Abstract
We investigated the relation between the two systems of visuospatial attention and working memory by examining the effect of normal variation in cholinergic and noradrenergic genes on working memory performance under attentional manipulation. We previously reported that working memory for location was impaired following large location precues, indicating the scale of visuospatial attention has a role in forming the mental representation of the target. In one of the first studies to compare effects of two single nucleotide polymorphisms (SNPs) on the same cognitive task, we investigated the neurotransmission systems underlying interactions between attention and memory. Based on our previous report that the CHRNA4 rs#1044396 C/T nicotinic receptor SNP affected visuospatial attention, but not working memory, and the DBH rs#1108580 G/A noradrenergic enzyme SNP affected working memory, but not attention, we predicted that both SNPs would modulate performance when the two systems interacted and working memory was manipulated by attention. We found the scale of visuospatial attention deployed around a target affected memory for location of that target. Memory performance was modulated by the two SNPs. CHRNA4 C/C homozygotes and DBH G allele carriers showed the best memory performance but also the greatest benefit of visuospatial attention on memory. Overall, however, the CHRNA4 SNP exerted a stronger effect than the DBH SNP on memory performance when visuospatial attention was manipulated. This evidence of an integrated cholinergic influence on working memory performance under attentional manipulation is consistent with the view that working memory and visuospatial attention are separate systems which can interact.
INTRODUCTION
Beginning with Cowan (1988), there has been an impetus toward a more comprehensive understanding of the normal interaction between attention and memory. There is evidence that separate storage and selection processes exist, but increasing recognition that these processes interact (Awh, Vogel, & Oh, 2006). Newer theories of working memory argue that the focus of visuospatial attention plays a central role within working memory, although the theories do not agree on the specifics of that role (Cowan, 2001; Engle, Kane, & Tuholski, 1999). One approach has examined how the focus of visuospatial attention influences the limited-capacity store of working memory (Conway, Cowan, & Bunting, 2001; Baddeley, 1992). Using such an approach, we found that visual working memory for a target location is more accurate when the target was preceded by smaller compared to larger precues (Greenwood, Lambert, Sunderland, & Parasuraman, 2005). That finding extended current views of the relation between visuospatial attention and working memory by showing that dynamic adjustments of the scale of visuospatial attention play a fundamental role in controlling the encoded representation in working memory.
Neuroimaging and electrophysiological studies have also revealed that the focus of visuospatial attention exerts modulatory effects on posterior working memory stores. This has been seen in inferior temporal (Ranganath, 2006) as well as striate and extrastriate regions (Vogel, McCollough, & Machizawa, 2005; Super, Spekreijse, & Lamme, 2001). Several authors recently reviewed the evidence bearing on the role of prefrontal cortex (PFC) in working memory (Postle, 2006; Curtis & D’Esposito, 2003), concluding PFC does not provide temporary storage of information, but rather exerts attention-based control over the posterior stores of working memory. Consistent with that view is human evidence that manipulations of visuospatial attention induce PFC to modulate extrastriate-generated ERPs in the P1 latency range (Vogel et al., 2005; Yago, Duarte, Wong, Barcelo, & Knight, 2004; Foxe & Simpson, 2002). Electrophysiological and electromagnetic recordings showed that directing the focus of attention did not alter the initial striate response measured in fMRI, but did enhance later striate activity (140–250 msec) (Noesselt et al., 2002). Thus, there is growing evidence that the attentional focus and working memory, although separate systems mediated in different brain regions, clearly interact.
Although fMRI and ERP studies have helped identify the spatio-temporal properties of brain networks underlying visuospatial attention and working memory, these techniques cannot speak directly to the innervation of these networks or to the way gene expression influences such innervation. Pharmacological and genetic methods offer another avenue for examining these questions (Posner, Rothbart, & Sheese, 2007; Fossella & Casey, 2006; Parasuraman & Greenwood, 2004; Sarter & Bruno, 2004; Greenwood & Parasuraman, 2003; Everitt & Robbins, 1997). However, the specific neurotransmission systems involved in the influence of attention on working memory stores are not known. As reviewed below, the nicotinic cholinergic system is an important mediator of visuospatial attention, whereas noradrenergic and dopaminergic systems have been shown to be important mediators of working memory. There is substantial animal evidence reporting a role for muscarinic and nicotinic receptors in cognition generally, including attention, learning, and memory (Levin, McClernon, & Rezvani, 2006). However, some of the paradigms commonly used in rodent studies of working memory require both attention and working memory, for instance, the radial-arm maze. Moreover, there is evidence that the influence of cholinergic agents on delay-period effects on memory tasks in primates are exerted through visuospatial attention (Furey et al., 2000; Voytko, 1996; Robbins et al., 1989).
Molecular genetics offers one way to investigate the neurotransmission systems involved in attentional modulation of working memory, viz, by using allelic association methods in the context of performance on visuospatial attention and working memory tasks. Such an approach does not involve the direct manipulation of neurotransmission systems used in pharmacological studies, but rather examines the cognitive consequences of normal variation in neurotransmission genes on the efficiency of those systems. Several groups have used this approach with enzyme and receptor genes (Greenwood, Fossella, & Parasuraman, 2005;Greenwood, Lambert, et al., 2005; Parasuraman, Greenwood, Kumar, & Fossella, 2005; Goldberg & Weinberger, 2004;Greenwood & Parasuraman, 2003; Fossella et al., 2002).
In a previous investigation, we observed that whereas working memory was modulated by variation in a noradrenergic gene but not by variation in a nicotinic cholinergic gene, shifting (Parasuraman et al., 2005) and scaling (Greenwood et al., 2006) of visuospatial attention were modulated by variation in a nicotinic gene but not a noradrenergic gene. We hypothesized that if a processing overlap exists between visuospatial attention and visual working memory (Greenwood, Lambert, et al., 2005; Cowan, 2001; Awh, Jonides, & Reuter-Lorenz, 1998), then when visuospatial attention is experimentally manipulated within working memory, memory performance should be modulated by variation in both cholinergic and noradrenergic genes. To test this, we measured the effect of normal variation in noradrenergic and cholinergic neurotransmission genes in a working memory task in which the focus of visuospatial attention was experimentally manipulated.
Selection of genes for studying the mediation of visuospatial attention and working memory in people can be guided by what is known of the pharmacology of those processes. Several groups have reported that working memory performance is influenced by dopamine (DA) D1 receptor availability (Abi-Dargham et al., 2002; Castner, Williams, & Goldman-Rakic, 2000). Noradrenergic agonists also influence both working memory and PFC activity. For example, Avery, Franowicz, Studholme, van Dyck, and Arnsten (2000) observed that an alpha-2A adrenoreceptor agonist improved working memory performance and increased blood flow in prefrontal but not temporal cortex in monkeys. With regard to visuospatial attention, disengagement of attention from an invalidly cued location is influenced by cholinergic agonists. In both monkeys and humans, administration of nicotine selectively enhanced the effects of invalid cues on performance (Witte, Davidson, & Marrocco, 1997) and reduced effects of cue validity overall (Thiel, Zilles, & Fink, 2005). Abstinence from nicotine in smokers also selectively enhanced effects of invalid cues (Shirtcliff & Marrocco, 2003).
Based on this evidence of nicotinic cholinergic mediation of visuospatial attention and noradrenergic and dopaminergic mediation of working memory, we previously tested hypotheses concerning the effect of normal variation of genes in the noradrenergic and cholinergic neurotransmission pathways on working memory and visuospatial attention, respectively. We reported that normal variation in a gene that encodes dopamine beta hydroxylase (DBH), an enzyme that converts DA to norepinephrine (NE) in synaptic vesicles, modulated working memory for location. The G allele of the 444 G/A SNP (rs#1108580) in the DBH gene was previously associated with higher DBH enzyme activity levels in serum (Cubells et al., 1998) and reduced risk of paranoid ideation in depressed patients (Wood, Joyce, Miller, Mulder, & Kennedy, 2002). We found that the G allele was associated with superior spatial working memory performance but did not modulate visuospatial attention, either attentional shifting (Parasuraman et al., 2005) or attentional scaling (Greenwood, Fossella, et al., 2005). With regard to genes with a role in attention, we reported that the ability to shift visuospatial attention was modulated by normal variation in a nicotinic receptor gene. The alpha4/beta2 nicotinic cholinergic receptor is the most widely distributed nicotinic receptor in the CNS (Flores, Rogers, Pabreza, Wolfe, & Kellar, 1992). The CHRNA4 gene codes the alpha4 subunit of the nicotinic cholinergic receptor and has an SNP (termed 1545 C/T, rs#1044396) previously found to be associated with nicotine addiction (Feng et al., 2004) but not with attention deficit hyperactivity disorder (Kent et al., 2001). Shifting the focus of visuospatial attention was modulated by the CHRNA4 rs#1044396 C/T SNP but not by the DBH rs#1108580 G/A SNP (Parasuraman et al., 2005). Adjusting the size (scale) of the focus of visuospatial attention (Greenwood, Sunderland, Putnam, Levy, & Parasuraman, 2005; Greenwood & Parasuraman, 2004) was modulated by the same CHRNA4 SNP but not by the DBH SNP (Greenwood, Lambert, et al., 2005).
