear kinases rephosphorylate NFAT leading to export and cytoplasmic retention.
Numerous serine/threonine protein kinases have been identified as regulators of NFAT activity and are subdivided into maintenance and export kinases (Fig. 2), which function to keep NFAT in the cytoplasm by retaining it or promoting its export from the nucleus, respectively. Export kinases include glycogen synthase kinase 3 (GSK-3) which phosphorylates the SP2 and SP3 motifs of NFAT 1 and NFAT2, an event that requires prior phosphorylation of NFAT by the priming kinase protein kinase A (PKA) 27, 28. GSK-3 is a constitutively active kinase that is phosphorylated and inactivated by PI 3-K and Akt signaling, one of the most frequently deregulated pathways in human tumors 29. As discussed later, this provides a point of cross-talk between PI 3-K and NFAT signaling that is predicted to have important consequences for tumorigenesis. Casein kinase 1 (CK1) functions as both an export and maintenance NFAT kinase and phosphorylates the SRR1 region 30, 31. The mitogen-activated protein kinase (MAPK) pathway is also frequently hyperactive in human cancers, and in this context it is noteworthy that JNK (c-Jun N-terminal kinase) and p38 MAPKs phosphorylate the SRR region of NFAT2 and NFAT1 respectively 32, 33.
A distinct class of maintenance and export kinases was recently revealed when the dual-specificity tyrosine-phosphorylation regulated kinases (DYRK) emerged from an siRNA screen in Drosophila as modifiers of NFAT subcellular localization 34. DYRK1 and DYRK2 phosphorylate NFAT1 on the SP3 motif, thus priming the subsequent phosphorylation of the SP2 and SRR1 motifs by CK1 and GSK-3 34, 35. DYRK1 functions as an NFAT export kinase, whereas DYRK2 phosphorylates NFAT in the cytoplasm and functions as a maintenance kinase (Fig. 2). Thus, several distinct NFAT kinases exist to maintain the precise subcellular localization of NFAT and in turn transcriptional activity. Although there is now ample evidence that NFAT family members are targeted by distinct maintenance and export kinases, it is likely that additional kinases that regulate NFAT subcellular localization have yet to be identified. In the context of carcinoma progression, identification of protein kinases that control NFAT activation and that are frequently deregulated in human carcinoma may provide important new information and possible novel therapeutic targets.
In addition to phosphorylation, distinct post-translational modifications have been reported for NFAT family members. Sumoylation of NFAT1 and NFAT2 isoforms provides a separate mechanism of cytoplasmic-nuclear trafficking as it results in nuclear retention of the transcription factors 36, 37. Moreover, NFAT1 is also ubiquitinated by the E3 ubiquitin ligase murine double minute 2 (MDM2) downstream of Akt and GSK-3 signaling in breast cancer cells 38,39. Whether all NFAT isoforms are modified by ubiquitination and subsequent degradation by the proteasome remains to be determined. Taken together, the sensitivity to calcium flux, the existence of several NFAT kinases and diverse post-translational modifications demonstrate the complexity of the mechanisms leading to NFAT activation.
NFAT signaling in tumor cell transformation and proliferation
Single NFAT isoform knockout mice (BOX 2) have indicated that there is a broad range of targets for these transcription factors and suggest redundancy, but also indicate that isoform-specific differences exist among NFAT1-4. This obviously hampers the use of single or double NFAT knockout mice for cancer-related studies in adult mice, as a lack of an observable phenotype in whole animal knockouts could be due to redundancy, whereas the profound immunological disorders in double knockout mice limits the analysis of tumor progression in adult animals. Thus, studies on the role of NFAT transcription factors in cancer progression to date have largely been restricted to in vitro or cell-based assays. However, isoform-specific functions of distinct NFAT family members in settings of proliferation and tumorigenesis have been identified.
Box 2. Lessons from NFAT Knockout Mice.
The considerable sequence similarity among NFAT isoforms as well as their mode of regulation by calcineurin and nuclear export and maintenance kinases would suggest a high degree of redundancy. This is borne out by the relatively mild phenotypes of the individual NFAT knockout mice. However, some studies have revealed isoform-specific roles in development. NFAT1 null mice display hyperproliferation of splenic B and T cells due to lack of FasL expression and therefore escape from cell death 99. This is concomitant with a reduction in IL-4 suggesting that NFAT1 is a positive regulator of cytokine production, consistent within vitrostudies. Conversely, NFAT2 null mice show defects in heart valve morphogenesis associated with an abnormal cardiac septum, providing evidence for an indispensable role for NFAT2 in cardiac development 100. NFAT4 null mice exhibit abnormal development of myofibers as well as reduced thymocyte numbers due to suppression of Bcl-2 101. Only when more than one NFAT isoform is eliminated are more pronounced phenotypes evident, particularly in the immune system. NFAT1/2 null mice have profound defects in cytokine production and cytolytic activity 102. NFAT3/4 null mice are embryonic lethal due to defects in angiogenesis characterized by vessel instability and disorganization 103. NFAT1/4 null mice have a profound lymphoproliferative disorder 104.
Among the first studies implicating NFAT in proliferation was in fibroblasts where constitutively active NFAT2 induced cell transformation and colony formation 40. Similarly, proliferation and anchorage-independent growth of pancreatic tumor cells is dependent on calcineurin activity and NFAT2 in a manner dependent on the induction of c-Myc, consistent with the finding that pancreatic cancers show a high level of nuclear NFAT2 41. More recently, distinct and opposing roles for NFAT1 and NFAT2 in tumorigenesis were revealed whereby NFAT1 acts as a tumor suppressor and NFAT2 functions as an oncogene 42. Although NFAT1 and NFAT2 are 72% identical in the carboxyl-terminal DNA binding domain, the functional differences between these two isoforms lie within this region. In fibroblasts, constitutively active NFAT1 induced cell cycle arrest and apoptosis and inhibited RasV12 induced transformation, whereas constitutively active NFAT2 increased proliferation and transformation. Similar findings are observed in mice, where NFAT1 null mice are more susceptible to chemically-induced carcinogenesis 42. These observations underscore the notion that NFAT1 and NFAT2 likely induce a non-overlapping subset of transcriptional targets that either suppress or promote cell growth, respectively.