There is also evidence that the cholinergic system does influence some aspects of memory. In general, nicotinic agonists benefit memory and nicotinic antagonists impair it. However, it appears that only certain types of memory are influenced and only under certain conditions (Dani & Bertrand, 2007). The work of Levin and Simon (1998) in rodents shows that such influences occur most often when the task is difficult or the animal cognitively impaired. Moreover, the 8-arm radial maze used in most rodent studies of working memory may require the effective use of attention as well as working memory. Consistent with that interpretation, Voytko et al. (1994) found that lesions of the nucleus basalis of Meynert (nbM) in monkeys did not impair performance on several working memory tasks (delayed nonmatch-to-sample, delayed response) but did impair the ability to shift visuospatial attention. Such findings led Voytko (1996) to conclude that cholinergic dysfunction in the primate basal forebrain cholinergic system leads to deficits of attention rather than memory. Everitt and Robbins (1997) and Robbins et al. (1989) have also argued that apparent cholinergic modulation of delay-period effects on performance may be due to cholinergic modulation of visuospatial attention rather than of working memory.
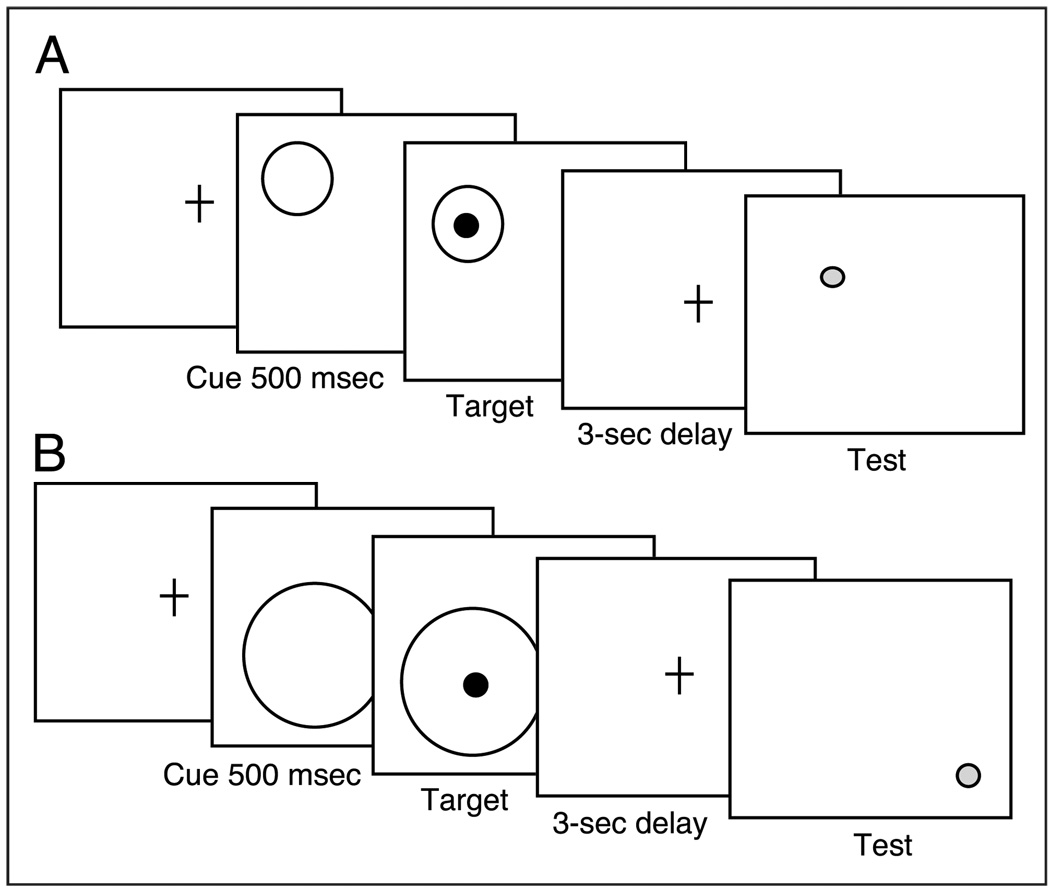
Understanding interactions between cognitive systems can be advanced by converging evidence from different methodologies. The interaction between visuospatial attention and working memory has been investigated behaviorally (e.g., Awh & Jonides, 1998), electrophysiologically (e.g., Vogel et al., 2005; Vogel & Machizawa, 2004), and pharmacologically (e.g., Everitt & Robbins, 1997; Voytko, 1996). In the present study, we investigate the question using genetics. In previous work, we have argued that evidence of genetic dissociation on cognitive performance indicates dissociation in the underlying innervation (Greenwood, Fossella, et al., 2005). In the present study, we apply that logic to investigate the interaction between visuospatial attention and working memory. We know from our previous observation of a double dissociation that CHRNA4 rs#1044396 modulates visuospatial attention, but not working memory, whereas DBH rs#1108580 modulates working memory, but not visuospatial attention (Parasuraman et al., 2005). What has not been investigated previously is the effect of these SNPs when working memory is manipulated by attention. Such an investigation allows us to move beyond cataloging the separate effects of neurotransmission SNPs on component processes of cognitive functions to greater understanding of the normal interaction between component processes. Based on our previously reported evidence of specificity in the effect of neurotransmission genes on visuospatial attention and working memory, we predicted that both cholinergic and noradrenergic neurotransmission SNPs would modulate performance when working memory was manipulated by attention. We manipulated visuospatial attention in the size—hence, the precision—of precues to target location and measured the accuracy of memory for that location after 3 sec (Figure 1). We also manipulated working memory difficulty by varying the distance between the target location and the test location when the two did not match.
Figure 1.
Illustration of task manipulating the scale of visuospatial attention at the location to be remembered over 3 sec. Circular precues varying in size (1.6°, 5.2°, and 8.1°) preceded a black target dot (100 msec duration) with an SOA of 500 msec. There was a requirement to remember dot location over a 3-sec delay. After the delay, a decision was required indicating whether the location of a red test dot matched that of the target dot. (A) Match trial, with test location matching target location. (B) Nonmatch trial illustrated with test dot at one of three distances (1.9°, 3.8°, 5.7°) from target location.
METHODS
Participants
Participants were recruited from Catholic University, George Mason University, and the Washington, DC community. All procedures were approved by the institutional review boards of each university and were performed in accordance with the 1964 Declaration of Helsinki. All persons gave informed consent prior to their inclusion in the study. Informed consent was obtained and vision was tested to ensure at least 20/30 vision (after correction, if necessary) on a Rosenbaum pocket screener. Participants included both young and old but the sample was not large enough to allow analysis by both age group and genotype. All participants were screened by questionnaire for neurologic and psychiatric illness. All participants were cognitively screened by means of the Wechsler WAIS Vocabulary subtest (Wechsler, 1981) and the Wechsler Memory Scale—Revised Logical Memory subtest (Wechsler, 1987). To eliminate individuals with a dementing illness, participants aged 65 years and older were additionally screened with the Mini-Mental State Exam. Demographic information for each SNP and for young and old groups is provided in Table 1. There were no significant differences in performance on these standardized neuropsychological tests whether individuals were grouped by CHRNA4 rs#1044396 C/T or DBH rs#1108580 G/A genotype.
Table 1.
Demographics of Participant Groups (Means and Standard Deviations)
| CHRNA4 rs#1044396 Genotype Groups | |||
|---|---|---|---|
| C/C | C/T | T/T | |
| Old | |||
| Sample size | 26 | 39 | 31 |
| Age | 70.0 (4.7) | 71.5 (5.1) | 71.7 (5.2) |
| Sex (F/M) | 11/15 | 27/12 | 15/16 |
| WAIS-R Vocabularya | 50.1 (8.1) | 58.3 (7.4) | 59.2 (7.4) |
| Logical memoryb | |||
| Immediate | 10.2 (3.8) | 10.7 (3.8) | 10.6 (4.4) |
| Delayed | 8.2 (4.2) | 8.4 (3.7) | 8.6 (5.3) |
| Mini-Mental State Examc | 29.0 (1.2) | 28.6 (1.3) | 27.8 (2.1) |
| Young | |||
| Sample size | 15 | 50 | 17 |
| Age | 20.0 (2.1) | 19.6 (1.7) | 20.1 (2.1) |
| Sex (F/M) | 12/3 | 37/13 | 10/7 |
| WAIS-R Vocabularya | 45.1 (11.9) | 47.3 (10.2) | 48.3 (6.6) |
| Logical memory (WMS-R)b | |||
| Immediate | 11.4 (3.7) | 11.1 (3.9) | 12.6 (3.6) |
| Delayed | 10.1 (3.5) | 9.9 (3.8) | 11.2 (3.7) |
| DBH rs#1108580 Genotype Groups | |||
| A/A | A/G | G/G | |
| Old | |||
| Sample size | 37 | 60 | 23 |
| Age | 71.9 (4.4) | 71.0 (5.5) | 70.5 (4.8) |
| Sex (F/M) | 14/23 | 36/24 | 15/8 |
| WAIS-R Vocabularya | 59.6 (7.8) | 59.6 (7.3) | 57.4 (7.2) |
| Logical memory (WMS-R)b | |||
| Immediate | 10.3 (3.9) | 11.1 (4.2) | 10.1 (3.9) |
| Delayed | 8.7 (4.3) | 8.6 (4.7) | 8.0 (3.6) |
| Mini-Mental State Examc | 27.8 (2.0) | 28.7 (1.8) | 28.5 (1.2) |
| Young | |||
| Sample size | 45 | 55 | 33 |
| Age | 19.9 (2.1) | 19.9 (2.0) | 19.5 (1.7) |
| Sex (F/M) | 30/15 | 35/20 | 25/8 |
| WAIS-R Vocabularya | 50.4 (10.9) | 48.0 (8.4) | 48.8 (8.8) |
| Logical memory (WMS-R)b | |||
| Immediate | 11.3 (3.1) | 12.4 (3.9) | 10.8 (4.4) |
| Delayed | 10.2 (3.0) | 11.2 (4.0) | 9.9 (4.6) |
Administered only to thoseolder than 65 years (Folstein, Folstein, & McHugh, 1975).
Genotyping
Buccal (cheek) swabs were obtained from each participant. Genomic DNA was purified from these swabs as directed by the manufacturer (MasterAMP TMBuccal Swab DNA Extraction Kit, Epicentre Technologies, Madison, WI). Participants were genotyped (double-blind) for a nicotinic receptor SNP (rs#1044396, originally termed CHRNA4 1545 C/T by Steinlein et al., 1997) and a noradrenergic enzyme SNP (rs#1108580, originally termed DBH 444 G/A; Cubells et al., 1998) as described below.
In order to analyze the CHRNA4 rs#1044396 C/T SNP, a PCR fragment 309 bp in length was amplified from genomic DNA in a reaction volume of 50 µl, using PTC-100 or PTC-200 thermal cyclers (MJ Research). The amplified PCR fragment was purified on AMPure magnetic beads (Agencourt) and the DNA sequence of this PCR fragment was determined by cycle sequencing with BigDye terminators on an ABI 310 capillary sequencer. We and others have previously shown that this method allows reliable determination of both homozygous and heterozygous genotypes (Hare & Palumbi, 1999).
In order to analyze the DBH rs#1108580 G/A SNP, we chose an alternative method that combined nested PCR, plus allele-specific Tm-shift primers and automated melting curve analysis (Wang et al., 2005). First an “external” PCR fragment was preamplified for 20 cycles in a reaction volume of 50 µl in PTC-100 or PTC-200 thermal cyclers (MJ Research). Then a 1-µl aliquot of the first-round PCR was reamplified with two or three “internal” primers (which primed inside the original PCR fragment), in a reaction volume of 15 µl on a Bio-Rad iCycler. The internal primers included two allele-specific Tm-shift primers, which were designed as described (Wang et al., 2005). The allele-specific Tm-shift primers were used both separately and together (i.e., in three PCR reactions), all of which also contained a common reverse primer. DBH alleles were scored by automated analysis of the melting curves of the PCR products (using Bio-Rad iCycler software). The assay is designed so that the two possible PCR products (incorporating one or the other allele-specific Tm-shift primer) differ in melting temperature because of a GC-tail that is included in the 5′ end of one of the allele-specific primers (Wang et al., 2005).
Nine participants were excluded for low accuracy (performance at a level less than chance). Seven of the excluded were over 65 years of age. For CHRNA4 rs#1044396 C/T, there were 41 C/C homozygotes, 89 C/T heterozygotes, 48 T/T homozygotes. For DBH rs#1108580G/A, there were 82 A/A homozygotes, 115 A/G heterozygotes, and 56 G/G homozygotes. SNPs in both samples were in Hardy–Weinberg equilibrium (p > .05 in respective χ2 tests).
Stimuli and Procedures
A cued working memory task was used (Figure 1). This task was designed to manipulate the accuracy of memory for location by varying the precision of location precues to that location. Following a 1-sec duration fixation cross, a circular cue appeared for 500 msec in 1 of 12 randomly selected locations on the screen and in 1 of 3 of visual angles in size (1.6°, 5.2°, and 8.1°). At cue offset, one black target dot (0.67° in diameter) appeared centered within the cue for 100 msec. On 10% of trials, the cues were invalid and the target appeared outside the cue. This condition was included to reduce predictability of the cue. Data from these trials were not analyzed, as there were insufficient numbers. When cues were valid, the target was always centered in the cue. At target offset, a 3-sec delay began during which time only the fixation cross was visible. After the delay, the screen cleared and a red test dot appeared either at the same location as the target dot (match trial) or at a different location (nonmatch trial). This trial type manipulation (match/nonmatch) provided a memory load manipulation. On nonmatch trials, the distance between the target location and the test dot varied (target–test distance [TTD]), being 1.9°, 3.8°, or 5.7° apart. The closest distance required the most accurate memory. The red test dot remained visible for 2 sec during which a same/different decision was required. Both accuracy and reaction time (RT) were measured but the instructions emphasized accuracy.
In an additional sample of participants not yet genotyped, the same task was administered, but with a no-cue condition added to the other conditions. This was done to confirm the beneficial nature of the cue on task performance. The duration of the fixation was lengthened on no-cue trials to preserve the timing between fixation and target onset.
RESULTS
All statistical tests were performed at the .05 level of significance. Repeated measures F values were corrected for violations from sphericity. The task was designed to assess effects of genotype on accuracy of memory for target location after a delay, under an attentional manipulation. Therefore, percent correct is the most useful measure. In addition, some analyses of the RT data were carried out.
Reaction Time Analyses
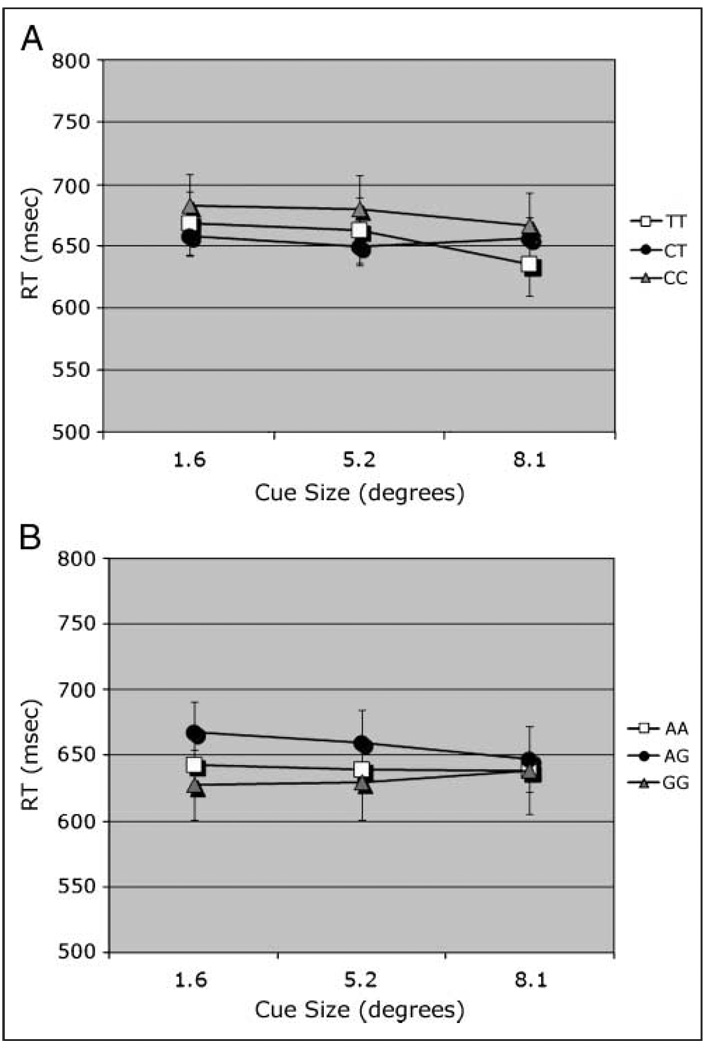
Median RTs were analyzed in an omnibus ANOVA for each SNP. To allow comparisons between match and nonmatch conditions, data were collapsed across TTD. When the data were grouped by CHRNA4, there was no main effect of genotype. Median RTs were slower on nonmatch compared to match trials [F(1, 174) = 4.05]. RT was speeded slightly but significantly [F(2, 348) = 5.04] as the size of the cues increased (667, 661, 654 msec for sizes 1.6°, 5.2°, and 8.1°, respectively). Cue size interacted with CHRNA4 genotype [F(4, 348) = 2.49; Figure 2A]. There was also a nonsignificant trend in the interaction of Trial type × Cue size × Age group [F(4, 348) = 1.91, p < .11]. There were no other significant effects. Nonmatch RT data were analyzed over levels of TTD, revealing no effect of CHRNA4 genotype.
Figure 2.
RT measure. (A) Interaction of Cue size × CHRNA4 1044396 in an omnibus analysis (collapsed across TTD). (B) Interaction of Cue size × DBH rs#1108580 × Trial type; match trial plotted.