NFAT is also implicated in the induction and progression of hematologic malignancies. Active nuclear NFAT2 is found in cases of Burkitt lymphoma, diffuse large B cell lymphoma and in aggressive T cell lymphoma 5, 43, 44. In experimental settings of T cell acute lymphoblastic leukemia (T-ALL), NFAT activation is calcineurin-dependent and pharmacological inhibition of calcineurin reverses cell growth and induces apoptosis 5, 44. Consistent with these findings, inhibition of calcineurin causes disease regression in mouse models of leukemia 5. Considering the major role of the calcineurin-NFAT axis in immune cell signaling, these findings are not surprising. What has remained elusive, however, are the genetic and epigenetic mechanisms that drive constitutive nuclear localization of NFAT and in turn the genes that drive these malignancies.
NFAT also plays an important role in maintaining the balance between quiescence and proliferation in stem cells 45. In stem cells undergoing a transition to a proliferative state, NFAT2 acts downstream of bone morphogenetic protein (BMP) as a transcriptional repressor of the CDK4 (cyclin-dependent kinase 4, required for cell cycle progression), thus maintaining a state of quiescence in the stem cell population. This may have fundamental consequences for tumor progression, as metastatic tumor cells undergo the epithelial to mesenchymal transition (EMT) and gain the properties of stem cells, providing metastatic tumor cells self-renewal capacity 46.
NFATs modulate epithelial cell invasive migration
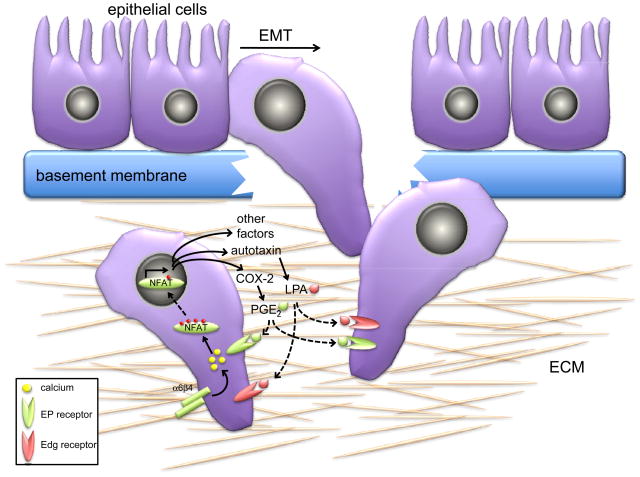
Acquisition of motile and invasive properties is concomitant with the mesenchymal phenotype displayed by tumor cells subsequent to the EMT 47. Recent studies point to an important role for NFAT in modulating invasive migration, particularly in breast cancer (Fig. 3). Expression of active NFAT1 promotes migration and invasion of breast cancer cells through Matrigel in vitro, whereas expression of NFAT5 promotes migration but not invasion 4, again suggestive of NFAT isoform-specific differences that are likely due to the induction of a non-overlapping subset of genes. In human breast epithelial cells, the non-canonical Wnt ligand Wnt5a, which is known to suppress metastatic progression48 blocks NFAT activation coincident with attenuated migration by a mechanism that depends in part on the binding of CK1 49. Conversely, elevated expression of NFAT1 and NFAT5 is observed in invasive human ductal breast carcinoma cells lines and also in patients with invasive breast cancer, and this correlates with expression of the α6β4 integrin, which in carcinoma is released from hemidesmosomes and associates with the actin cytoskeleton, consistent with enhanced expression of α6β4 detected in patients with advanced breast cancer 50, 51. A very high frequency of breast cancer patients harbor activating mutations in PIK3CA, the gene that encodes the catalytic subunit of PI 3-K 29. Downstream of PI 3-K, the Akt1 serine/threonine kinase attenuates NFAT activity by a mechanism that depends in part on the ubiquitination of NFAT by MDM2 38. The net effect is reduction of invasive migration of breast cancer cells, such that a gain of function signal (activation of Akt1) results in a loss of function downstream event (NFAT ubiquitination and degradation) and loss of function phenotype (inhibition of invasion).
Figure 3. NFAT promotes tumor cell migration through paracrine and autocrine mechanisms.
Subsequent to genetic and epigenetic deregulation, epithelial cells that reside on the basement membrane undergo a fundamental change in morphology adopting a mesenchymal architecture, concomitant with acquisition of a motile phenotype. This ordered series of events is collectively termed the epithelial to mesenchymal transition (EMT). Subsequently, the tumor cells degrade the basement membrane and this facilitates cancer invasion into connective tissue that is comprised primarily of extracellular membrane (ECM) proteins. NFAT promotes migration and invasion through multiple non-redundant mechanisms. Engagement of integrins such as α6β4 on tumor cells promotes NFAT nuclear translocation likely through calcium flux. Nuclear NFAT transactivates numerous genes, including those that encode the autotaxin and cyclooxygenase (COX-2) proteins. Autotaxin and COX-2 are secreted proteins and catalyze the synthesis of lysophosphatidic acid (LPA) and prostaglandin E2 (PGE2) respectively. Both LPA and PGE2 are potent motogens and mitogens that act in both paracrine and autocrine signaling through binding Edg (endothelial differentiation gene) and prostaglandin E2 (EP) receptors, respectively, to promote the invasive migration of tumor cells through the ECM.