When the data were grouped by DBH genotype, there was no effect of DBH. There was a significant main effect of cue size [F(2, 500) = 4.88], with RT decreasing as cue size increased (645, 641, and 631 msec for sizes 1.6°, 5.2°, and 8.1°, respectively). There were significant interactions of Trial type × Cue size [F(2, 500) = 3.51] and DBH × Trial type × Cue size [F(4, 500) = 2.41]. The latter interactions can be attributed to a genotype effect on match trials, which decreased with cue size (Figure 2B). Nonmatch RT data were analyzed over levels of TTD, revealing no effect of DBH genotype.
Accuracy Analyses
Accuracy ratios (defined as number correct/number presented) were subjected to repeated measures ANOVA with factors as described below.
Age Effects
Because the sample was heterogeneous with regard to age, effect of age group was initially assessed. An omnibus ANOVA compared performance of young and old (between-subjects factor of age group) on the within-subjects factor of trial type (match, nonmatch). As only nonmatch trials had levels of TTD, data were averaged over those distances on nonmatch trials. The age groups did not differ on working memory accuracy overall, but there was a significant interaction of Trial type × Age group [F(1, 252) = 3.96]. The old group was slightly more accurate than the young on match trials (means of 0.882 for old, 0.867 for young), but slightly less accurate on nonmatch trials (means of 0.919 for old and 0.924 for young). There were no other significant interactions with age group. Accuracy was lower on match trials overall [task type: F(1, 252) = 83.03] and highest following the medium-sized cue [cue size: F(2, 504) = 3.87]. These effects interacted [F(2, 504) = 4.78]. Despite the absence of age effects, it remains possible that age might drive some of the genotype effects. To assess that possibility, effects of age group were analyzed for each SNP. There were no main effects of age group and no interactions involving age group and genotype. The criterion used for this analysis was p < .10. In light of the weak effects of age, and the low numbers in some cells when the sample was divided by both genotype and age, subsequent analyses were conducted without age group as a factor.
CHRNA4 C/T (rs#1044396)
Because TTD could be varied only under nonmatch conditions, an omnibus ANOVA compared trial type (match, nonmatch) by averaging across TTD on nonmatch trials. The within-subjects factor was trial type (match, nonmatch). The between-subjects factor was CHRNA4 rs#1044396 genotype (T/T, T/C, C/C).
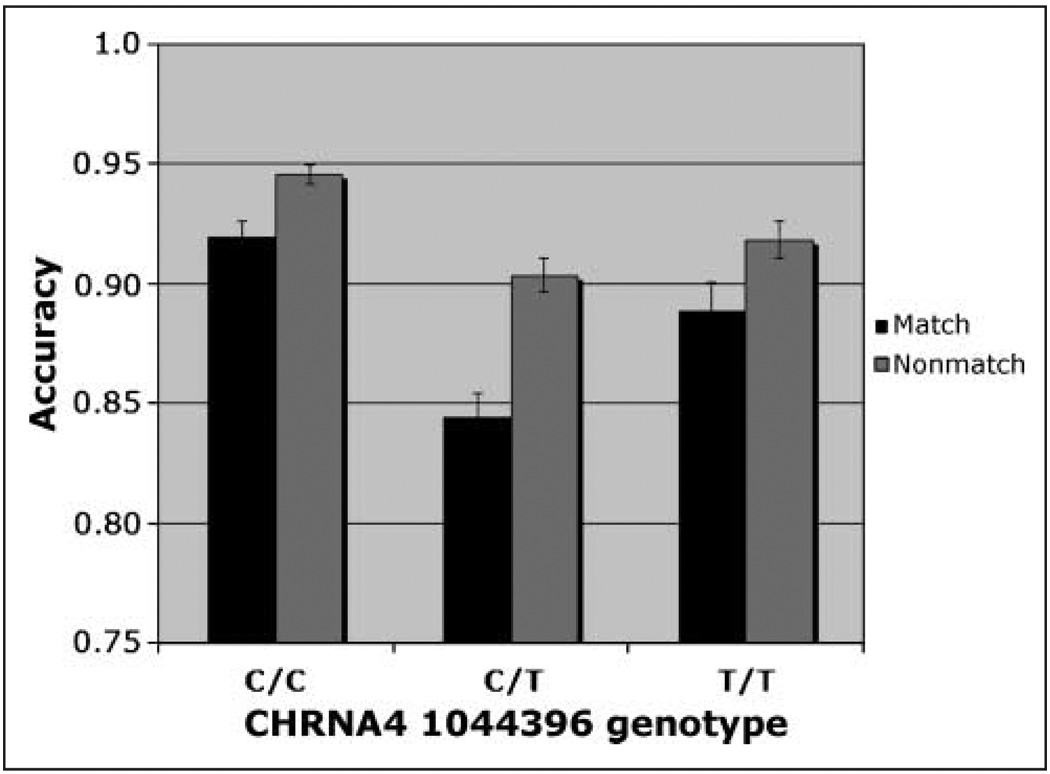
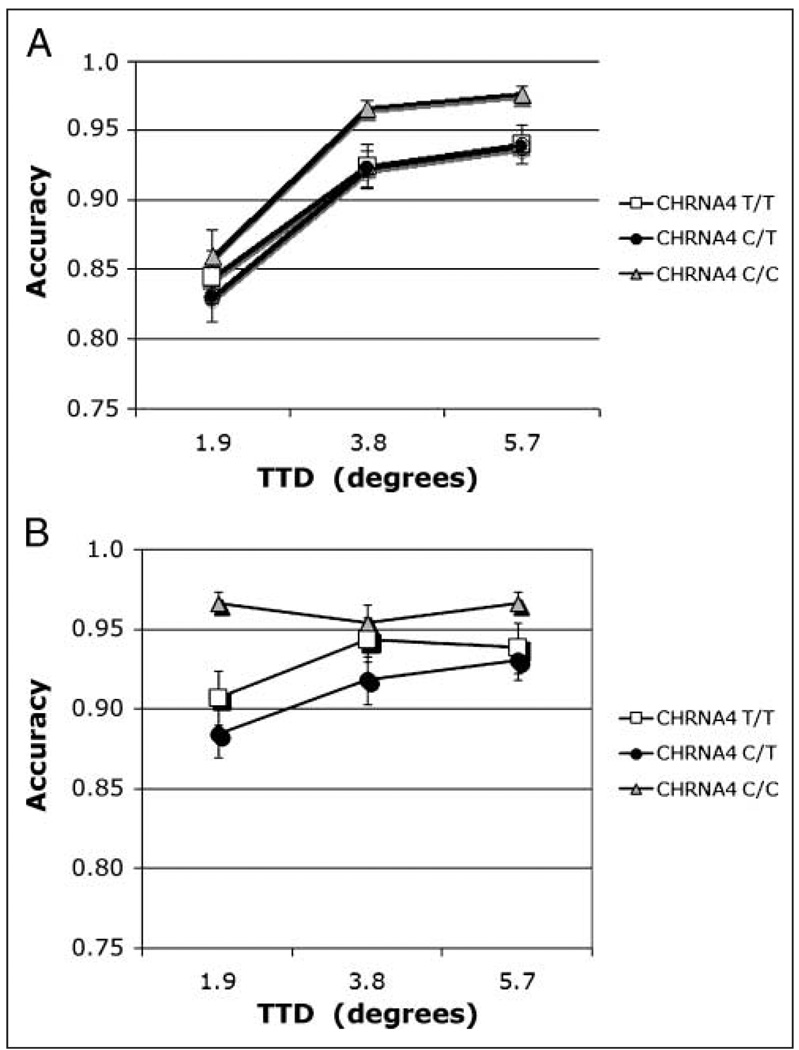
The omnibus analysis required collapsing across TTD to allow comparisons between match and nonmatch conditions. This analysis revealed significant main effects of CHRNA4 genotype [F(2, 174) = 4.15, η2 = .05], trial type [F(1, 174) = 37.40], and cue size [F(2, 348) = 7.29]. Accuracy was highest in the C/C homozygotes (mean = 0.93). Both the Trial type × Cue size interaction [F(2, 348) = 5.23] and the Trial type × CHRNA4 genotype interaction [F(2, 174) = 3.21, η2 = .01] were significant. The three-way interaction was not significant. The C/C homozygotes were most accurate and the C/T heterozygotes were least accurate, particularly on match trials. Although heterozygotes are expected to perform at an intermediate level between homozygote groups (as we have reported previously; Greenwood, Fossella, et al., 2005), we do not invariably observe that result (e.g., Greenwood, Lambert, et al., 2005). Based on the Trial type × CHRNA4 interaction (Figure 3), simple main effects of genotype were calculated at each level of trial type.
Figure 3.
Accuracy. Interaction of CHRNA4 genotype × Trial type in an omnibus analysis (collapsed across TTD) of individuals genotyped for CHRNA4 rs#1044396 C/T.
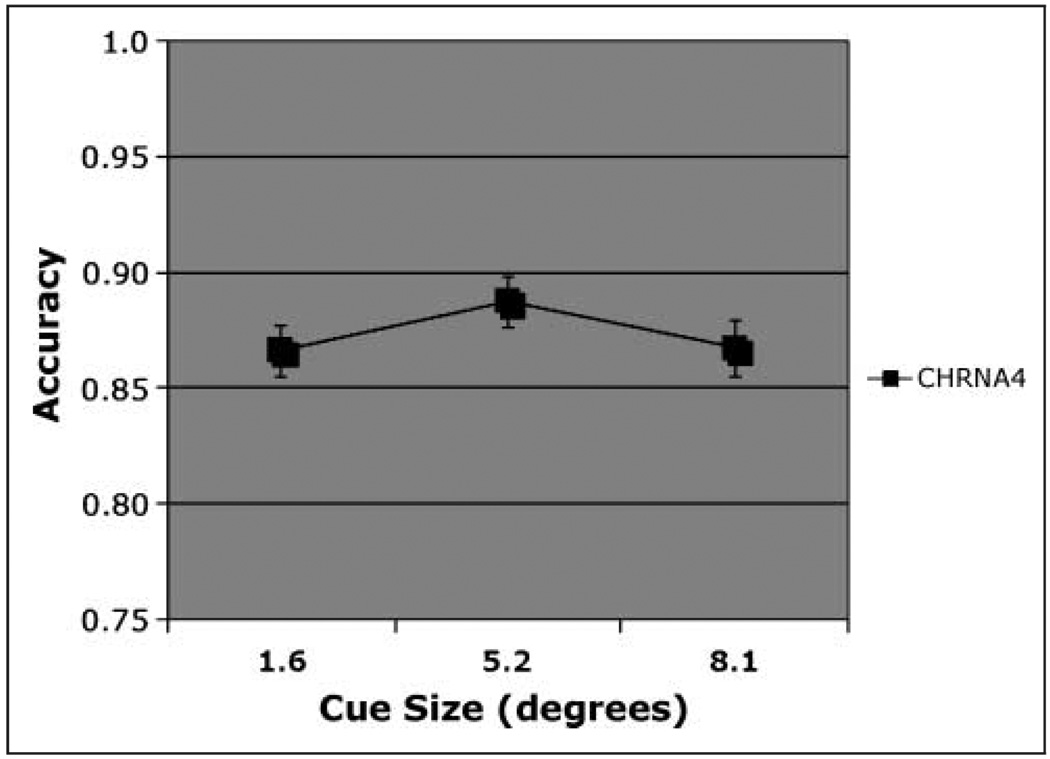
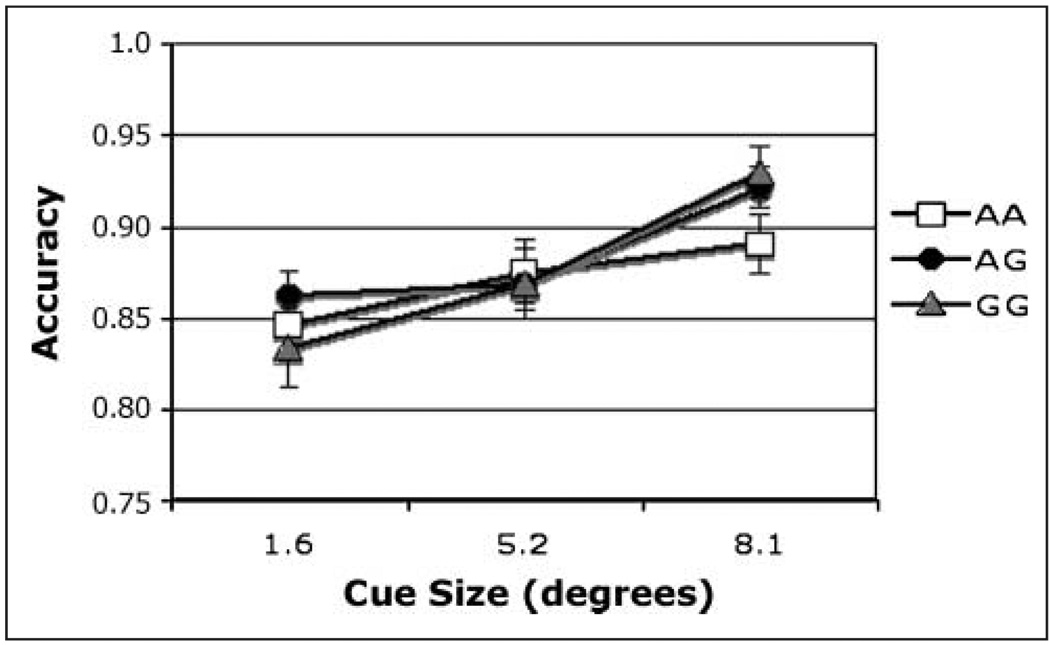
On match trials, there was a significant effect of CHRNA4 genotype [F(2, 174) = 4.76, η2 = .055], with accuracy highest in C/C homozygotes. Simple effects of CHRNA4 reveal that, after using a Bonferroni adjustment for multiple comparisons, only the C/C genotype differed significantly from the C/T genotype (mean difference = 0.095). Using a formula for unequal samples (Cohen, 1988), the size of the main effect of CHRNA4 was calculated to be 0.25. In light of the concern about false positives in candidate gene studies, it can be noted that this effect size is close to the value of 0.29 recently reported in a meta-analysis of the effect of the COMT val158met polymorphism on the Wisconsin Card Sort Task, a test of executive function (Barnett, Jones, Robbins, & Muller, 2007). With regard to cue size, accuracy was highest following the medium-sized cue [Figure 4; F(2, 348) = 4.07], consistent with our previous report on this task from a different population (Greenwood, Lambert, et al., 2005). These two factors did not interact.
Figure 4.
Accuracy. Match condition, main effect of cue size on accuracy in individuals genotyped for CHRNA4 rs#104439 C/T.
On nonmatch trials, CHRNA4 C/C homozygotes were again the most accurate but that was only a nonsignificant trend (p = .07). Accuracy increased with cue size [F(2, 348) = 14.19]. A full analysis of nonmatch trials was also conducted, without the constraints imposed by the omnibus analysis.
Full Analysis of Nonmatch Trials
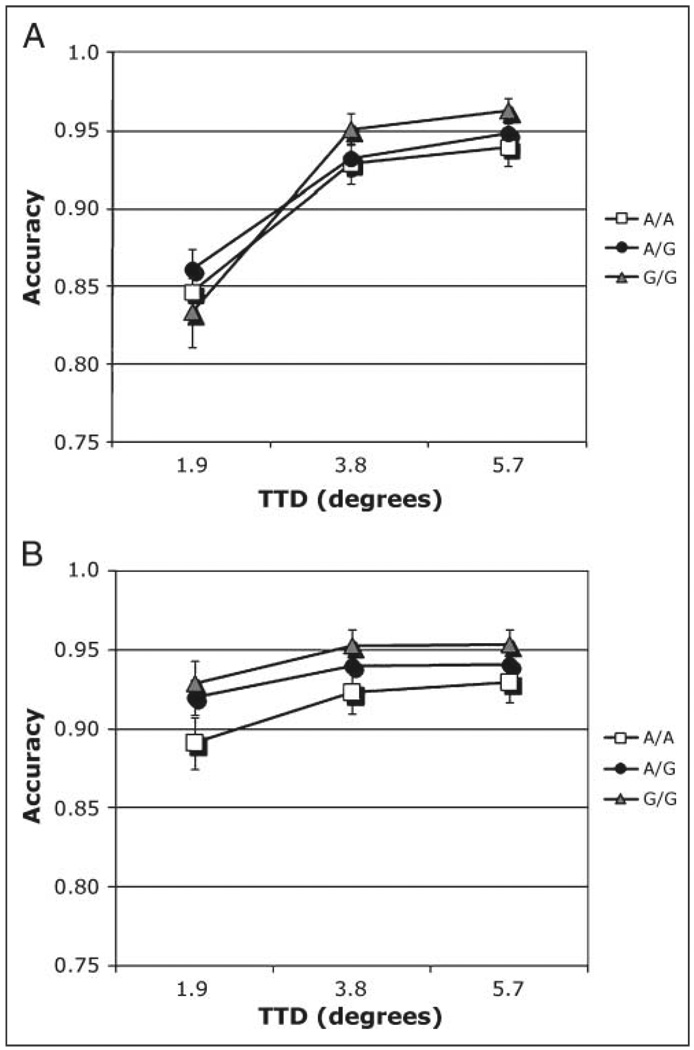
A full analysis was carried out on nonmatch trials, with CHRNA4 genotype as the between-subjects factor and cue size and TTD as within-subjects factors. Memory accuracy was highest in the C/C homozygotes but that was only a nonsignificant trend (p = .07). Accuracy increased with cue size [F(2, 348) = 14.19], but that effect was strongest when target and test stimuli locations were close together, making the match/nonmatch judgment difficult. These results were reflected in a main effect of TTD [F(2, 348) = 106.39] and a TTD × Cue size interaction [F(4, 696) = 25.41]. Overall, performance improved as cue size increased and as TTD increased. These factors interacted with CHRNA4 genotype [Cue size × TTD × CHRNA4: F(8, 696) = 1.96, η2 = .01). This interaction was analyzed using simple main effects. Simple main effects of TTD and genotype at each level of cue size were conducted to determine the source of that interaction. Results from the smallest cue are plotted in Figure 5A and from the largest cue in Figure 5B. The two smaller cue size conditions did not yield significant effects. At the largest cue size, main effects of CHRNA4 [F(2, 175) = 3.56], TTD [F(2, 350) = 8.61], and their interaction were significant [Figure 5A and B; F(4, 350) = 3.56]. When adjusted for the number of comparisons, the interaction remained significant. Following the large cue, C/C homozygotes were more accurate at the closest 1.9° TTD compared to the other genotype groups. This suggests that the C/C genotype obtained greater benefits from the large cue when the discrimination was difficult.