The mechanism by which NFAT functions as a pro-invasion transcription factor likely lies with the transcriptional program of genes that are induced in tumor cells. NFAT induces the transcription of cyclooxygenase 2 (COX-2, also known as PTGS2) in breast epithelial cells, and this is required for the ability of NFAT to promote invasive migration 52. COX-2 catalyzes the synthesis of prostaglandins such as prostaglandin E2 (PGE2). Breast cancer cells display reduced invasion with knockdown of COX-2, or treatment with COX inhibitors such as NSAIDS (non-steroidal anti-inflammatory drugs), whereas increased expression of COX-2 or addition of PGE2 to cells enhances cell invasion 52. This suggests that PGE2 might function in a cell autonomous or paracrine manner to influence invasive migration of epithelial cells (Fig. 3).
NFAT also induces the transcription of the autotaxin gene (also known as ENPP2, exonucleotide pyrophosphatase and phosphodiesterase-2) in breast epithelial cells 53. Autotaxin is a secreted protein and interconverts lyso-phosphatidylcholine into lyso-phosphatidic acid (LPA). LPA is potent mitogen and motogen for breast cancer cells (Fig. 3) 54. Autotaxin is highly upregulated in cells expressing the α6β4 integrin in an NFAT-dependent manner 53. Importantly, autotaxin is also significantly upregulated in breast cancer metastases 55. Transgenic mice expressing autotaxin or the receptors for LPA (LPA-R, also know as the endothelial differentiation gene (Edg) family) in the mammary epithelium display a high frequency of invasive and metastatic carcinoma 56. Similarly, LPA analogues that act as antagonists for autotaxin and LPA-R receptors reduce breast cancer cell migration in vitro and significantly inhibit tumor burden in breast cancer xenografts in mice 57. Because PGE2 and LPA are secreted molecules, they have the capacity to act in a cell autonomous manner by directly binding to cell surface receptors of epithelial cells and thus promote cell migration, presumably by engaging cell signaling pathways that elicit remodeling of the actin cytoskeleton, a pre-requisite for cell motility (Fig. 3).
One would also expect that for NFAT to function as a pro-invasion transcription factor, it would induce the transcription and secretion of matrix metalloproteinases (MMPs) that are required for efficient basement membrane proteolysis during tumor invasion and metastasis 58. While there is some evidence that NFAT is required for MMP induction in myocytes and mesangial cells, to date no studies have addressed MMP regulation by calcineurin-NFAT signaling in settings of carcinoma progression. Similarly, secreted factors that are released within the tumor and its microenvironment are also likely to act in a paracrine manner, particularly in the case of COX-2 and PGE2 which have a profound influence on endothelial cell growth leading to angiogenesis.
Regulation of tumor angiogenesis by the NFAT axis
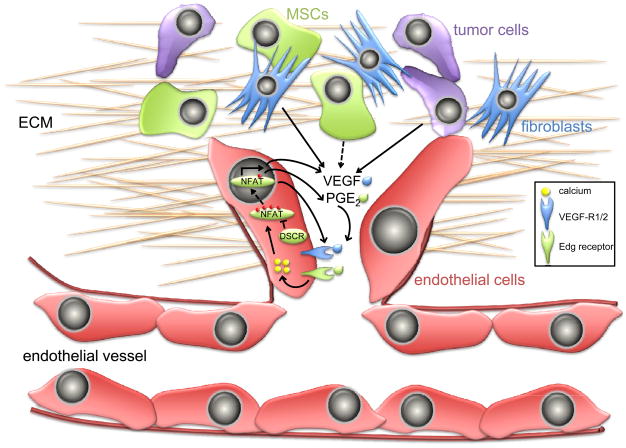
In addition to providing solid tumors with the nutrients and oxygen necessary for tumor cells to survive and proliferate, the vasculature also provides tumor cells a mechanism with which to disseminate and metastasize to distant organs such as lungs, liver, bone and brain. As first recognized in the NFAT3/4 null mice (BOX 2), calcineurin-NFAT signaling is essential for angiogenesis and the formation of an intact vasculature during development. It is therefore not surprising that NFAT signaling also profoundly affects tumor angiogenesis in humans. Angiogenesis involves organization and subsequent branching of endothelial cells as well as recruitment of vascular smooth muscle cells; a process that requires cell proliferation, migration and differentiation 59. A pre-requisite for tumor angiogenesis is the stimulation of endothelial cell proliferation, achieved in part by the vascular endothelial growth factor (VEGF), also an endothelial cell permeability factor 60. As with most mitogens, VEGF stimulates receptor-mediated activation of PLCγ leading to an increase in intracellular calcium, calcineurin activation and NFAT nuclear translocation 61 (Fig. 4). In turn, this leads to the transactivation of genes that are essential for angiogenesis, such as COX-2 resulting in synthesis of PGE2, a critical mediator of tumor cell and endothelial cell migration and tube formation 62. COX-2 has emerged as a key enzyme in metastatic dissemination of most human tumors, in particular breast cancer cell infiltration to the lungs and brain 63, 64. Activation of NFAT by VEGF in endothelial cells also induces the transcription of tissue factor (TF, also known as F3), an important initiator of blood coagulation and angiogenesis 65. Similarly, NFAT induces granulocyte-macrophage colony-stimulating factor (GM-CSF in endothelial cells and monocytes, which is important for their differentiation and survival 66.
Figure 4. NFAT promotes tumor angiogenesis.
The vascular endothelial growth factor (VEGF) is secreted into the tumor microenvironment by multiple distinct resident cell types, including endothelial cells, fibroblasts and the tumor cells themselves. If not immediately utilized, VEGF is tethered to the extracellular matrix (ECM), and signals that stimulate the angiogenic switch during tumor progression activate mesenchymal stem cells (MSCs), secretion of matrix metalloproteases and release VEGF from the ECM. VEGF then binds VEGF-receptors (VEGF-R1 and/or 2), leading to an increase in intracellular calcium that promotes the nuclear translocation of NFAT. Activation of NFAT in endothelial cells induces the transcription of VEGF and VEGF-R that function in an autocrine loop. NFAT also induces COX-2 in endothelial cells leading to synthesis of PGE2, which binds to Edg receptors. Both VEGF and PGE2 stimulate endothelial cell proliferation, migration and ultimately vessel formation. Endogenous inhibitors of calcineurin-NFAT, such as DSCR1 (Down’s syndrome candidate region 1) block endothelial cell NFAT activation and are potent inhibitors of tumor angiogenesis.
The function of NFAT in tumor angiogenesis is best illustrated by studies in animal models of cancer progression as well as analysis of human pathophysiologies associated with deregulated NFAT activation. Infantile hemangiomas are rapidly growing areas of disorganized blood vessels that are dependent on VEGF signaling for growth. Suppressed transcription of VEGFR1 (VEGF-receptor 1) in these lesions is mediated by reduced activity of NFAT, and in turn this elicits an increase in VEGF-dependent VEGFR2 activation which ultimately causes lesion formation 67. Normalization of VEGF or VEGFR2 activity could provide an effective strategy for hemangioma treatment in children afflicted with the most aggressive forms of this disease.
Patients with Down’s syndrome who achieve adulthood have a strikingly lower incidence of cancer than the normal population 68. This led to the speculation that one or more of the 231 genes elevated in the extra copy of chromosome 21 in Down’s cases might posses tumor suppressor activities. One of these genes is the calcineurin suppressor DSCR1. DCSR1 loss in mice seems to suppress proliferation and increase apoptosis of endothelial cells 6. Conversely, increased expression of DCSR1 results in a reduction of tumor angiogenesis through the suppression of NFAT activation and decreased VEGF signaling in endothelial cells 69. This is consistent with an examination of Down’s syndrome patients that reveals an upregulation of DSCR1 and also the NFAT maintenance kinase DYRK1A that inactivates NFAT by promoting nuclear export 69. Similarly, cells over-expressing DCSR1 and DYRK1A display reduced VEGF-mediated proliferation 69, 70. These findings provide a molecular explanation for the reduced incidence of cancers in Down’s syndrome patients with trisomy of chromosome 21, and underscore the importance of VEGF and NFAT signaling in tumor progression.
Endothelial cells also comprise lymphatic vessels that are responsible for returning interstitial fluid to circulation. In breast, lung and gastrointestinal tumors, cells metastasize through lymphatic vessels 71. It is not clear if solid tumors promote localized lymphangiogenesis or utilize pre-existing vessels to metastasize. Regardless, many lymphangiogenic factors such as VEGFC influence tumor progression 72, 73. In this context, NFAT2 has been shown to modulate lymphangiogenesis, specifically the patterning process and subsequent valve formation after the initial sprouting of lymphatic endothelial cells 74, 75. In this mechanism, NFAT2 functions downstream of VEGF through interactions with lymphangiogenic promoting factors such as prospero homeobox 1 (PROX1), podoplanin, forkhead box C2 (FOXC2) and VEGFR3. Thus, NFAT regulates both tumor angiogenesis and lymphangiogenesis.
Chemokines and immune cell infiltration
It is well-established that in immune cells, NFAT directly induces the transcription of chemokines 24. Inflammatory chemokines also function as chemoattractants for leukocytes, enhancing the migration and recruitment of monocytes and neutrophils to sites of tissue damage 76, 77. Chemokines such as CXCL12 (also known as SDF1a) and CCL21 and their receptors CXCR4 and CCR7 are highly expressed in advanced breast cancer and mediate breast cancer metastasis by promoting chemotactic and invasive migration of epithelial cells 78, 79. Although presently unknown, any role of NFAT in chemokine signaling and metastatic dissemination in the tumor microenvironment is likely to be complex. Infiltrating immune cells, as well as mesenchymal stem cells that are localized in breast carcinomas, secrete chemokines that may function in a paracrine manner to influence tumor cell invasion and ultimately metastasis 80 (Fig. 5). However, as NFAT is also active in the tumor cells themselves, chemokines could influence metastasis in a cell-autonomous manner. Regardless, NFAT signaling in the tumor microenvironment is likely to have a significant impact on chemokine signaling. This is borne out by mouse models of leukemia and lymphoma which reveal hyperactivation of NFAT as a result of paracrine signaling in the tumor microenvironment 5, 44. Similarly, infiltrating macrophages in the tumor microenvironment are directly associated with tumor cells and participate in a paracrine signaling loop between tumor cells that express epidermal growth factor (EGF)-receptor and macrophages that secrete EGF and CSF1 (colony-stimulating factor-1), which in turn promote tumor cell migration.81 Since NFAT induces CSF1 in both monocytes and endothelial cells, presumably the function of this paracrine signaling loop is to promote epithelial cell migration, consistent with the finding that tumor-associated macrophages are located adjacent to the tumor vasculature, the route of metastatic dissemination.
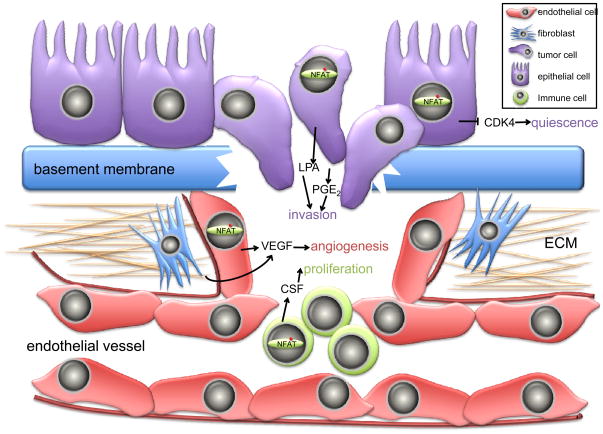
Figure 5. Multiple roles for NFAT in the heterotypic interactions of the tumor microenvironment.