Figure 5.
(A, B) Accuracy. Nonmatch condition, interaction of Cue size × TTD in individuals genotyped for CHRNA4 rs#1044396 C/T. (A) Cue size 1 = 1.6°. (B) Cue size 3 = 8.1°.
DBH rs#1108580 G/A
Omnibus
Collapsing across TTD allowed comparisons between match and nonmatch conditions (trial type) in one omnibus analysis. That analysis revealed no main effect of DBH genotype. There were significant main effects of trial type [F(1, 250) = 82.43] and cue size [F(2, 500) = 3.67], which interacted [Trial type × Cue size: F(2, 500) = 5.17]. Trial type also interacted with DBH [F(2, 250) = 4.58, η2 = .013]. Based on the interaction of Trial type × DBH, separate analyses were carried out for DBH at each level of trial type (match and nonmatch).
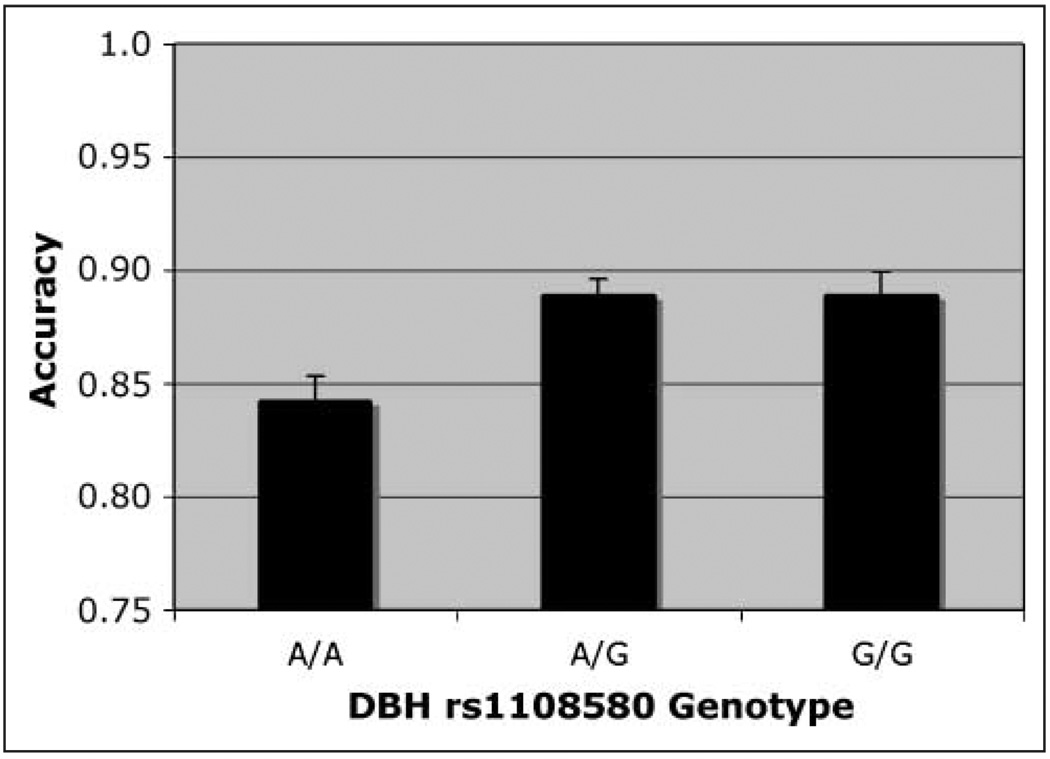
On match trials, when the test stimulus appeared at the target location, accuracy was higher in the DBH rs#1108580 G carriers and lower in the A/A homozygotes [Figure 6; F(2, 250) = 3.55]. Simple main effects analysis revealed that only A/A and A/G differed significantly. Using a formula for unequal samples (Cohen, 1988), the effect size (f) was calculated to be .18, producing power of .75 for the sample. As with CHRNA4 analyses, the best performance was seen with the medium-sized cue, but that effect was not significant and there was no interaction.
Figure 6.
Accuracy. Match condition, main effect of DBH rs#1108580 genotype on accuracy in simple effects analysis.
On nonmatch trials (collapsed across TTD), simple effects of DBH revealed that the only significant effect was due to an increase in accuracy with cue size [F(2, 250) = 12.47].
Full Analysis of Nonmatch Trials
A full analysis was carried out on nonmatch trials, when the test stimulus appeared in a different location from the target. DBH genotype was the between-subjects factor, whereas cue size and TTD were within-subjects factors. There was no main effect of DBH on nonmatch trials. As with the CHRNA4 SNP, accuracy increased with cue size [F(2, 500) = 12.47]. There was also a main effect of TTD [F(2, 500) = 161.24]. The effects of cue size were strongest under difficult task conditions when target and test were close together [Cue size × TTD: F(4, 1000) = 31.30]. Under those conditions, the large precue was associated with better performance. This effect interacted with DBH genotype [DBH × Cue size × TTD: F(8, 1000) = 2.55, η2 = .006]. The interaction was analyzed by simple effects of TTD and DBH at each level of cue size. On nonmatch trials when the cue was smallest, accuracy increased with TTD [Figure 7A; F(2, 500) = 136.18], being highest in G allele carriers at longer distances [DBH × TTD: F(4, 500) = 2.50]. For the intermediate cue size, accuracy increased with TTD [F(2, 500) = 105.44]. For the large cue size, the only significant effect was of TTD [F(2, 500) = 18.94]. Under that condition, both G allele carrier groups (G/G and A/G) performed better than the A/A homozygotes (Figure 7B), but that effect was not significant. The DBH × TTD interaction at the smallest cue size did not remain significant after Bonferroni correction. These plots suggest that all participants had reduced accuracy when test and target were very close. However, this effect was reduced when the cue was large. To further understand this interaction, we calculated the simple effects of DBH × Cue size at the shortest target–test difference. Accuracy increased with cue size [F(2, 500) = 43.96] and G allele carriers benefited more from the largest cue than the A/A group [Figure 8; F(4, 500) = 3.18]. This result did survive Bonferroni correction.
Figure 7.
(A. B) Accuracy. Nonmatch condition, interaction of Cue size × TTD × Genotype in individuals genotyped for DBH rs#1108580 SNP. (A) Cue size 1 = 1.6°. (B) Cue size 3 = 8.1°.
Figure 8.
Nonmatch condition, interaction of Cue size × TTD × genotype in individuals genotyped for DBH rs#1108580 SNP. Data from the shortest TTD (1.9°) plotted as a function of cue size and genotype.
Direct Comparison of Both SNPs
An exploratory analysis was carried out on people genotyped for both SNPs which allows comparison of the two SNPs in one analysis. This was considered exploratory because of the small size of some of the cells. The largest group (DBH A/G and CHRNA4 C/T) had 37 individuals, whereas the smallest group (DBH G/G, CHRNA4 T/T) had only 7 individuals. In an omnibus analysis involving both SNPs and collapsed across TTD, there was a main effect of CHRNA4 [F(2, 170) = 4.37, η2 = .05], but no effect of DBH and no interaction between the two genotypes. As in the above analyses, there were main effects of cue size [F(2, 340) = 5.86] and trial type [F(1, 170) = 28.35] and an interaction between them [F(2, 340) = 5.17]. The Trial type × CHRNA4 [F(2, 170) = 3.54, η2 = .014] interaction was also significant. The latter interaction justified separate analysis of the full nonmatch conditions and both SNPs. This revealed again a main effect of CHRNA4 [F(2, 170) = 2.93] but not of DBH. All other main effects were significant [cue size: F(2, 340) = 13.35; TTD: F(2, 340) = 97.05; their interaction: F(4, 680) = 22.24]. There were no interactions with either SNP, although the interaction of Cue size × TTD × DBH was a nonsignificant trend (p = .09).
Because the original design did not include a “no-cue” condition, an analysis was carried out on a separate sample of people who were administered the same task with a no-cue condition added (described in Methods). This new sample of 54 individuals (42 young, 12 old) has not as yet been genotyped, but is included here to demonstrate the overall benefits of the cue for working memory performance. Under match conditions, accuracy was lowest in the no-cue condition (mean = 0.753) and highest in the 5.2° (middle) cue size condition, the latter being similar to cue size effects plotted in Figure 3. Under nonmatch conditions, accuracy was highest in the no-cue condition (mean = 0.89), and lowest in the small cue (1.6°) condition [mean = 0.85, F(3, 159) = 4.37]. As in the analyses above, accuracy was lowest when test and target were closest [F(2, 106) = 98.59]. Those factors interacted [F(6, 318) = 12.96], such that the lowest accuracy overall was seen under no-cue conditions when TTD was smallest (mean = 0.791) and the highest accuracy overall was seen following the largest cue when TTD was greatest (mean = 0.896). Thus, precues do benefit working memory performance. Small cues benefit memory on match trials and large cues benefit memory on nonmatch trials when test and target locations are widely separated.