Multiple non-redundant mechanisms function to control the specific roles of NFAT transcription factors in tumor progression. In non-tumorigenic epithelial cells, quiescence is achieved by multiple mechanisms, including the NFAT-dependent suppression of cyclin-dependent kinases (CDK4) that are required for proliferation. During tumorigenesis, cells acquire a motile and invasive phenotype that is in part dependent on the upregulation of NFAT activity that in turn stimulates the synthesis and secretion of pro-motility factors such as lysophosphatidic acid (LPA) and prostaglandin E2 (PGE2). Intravasation of tumor cells into the vasculature is only possible subsequent to the angiogenic switch. NFAT promotes angiogenesis through secretion of pro-angiogenic factors such as the vascular endothelial growth factor (VEGF) that are secreted by endothelial cells and fibroblasts in an NFAT-dependent manner. Infiltrating immune cells are mobilized to the tumor microenvironment by chemotaxis mechanisms that depend on NFAT activation, and in turn secrete local factors such as colony-stimulating factor-1 (CSF-1) that promote proliferation.
Targeting the NFAT pathway in cancer therapy
NFAT has long been considered an ideal target for therapeutic intervention in immune responses. Two structurally unrelated inhibitors, CsA and FK506 are potent inhibitors of calcineurin-NFAT and are widely used as immunosuppressive agents in tissue/organ transplant to prevent rejection, and also for treatment of autoimmune disease 82. Both CsA and FK506 prevent NFAT nuclear translocation by interfering with calcineurin activation 83. Specifically, CsA and FK506 bind to the immunophilin proteins cyclophilin A (CyPA) and FKBP12, respectively, and both complexes directly bind calcineurin and inhibit phosphatase activity 84, 85. By interfering with calcineurin activity, both CsA and FK506 block the dephosphorylation of numerous other substrates in addition to NFAT86 (Fig. 1). This probably explains the neuro- and nephrotoxicitiy as well as complications from diabetes and high blood pressure observed in the clinic87. Nonetheless, one would predict that as potent calcineurin-NFAT inhibitors, both CsA and FK506 could be effective cancer therapeutics. Somewhat paradoxically, there is actually a significant increase in cancer incidence in patients on long-term immunosuppressive treatments 88. The explanation for this observation is two-fold: first, the increased cancer incidence is likely due to decreased immune surveillance leading to reactivation of previously quiescent Epstein Barr Virus (EBV)-transformed positive B cells and; second, other targets of calcineurin exist that function to modulate phenotypes associated with cancer, such as proliferation and survival. Moreover, systemic administration of CsA and FK506 would affect the entire milieu of the tumor microenvironment with pleiotropic effects on cellular pathophysiology. It is more reasonable to assume that effective NFAT therapy in cancer will have to come from targeted therapy for the tumor endothelium or the tumor cells themselves, as these are the primary compartments where attenuation of NFAT activity is predicted to block or even reverse the tumorigenic phenotype.
Because of the caveats associated with CsA and FK506, other more selective NFAT inhibitors have been developed. A peptide that interferes with the calcineurin-NFAT interaction, termed VIVIT, potently blocks NFAT dephosphorylation and nuclear translocation 89, 90, prolongs graft survival in mice 91 and also attenuates breast cancer cell invasion 4. Although the use of peptides as signaling antagonists in therapy is problematic due to delivery and stability, it will be useful to determine the efficacy of VIVIT peptides in mouse models of carcinoma progression. Small molecule inhibitors of NFAT hold more promise for therapy, and in this context inhibitors that are similar in structure and function to CsA and FK506, but exhibit fewer side effects, have been developed. L-732531, an analog of FK506, exhibits less kidney toxicity and similarly ISATX47 is a potent and less toxic analog of CsA 92, 93. Again, mouse studies are required to determine if these inhibitors demonstrate any efficacy at preventing or reversing tumorigenesis in mouse models of cancer, beyond their well-documented activities in immune suppression.
As discussed above, DSCR1 is an endogenous inhibitor of the NFAT pathway. In DSCR1 null mice calcineurin is hyperactivated, thereby suppressing cell proliferation and increasing apoptosis 6, 69. A small-molecule strategy targeting the DCSR1/calcineurin interaction could prove to be effective in settings of cancer therapy. Exploiting the interaction between calcineurin and DSCR1 with drugs would in both cases effectively block NFAT activity in the tumor microenvironment, and in turn attenuate the release of factors into the vasculature that promote proliferation and metastatic dissemination. As more information accumulates on the regulation of NFAT in cancer, other strategies are likely to emerge. For example, targeting the NFAT nuclear export kinases such as GSK-3 would be predicted to phenocopy inhibition of calcineurin. As GSK-3 activity is inhibited by the PI 3-K and Akt pathway, frequently hyperactive in most human solid tumors, this results in diminished GSK-3 activity and in turn constitutive NFAT nuclear localization. Thus, inhibitors of PI 3-K or Akt might be expected to reverse GSK-3 inhibition, promote NFAT export and terminate transcriptional activity. Ultimately, any new therapeutic strategy targeting NFAT will have to take into account the pleiotropic functions of NFAT in all cell types within the tumor microenvironment. Moreover, targeted cancer therapy for NFAT will have to take into account the contribution of isoform-specific functions in cancer phenotypes.
Conclusions and future perspectives
In the past few years evidence has accumulated that points to a key role for NFAT transcription factors in cancer progression. Indeed NFAT function is not restricted to the immune system, as originally thought, instead these ubiquitously-expressed transcription factors control numerous responses in all cells and tissues. However, a pre-requisite for NFAT activation in all cells is nuclear translocation and DNA binding, typically in cooperation with other binding partners. NFAT activity is important for fibroblast proliferation and survival, epithelial tumor cell invasive migration and endothelial cell growth and angiogenesis. However, many questions remain, and answers to these questions are predicted to not only reinforce the notion that the NFAT pathway is a key signaling axis in cancer progression, but may also provide novel therapeutic avenues for clinical intervention. Most pressing is the development and use of mouse models of cancer in which NFAT isoforms are either deleted or activated in specific cell types or compartments in the microenvironment to evaluate the consequences for tumor initiation and progression. The existence of isoform-specific functions for NFAT family members in phenotypes such as proliferation and tumor suppression has been shown, but the mechanisms responsible for these distinctions have yet to be determined. Similarly, although a handful of genes such as COX-2 and autotaxin have been identified as mediators of the NFAT signal in tumor and endothelial cells, it is likely that numerous other genes, most likely soluble mitogens or motogens, have yet to be described. In this context, genome profiling of tumor cell lines and tumor tissues in which NFAT is active are likely to yield a wealth of new information. It is possible that mutations and/or amplifications in NFAT maintenance and export kinases exist in human cancers, consistent with the reported constitutive NFAT nuclear localization seen in breast cancers. Similarly, mutations or amplifications in NFAT isoforms may exist, although based on recent cancer genome sequencing studies these are not likely to occur at very high frequencies. Instead, activation of NFAT in cancer probably occurs by mechanisms that drive or retain NFAT in the nucleus. Answers to these pressing questions may hold promise for the development of drugs that specifically target NFAT signaling in human cancer.
At a glance
NFAT (nuclear factor of activated T cells) is a family of closely related transcription factors that are ubiquitously expressed in mammalian cells and tissues. NFAT1-4 are regulated by the calcium-sensitive phosphatase calcineurin that induces nuclear translocation and transcriptional activation.
NFAT transcriptional activity is regulated primarily by phosphorylation that in turn determines subcellular localization. Maintenance kinases such as DYRK2 and CK1 phosphorylate cytoplasmic NFAT and prevent nuclear translocation, whereas export kinases such as DYRK1 and GSK-3 phosphorylate nuclear NFAT and promote its export.
Overexpression and increased transcriptional activity of NFAT isoforms has been detected in various human solid tumors and cell lines as well as hematological malignancies. This leads to the induction of genes that promote cellular phenotypes that are associated with tumor progression, such as a proliferation, survival, migration and invasion.
NFAT isoforms promote the migration and invasion of tumor cells, prerequisites for metastatic dissemination. These phenotypes are mediated by the transcriptional induction of NFAT target genes in tumor cells, such as PGE2 and LPA.
NFAT is directly implicated in promoting tumor angiogenesis. In endothelial cells NFAT is activated by VEGF and promotes vessel formation by inducing pro-angiogenic genes such as cyclooxygenase 2.
NFAT activation in tumor cells and the tumor microenvironment induces soluble factors that function in both paracrine and autocrine mechanisms to promote tumor progression.
Inactivation of NFAT decreases tumor formation. This is consistent with pathophysiological settings of enhanced expression of the calcineurin inhibitor DSCR1 which attenuates NFAT activation and reduces tumor incidence.
Inhibition of NFAT activation using small molecule inhibitors is predicted to suppress tumorigenesis. Paradoxically, patients receiving immunosuppressive therapy that blocks NFAT activity have a higher incidence of cancer.
Future cancer therapy targeting NFAT must take into account the cell-type specific phenotypes associated with deregulated NFAT activation.
Acknowledgments
We thank members of the Toker laboratory for many useful discussions and suggestions. We apologize to colleagues whose work could not be cited due to space limitations. The Toker laboratory is funded by grants from the US National Institutes of Health and National Cancer Institute, the Department of Defense and the Susan G. Komen Breast Cancer Foundation.
Glossary
- EMT
epithelial to mesenchymal transition, a complex process whereby genetic and epigenetic events lead to epithelial cells acquiring a mesenchymal architecture concomitant with enhanced cell motility. Typically associated with loss of E-cadherin expression
- Hemangioma
a benign self-involuting mass of proliferating endothelial cells that typically presents in children
- Hemidesmosomes
rivet-like structures found in epithelial cells and also keratinocytes that attach cells to the extracellular matrix. In epithelial cells, hemidesmosomes couple to integrins to promote attachment of cells to the ECM
- Priming Kinase
A ser/thr kinase that phosphorylates specific residues in proteins that in turn allow for the subsequent phosphorylation of additional residues downstream of the priming sites, typically mediated by distinct kinases. Casein kinases are examples of priming kinases
- Rel-family transcription factors
also known as the NF-κB family, these transcription factors share an amino-terminal REL-homology domain that is responsible for nuclear localization, dimerization and DNA binding
- Sumoylation
similar to ubiquitination in that proteins are post-translationally modified with SUMO, the small ubiquitin-like modifier. Unlike ubiquitination, SUMOylation does not target proteins for degradation, instead it facilitates nuclear-cytoplasmic shuttling, transcription, and cell cycle progression
- Chemokines
a family of secreted cytokines that regulate both immune surveillance as well as inflammatory responses upon infection
- Store-operated channels
calcium channels in the plasma membrane that allow influx of extracellular calcium in response to emptying of intracellular calcium stores such as the ER. Also known as CRAC channels
- VEGF-R
Receptor tyrosine kinases on the surface of endothelial cells that activate downstream signaling pathways subsequent to binding VEGF. VEGF-R1 is also known as Flt-1, VEGF-R2 is also known as KDR/FLK1
- Angiogenic switch
the transition of a non-vascularized solid tumor to a highly vascularized state following recruitment of blood vessels
Biographies
Alex Toker received his BS from King’s College and his PhD from the National Institute for Medical Research, London. He conducted post-doctoral research in the laboratory of Lewis Cantley in the Department of Cell Biology, Harvard Medical School and Division of Signal Transduction, Beth Israel Deaconess Medical Center. His first faculty appointment was as Staff Scientist at the Boston Biomedical Research Institute. In 2000 he joined the faculty of Beth Israel Deaconess Medical Center and Harvard Medical School in the Department of Pathology where he is currently an Associate Professor. Research in his laboratory focuses on signal transduction pathways mediated by protein kinases, integrins and transcription factors and how they modulate breast cancer progression.