DISCUSSION
We investigated effects of normal variation in nicotinic and noradrenergic neurotransmission genes on attentionally modulated working memory by comparing effects of two SNPs on memory performance in the same task. We previously reported that working memory was modulated by a noradrenergic but not by a nicotinic SNP (Parasuraman et al., 2005), whereas the focus of visuospatial attention was modulated by the same nicotinic but not by the noradrenergic SNP (Greenwood, Fossella, et al., 2005; Greenwood, Lambert, et al., 2005; Greenwood, Sunderland, et al., 2005; Parasuraman et al., 2005). These findings are consistent with separate lines of evidence from humans showing working memory is modulated by dopaminergic and noradrenergic systems, whereas visuospatial attention is modulated by cholinergic, specifically nicotinic, systems (reviewed below). Based on this, we hypothesized that both neurotransmission systems would modulate working memory performance when the two systems were required to interact by manipulation of working memory by visuospatial attention.
The results supported this prediction, although more strongly for CHRNA4 than for DBH. Among people genotyped for CHRNA4, the C/C homozygotes showed the best performance on match trials and following large cues on nonmatch trials. Among people genotyped for DBH, the best memory performance was seen in DBH G allele carriers, although that reached significance only on match trials and on nonmatch trials in an interaction with TTD and cue size. Moreover, a preliminary analysis does not indicate that the effects of those SNPs interacted. Although the finding that both nicotinic and noradrenergic SNPs modulate working memory under attentional manipulation may seem predictable in hindsight, it should be noted that studies using chemical and lesion methods to manipulate cholinergic and noradrenergic systems have obtained variable results, with evidence of both synergism (Hasselmo, Linster, Patil, Ma, & Cekic, 1997) and antagonism between the two systems (Ammassari-Teule, Maho, & Sara, 1991).
Consistent with our hypothesis, visuospatial attention did alter the influence of both SNPs on working memory performance, although the effect of variation in CHRNA4 was stronger than that of DBH. On Match trials, neither SNP altered the effect of manipulation of the focus of attention by cue size. On nonmatch trials, working memory accuracy increased with both cue size and TTD. When the cue was small and the test stimulus close to target location (hardest discrimination), accuracy was poor in all groups. However, performance improved as the scale of visuospatial attention increased with cue size, but mainly in individuals with the CHRNA4 C/C genotype (Figure 5) or who were DBH G allele carriers (G/G and A/G, Figure 8). Thus, effect of the beneficial genotypes was most evident when the task was hard and the cue size optimal for nonmatch conditions. Although both SNPs exerted this influence on working memory accuracy on nonmatch trials, CHRNA4 had the stronger effect. The significant effects of CHRNA4 and TTD at each level of cue size on nonmatch trials survived correction for multiple comparisons. The only test of DBH simple main effects that survived correction showed that the G/G and A/G groups were more accurate than the A/A group under the hardest condition when the cue size was large (Figure 8).
There was a weak relation between RT and accuracy. For CHRNA4, RT to the comparison stimulus was slowest in C/C homozygotes, which showed the highest accuracies, although the main effect was not significant. The difference between C/C and T/T homozygote groups was 14 msec at the smallest cue and 31 msec at the largest cue. However, this was due to speeding up of the T/T group rather than slowing of the C/C group (Figure 2A). The significant interaction is due to this crossover of the C/T and T/T groups from the intermediate to the largest cue. For DBH, Figure 2B shows that the G/G genotype group was fastest at the smallest cue (where the highest accuracies were seen), but only on match trials (three-way interaction of DBH × Trial type × Cue size). There was no main effect of DBH genotype on RT. Therefore, it is acknowledged that the results for CHRNA4 are weakly consistent with a speed–accuracy tradeoff. Nevertheless, that concept may be most relevant to the initial processing of a stimulus, not, as here, to processing the mental representation of a stimulus. It is unlikely that comparison of a stimulus with a mental representation after the 3-sec delay would be subject to such a tradeoff. Participants are not making a perceptual decision, they are comparing a percept with a stored mental representation.
The present results extend our understanding of the neurotransmission systems important in working memory. Our previous study found strong noradrenergic but no cholinergic modulation of a working memory task without an attentional manipulation (Parasuraman et al., 2005), whereas the present study shows cholinergic and weaker noradrenergic modulation of a working memory task with an attentional manipulation. (There was a power of 0.75 to detect a main effect of DBH rs#1108580 genotype in the match analysis in the present study.) The previous task and the present task used the same stimuli and delays. However, the previous task required retention of one, two, or three locations, whereas the present task required retention of just one location. We argue that the important factor in determining whether the nicotinic SNP modulated working memory was the presence of an attentional manipulation. Nevertheless, it is possible—although somewhat harder to explain—that the stronger manipulation of working memory load in the previous task somehow suppressed the effect of the nicotinic SNPs in that study.
We also confirmed that the optimal scale of visuospatial attention for influencing working memory varies with task demands, being smaller when test and target are at the same location on match trials, but larger when test and target appear at different locations. Our finding that the cue size manipulation produced the best performance following the medium-sized cue on match trials is consistent with our previous findings from a different population (Greenwood, Lambert, et al., 2005) but stands in contrast to findings from visual search paradigms in which the best performance follows the smallest cue (Greenwood & Parasuraman, 1999, 2004; Eriksen & St James, 1986). That the optimal scale of the attentional focus is larger when encoding and retention are required compared to when only search is required, indicates that attentional scaling may control the form of the encoded mental representation, perhaps by encoding a portion of ground along with the figure.
It is of interest that the CHRNA4 C/C genotype associated in our previous work with the strongest effects of spatial cueing in visual search (Greenwood, Fossella, et al., 2005; Greenwood, Lambert, et al., 2005; Greenwood, Sunderland, et al., 2005) is the genotype associated with more accurate working memory accuracy in the present study. This finding links dependence on spatial cues with better working memory. Bleckley, Durso, Crutchfield, Engle, and Khanna (2003) reported that people with high working memory capacity show more flexible allocation of the focus of visuospatial attention compared to those with low working memory capacity. The present findings are consistent with that finding.
The present design cannot determine whether the effect of visuospatial attention was exerted on encoding or retention of location, or both. Presumably, discrimination accuracy in this task reflects the result of comparing the stored representation of target location with the observed test location. That this measure is sensitive to the size of precues presented some 3.6 sec earlier in the trial suggests that the stored representation of the target could have been enhanced at encoding or during the retention interval (maintenance). Recent studies find that both encoding and maintenance stages of spatial working memory benefit from visuospatial attention (Matsukura, Luck, & Vecera, 2007; Griffin & Nobre, 2003). Our previous and present work adds to this nascent literature by showing: (a) memory performance is affected by the scale of visuospatial attention; (b) the optimal scale of attention for working memory is small when stimuli are predictably located but large when stimuli are unpredictably located; (c) an optimally sized attentional scale at encoding improves later memory for location; (d) normal variation in neurotransmission genes influences the strength of the effect of visuospatial attention on working memory accuracy, perhaps by heightening the perceptual benefit accruing to an attended target when the focus of attention is optimally scaled. The present work also emphasizes the particular importance of cholinergic neurotransmission for attention-based control of the encoded representation in working memory.
We can consider our present findings in the context of pharmacological evidence that acetylcholine (ACh) and NE can work synergistically (Gu, 2002; Hasselmo et al., 1997), showing both neurotransmission systems are involved when tasks require deployment of visuospatial attention as well as memory. We review these different strands of evidence and argue (a) that normal genetic variation in neurotransmission genes has specific behavioral effects and (b) that the relative importance of cholinergic or noradrenergic efficiency on a given task varies with the relative demands made by that task on processes of attention and memory.
There is considerable evidence bearing on the neurotransmission systems involved in processes of working memory and visuospatial attention. Work in rodents indicates that nicotinic agents influence both memory (Levin & Simon, 1998) and sustained attention (Rezvani, Bushnell, & Levin, 2002). Levin, Kaplan, and Boardman (1997) argue from their work in rodents that nicotine boosts the performance of working memory specifically. However, other investigators have argued that in primates the basal forebrain cholinergic system influences visuospatial attention but not memory (Everitt & Robbins, 1997; Voytko et al., 1994). Consistent with that view, there is human evidence that noradrenergic agents modulate working memory, whereas cholinergic agents modulate visuospatial attention. DA D1 receptor availability has been found to be correlated with working memory performance in humans and monkeys (Abi-Darghamet al., 2002; Castner et al., 2000) and alpha-2A adrenoreceptor agonists modulate both working memory performance and blood flow in dorsolateral PFC in monkeys (Franowicz & Arnsten, 2002; Avery et al., 2000; Mao, Arnsten, & Li, 1999). Suggesting specificity, a noradrenergic agonist has been shown to modulate an alerting effect but not an attentional cue validity effect in monkeys (Witte & Marrocco, 1997).