Maria Mancini received her BS from the University of New England and her PhD from the University of Maine, Orono where she studied the role of TGFβ signaling in developmental and pathological angiogenesis. She is currently a post-doctoral research fellow in the laboratory of Alex Toker in the Department of Pathology at Beth Israel Deaconess Medical Center, Harvard Medical School. Her current research focuses on the mechanisms by which Akt and NFAT signaling promotes breast cancer progression.
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
Databases: The following terms in this article are linked on-line to: ENTREZ Gene: http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene
NFAT1 | NFAT2 | NFAT3 | NFAT4 | NFAT5 | NF-κB | FOS | JUN | PLCγ | STIM1 | ORAI1 | Calcineurin A | Calcineurin B | GSK-3 | CK1 | DYRK1A | DYRK2 | DSCR1 | AKT1 | PIK3CA | COX-2 | ENPP2 | VEGF | VEGFR1 | VEGFR2 | α6 | β4 | MDM2 | EDG1 | HIF1 | CSF1 | FKBP12
Further Information: Alex Toker laboratory homepage: http://www.tokerlab.com Access to the box links is available on-line.
References
- 1.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 (Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 2.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JP, et al. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–5. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 4.Jauliac S, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–4. doi: 10.1038/ncb816. This is the first study to demonstrate that NFAT is a pro-migration and invasion transcription factor in breast cancer. [DOI] [PubMed] [Google Scholar]
- 5.Medyouf H, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13:736–41. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- 6.Ryeom S, et al. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–31. doi: 10.1016/j.ccr.2008.02.018. These two studies reveal that DSCR1, a suppressor of calcineurin activity in endothelial cells, can function as a suppressor of tumor growth by attenuating NFAT activity and VEGF induction, as observed in Down’s syndrome patients who have elevated expression of DSCR1. [DOI] [PubMed] [Google Scholar]
- 7.Imamura R, et al. Carboxyl-terminal 15-amino acid sequence of NFATx1 is possibly created by tissue-specific splicing and is essential for transactivation activity in T cells. J Immunol. 1998;161:3455–63. [PubMed] [Google Scholar]
- 8.Chuvpilo S, et al. Multiple NF-ATc isoforms with individual transcriptional properties are synthesized in T lymphocytes. J Immunol. 1999;162:7294–301. [PubMed] [Google Scholar]
- 9.Chuvpilo S, et al. Alternative polyadenylation events contribute to the induction of NF-ATc in effector T cells. Immunity. 1999;10:261–9. doi: 10.1016/s1074-7613(00)80026-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42–8. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci U S A. 1999;96:7214–9. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–42. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang TT, Xiong Q, Graef IA, Crabtree GR, Chow CW. Recruitment of the extracellular signal-regulated kinase/ribosomal S6 kinase signaling pathway to the NFATc4 transcription activation complex. Mol Cell Biol. 2005;25:907–20. doi: 10.1128/MCB.25.3.907-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giffin MJ, et al. Structure of NFAT1 bound as a dimer to the HIV-1 LTR kappa B element. Nat Struct Biol. 2003;10:800–6. doi: 10.1038/nsb981. [DOI] [PubMed] [Google Scholar]
- 15.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356:801–4. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 18.Penna A, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–20. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 21.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 22.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–77. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–56. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Okamura H, et al. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell. 2000;6:539–50. doi: 10.1016/s1097-2765(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, McKeon F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature. 1999;398:256–60. doi: 10.1038/18473. [DOI] [PubMed] [Google Scholar]
- 27.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4. doi: 10.1126/science.275.5308.1930. The first study demonstrating that GSK-3 is a nuclear export kinase for NFAT. [DOI] [PubMed] [Google Scholar]
- 28.Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2000;3:3. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- 29.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Reviews Cancer. 2009 doi: 10.1038/nrc2664. in press. [DOI] [PubMed] [Google Scholar]
- 30.Okamura H, et al. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol Cell Biol. 2004;24:4184–95. doi: 10.1128/MCB.24.10.4184-4195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, et al. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–61. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- 32.Chow CW, Dong C, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol Cell Biol. 2000;20:5227–34. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22:3892–904. doi: 10.1128/MCB.22.11.3892-3904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwack Y, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–50. doi: 10.1038/nature04631. these two papers define DYRKs as the long sought-after export and maintenance kinases for NFAT. [DOI] [PubMed] [Google Scholar]
- 35.Arron JR, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. these two papers define DYRKs as the long sought-after export and maintenance kinases for NFAT. [DOI] [PubMed] [Google Scholar]
- 36.Nayak A, et al. Sumoylation of the transcription factor NFATc1 leads to its subnuclear relocalization and interleukin-2 repression by histone deacetylase. J Biol Chem. 2009;284:10935–46. doi: 10.1074/jbc.M900465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terui Y, Saad N, Jia S, McKeon F, Yuan J. Dual role of sumoylation in the nuclear localization and transcriptional activation of NFAT1. J Biol Chem. 2004;279:28257–65. doi: 10.1074/jbc.M403153200. [DOI] [PubMed] [Google Scholar]
- 38.Yoeli-Lerner M, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–50. doi: 10.1016/j.molcel.2005.10.033. In breast cancer cells, NFAT is ubiquitinated by the E3 ligase MDM2 that targets the transcription factor for degradation. [DOI] [PubMed] [Google Scholar]
- 39.Yoeli-Lerner M, Chin YR, Hansen CK, Toker A. Akt/Protein Kinase B and Glycogen Synthase Kinase-3{beta} Signaling Pathway Regulates Cell Migration through the NFAT1 Transcription Factor. Mol Cancer Res. 2009 doi: 10.1158/1541-7786.MCR-08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278:17246–54. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- 41.Buchholz M, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. Embo J. 2006;25:3714–24. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbs BK, Cruz AL, Werneck MB, Mognol GP, Viola JP. Dual roles for NFAT transcription factor genes as oncogenes and tumor suppressors. Mol Cell Biol. 2008;28:7168–81. doi: 10.1128/MCB.00256-08. In fibroblasts, distinct NFAT isoforms have non-overlapping roles as oncogenes and tumor suppressors as revealed in both in vitro studies and mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marafioti T, et al. The NFATc1 transcription factor is widely expressed in white cells and translocates from the cytoplasm to the nucleus in a subset of human lymphomas. Br J Haematol. 2005;128:333–42. doi: 10.1111/j.1365-2141.2004.05313.x. [DOI] [PubMed] [Google Scholar]
- 44.Pham LV, Tamayo AT, Yoshimura LC, Lin-Lee YC, Ford RJ. Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood. 2005;106:3940–7. doi: 10.1182/blood-2005-03-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 48.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–16. [PubMed] [Google Scholar]
- 49.Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26:6024–36. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin- containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion-lessons from thealpha 6 beta 4integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 52.Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J Biol Chem. 2006;281:12210–7. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- 53.Chen M, O’Connor KL. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene. 2005 doi: 10.1038/sj.onc.1208729. [DOI] [PubMed] [Google Scholar]
- 54.Stracke ML, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–9. [PubMed] [Google Scholar]
- 55.Yang SY, et al. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis. 2002;19:603–8. doi: 10.1023/a:1020950420196. [DOI] [PubMed] [Google Scholar]
- 56.Liu S, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–50. doi: 10.1016/j.ccr.2009.03.027. A very recent study that highlights the potent metastatic activity of autotaxin and its product LPA in breast cancer progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, et al. Dual Activity Lysophosphatidic Acid Receptor Pan-Antagonist/Autotaxin Inhibitor Reduces Breast Cancer Cell Migration In vitro and Causes Tumor Regression In vivo. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–75. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 60.Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312:522–6. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 62.Hernandez GL, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–20. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 64.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009 doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armesilla AL, et al. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol Cell Biol. 1999;19:2032–43. doi: 10.1128/mcb.19.3.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cockerill GW, et al. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood. 1995;86:2689–98. [PubMed] [Google Scholar]
- 67.Jinnin M, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–46. doi: 10.1038/nm.1877. NFAT activation is shown to lead to expression of VEGF-receptors and downstream signaling in hemangiomas, highly vascularized benign lesions typically seen in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–9. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 69.Baek KH, et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009 doi: 10.1038/nature08062. These two studies reveal that DSCR1, a suppressor of calcineurin activity in endothelial cells, can function as a suppressor of tumor growth by attenuating NFAT activity and VEGF induction, as observed in Down’s syndrome patients who have elevated expression of DSCR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin L, et al. Down syndrome candidate region 1 isoform 1 mediates angiogenesis through the calcineurin-NFAT pathway. Mol Cancer Res. 2006;4:811–20. doi: 10.1158/1541-7786.MCR-06-0126. [DOI] [PubMed] [Google Scholar]
- 71.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 72.Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 73.Karpanen T, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–90. [PubMed] [Google Scholar]
- 74.Kulkarni RM, Greenberg JM, Akeson AL. NFATc1 regulates lymphatic endothelial development. Mech Dev. 2009;126:350–65. doi: 10.1016/j.mod.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norrmen C, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185:439–57. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 77.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 78.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. Chemokine and chemokine receptors are shown to be highly upregulated and functional for metastatic dissemination of breast cancer cells. [DOI] [PubMed] [Google Scholar]
- 79.Karnoub AE, Weinberg RA. Chemokine networks and breast cancer metastasis. Breast Dis. 2006;26:75–85. doi: 10.3233/bd-2007-26107. [DOI] [PubMed] [Google Scholar]
- 80.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 81.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 82.Kaufman DB, et al. Immunosuppression: practice and trends. Am J Transplant. 2004;4 (Suppl 9):38–53. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 83.Martinez-Martinez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11:997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- 84.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–25. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 86.Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–72. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 87.Rezzani R. Cyclosporine A and adverse effects on organs: histochemical studies. Prog Histochem Cytochem. 2004;39:85–128. doi: 10.1016/j.proghi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 88.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371–3. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 89.Aramburu J, et al. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–37. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 90.Aramburu J, et al. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–33. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 91.Noguchi H, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004;10:305–9. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 92.Karanam BV, et al. Disposition of L-732,531, a potent immunosuppressant, in rats and baboons. Drug Metab Dispos. 1998;26:949–57. [PubMed] [Google Scholar]
- 93.Abel MD, et al. ISATX247: a novel calcineurin inhibitor. J Heart Lung Transplant. 2001;20:161. doi: 10.1016/s1053-2498(00)00290-4. [DOI] [PubMed] [Google Scholar]
- 94.Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254:703–6. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 95.Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci U S A. 2007;104:16176–81. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei C, et al. Calcium flickers steer cell migration. Nature. 2009;457:901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–34. doi: 10.1016/j.ccr.2008.12.019. This paper reveals that the STIM1 and Orai1 proteins, comprising the store operated calcium channels, promote calcium signals that are required for cell migration. [DOI] [PubMed] [Google Scholar]
- 98.Szado T, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci U S A. 2008;105:2427–32. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 100.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–4. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amasaki Y, et al. A constitutively nuclear form of NFATx shows efficient transactivation activity and induces differentiation of CD4(+)CD8(+) T cells. J Biol Chem. 2002;277:25640–8. doi: 10.1074/jbc.M201860200. [DOI] [PubMed] [Google Scholar]
- 102.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–35. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 103.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 104.Rengarajan J, Tang B, Glimcher LH. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naive T(H)cells. Nat Immunol. 2002;3:48–54. doi: 10.1038/ni744. [DOI] [PubMed] [Google Scholar]