In primates, including humans, cholinergic effects appear to be exerted selectively on visuospatial attention and not on memory. First, there is evidence of nicotinic cholinergic mediation of attention. Administration of nicotine to both humans and monkeys slowed responses on invalidly cued trials but not on validly cued trials (Witte et al., 1997). In human smokers, effects of cue validity increased with days of abstinence and were inversely related to salivary levels of the nicotine metabolite cotinine (Shirtcliff & Marrocco, 2003). Thiel et al. (2005) have also found selective effects of nicotine on discrimination following invalid cues in nonsmokers which were accompanied by reduced fMRI activation in the intraparietal sulcus and precuneus. Secondly, cholinergic effects appear to be exerted selectively on visuospatial attention. Neurotoxic inactivation of the nbM in the monkey basal forebrain, the major subcortical source of cortical ACh, disrupted performance in a cued visuospatial attention task but had no effect on several tasks of memory (Voytko, 1996; Voytko et al., 1994). Moreover, the attentional deficit observed was qualitatively similar to one observed in patients with Alzheimer’s Disease (Parasuraman, Greenwood, Haxby, & Grady, 1992), known to have marked cortical cholinergic depletion due to progressive degeneration of the nbM. Nicotine patches have been found to improve visuospatial attention, but not memory, in older people with memory impairment (White & Levin, 2004) and in AD patients (Sahakian, Jones, Levy, Gray, & Warburton, 1989). Thus, in humans and monkeys, there is evidence that working memory is selectively modulated by noradrenergic and dopaminergic systems, whereas visuospatial attention is selectively modulated by cholinergic systems.
Moreover, there is increasing evidence that cholinergic influences on working memory are exerted mainly under attentional load—that is, when the two systems interact. In a working memory task, Furey et al. (2000) found that during a 3-sec encoding period, the cholinergic agonist physostigmine enhanced the extrastriate response to faces and decreased the response to control stimuli. The differential effect of physostigmine was not seen during memory maintenance. The authors argued that the enhanced cholinergic activity improved working memory performance by selectively heightening perceptual processing of the relevant stimuli. In a subsequent study, Furey et al. presented overlapping stimuli of faces and houses and used a cue to direct the focus of visuospatial attention in advance to one of the two overlapping stimuli. Physostigmine speeded RT on trials after the shift in category, but not on the trial with the shift in category. Scopolamine had the opposite effect. Thus, enhancement or inhibition of cholinergic activity influenced ability to maintain selective attention on the cued category of target, but did not alter the ability to shift category (Furey, Pietrini, Haxby, & Drevets, 2008). This suggests that the cholinergic system has a large role in selective attention. Consistent with the work of Furey et al., scopolamine has been reported to cause deficiencies in delayed match-to-sample performance, but only when heightened attention was required at the longest delay (Robbins et al., 1997).
Considered as a whole, this work is consistent with an interpretation that the role of cholinergic systems in working memory is attentional. To test this directly, Robbins et al. developed a task which allowed separate assessment of attention and working memory. Scopolamine infused into PFC was found to impair memory but not attention in intact rats. Similarly, animals with selective Ig-G saporin lesions of the nucleus basalis magnocellularis (no scopolamine administered) showed impaired memory under high attentional load but not under low attentional load (Chudasama, Dalley, Nathwani, Bouger, & Robbins, 2004). These data indicate a role for basal cholinergic input to PFC in memory function when attention is required. Providing a possible explanation at the cellular level, a recent study showed that nicotinic receptors affect processes of long-term potentiation in PFC (Couey et al., 2007). Consistent with that finding are the increasing indications that human PFC plays a role in attentional modulation of working memory (Postle, 2006; Curtis & D’Esposito, 2003). The evidence of nicotine-driven enhancement of signal-to-noise ratio during PFC information processing (Couey et al., 2007) is consistent with the present finding of nicotinic receptor modulation of working memory under attentional load in humans. Nonetheless, further work will be needed to disentangle the cholinergic and noradrenergic influences on the interactions between visuospatial attention and working memory.
What is the physiological basis for the apparent influence of cholinergic and noradrenergic systems on working memory when attention is manipulated? In several studies, Hasselmo et al. (1997) has shown that when perfused directly into cortex, the effects of ACh and NE combine in an additive manner to produce dose-dependent suppression of synaptic potentials. In contrast to this synergism in ACh–NE physiological interactions, Dyon-Laurent, Herve, and Sara (1994), Ammassari-Teule et al. (1991), and Sara (1989) have reported antagonistic interactions in a set of learning and memory experiments. Learning and memory deficits, which developed subsequent to cholinergic lesions, were reduced by the administration of a noradrenergic alpha-2 agonist. From this and other evidence, Yu and Dayan (2005) have argued that ACh is involved in “expected uncertainty,” whereas NE is involved in “unexpected uncertainty.” They interpret evidence that cue validity effects vary inversely with level of nicotine or ACh in attentional cueing tasks (Phillips, McAlonan, Robb, & Brown, 2000; Witte et al., 1997; Voytko et al., 1994) as reflecting cholinergic suppression of cueing effects. On the other hand, they interpret findings that NE modulates performance on tasks with unpredictable changes in cue–target relationship (Devauges & Sara, 1990) as reflecting noradrenergic-induced change in cue– target associations. The model advanced by Yu and Dayan would predict a role for cholinergic neuromodulation in any task using attentional cueing, consistent with the present findings.
These results can also be viewed in the context of the role of visuospatial attention in working memory. Several theorists have argued that the focus of visuospatial attention is integral to working memory (Cowan, 2001; Awh et al., 1998). Elsewhere, we have reported that dynamic adjustments of the scale of visuospatial attention in response to task demands (Greenwood & Parasuraman, 2004) have a role in controlling the encoded representation in working memory (Greenwood, Lambert, et al., 2005). Using two independent samples, we have shown that the scale of visuospatial attention deployed around a target affects memory for location of that target stimulus. The present study shows that variation in the gene controlling a subunit of the most common nicotinic receptor—the alpha4/beta 2 nicotinic ACh receptor (Flores, DeCamp, Kilo, Rogers, & Hargreaves, 1996)—modulates that effect of attention on memory. This indicates the importance of cholinergic neurotransmission for attention-based control of the encoded representation in working memory. As such, the present results are consistent with theories that argue for a relation between visuospatial attention and working memory (Cowan, 2001; Engle et al., 1999). The present results go beyond those theories by showing that cholinergic mediation of working memory is particularly important when working memory is explicitly modulated by the focus of visuospatial attention. This indicates that visuospatial attention and working memory are separate systems which can interact.
In light of the concern about false positives in candidate gene studies, it can be noted that the effect size we report for CHRNA4 C/T of .25 is close to the effect size of .29 recently reported in a meta-analysis of the effect of the well-studied COMT val158met polymorphism on the Wisconsin Card Sort Task (Barnett et al., 2007). We have now observed effects of the CHRNA4 rs#1044396 SNP on visuospatial attention in two large and independent samples (Espeseth et al., 2006; Greenwood, Fossella, et al., 2005; Parasuraman et al., 2005). Effects of this SNP have been reported by other investigators on fMRI measures of an attentional manipulation (Winterer et al., 2007). Auditory and visual ERPs likewise reflect polymorphic variation in this SNP (Espeseth, Endestad, Rootwelt, & Reinvang, 2007).
There are some limitations of the present study. The sample size, although large for the psychological literature, allowed only a preliminary full comparison of the two SNPs in one analysis and did not allow analysis of combined age and genotype effects. Also, although our interpretations are supported in the human literature, we acknowledge that our findings cannot completely rule out cholinergic modulation of working memory in the absence of attentional manipulations. Although the present findings will need to be replicated with larger samples, they are notable for showing that the effect of visuospatial attention on working memory is modulated by normal genetic variation in separate neurotransmission systems.
Acknowledgments
This work was supported by NIA grant AG19653 to R. P. and the Virginia Center on Aging grant ARDRAF 06-2 to P. M. G. We thank Ryan McGarry and Ruchi Kapani for assistance with data collection and management.
REFERENCES
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammassari-Teule M, Maho C, Sara SJ. Clonidine reverses spatial learning deficits and reinstates theta frequencies in rats with partial fornix section. Behavioural Brain Research. 1991;45:1–8. doi: 10.1016/s0166-4328(05)80174-3. [DOI] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Spatial working memory and spatial selective attention. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT Press; 1998. pp. 353–380. [Google Scholar]
- Awh E, Jonides J, Reuter-Lorenz PA. Rehearsal in spatial working memory. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: Dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learning and Memory. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Conway AR, Cowan N, Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychological Bulletin. 1988;104:163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114–185. [DOI] [PubMed] [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, et al. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Human Genetics. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behavioural Brain Research. 1990;39:19–28. doi: 10.1016/0166-4328(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Dyon-Laurent C, Herve A, Sara SJ. Noradrenergic hyperactivity in hippocampus after partial denervation: Pharmacological, behavioral, and electrophysiological studies. Experimental Brain Research. 1994;99:259–266. doi: 10.1007/BF00239592. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW, editors. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Eriksen CW, St James JD. Visual attention within and around the field of focal attention: A zoom lens model. Perception & Psychophysics. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Endestad T, Rootwelt H, Reinvang I. Nicotine receptor gene CHRNA4 modulates early event-related potentials in auditory and visual oddball target detection tasks. Neuroscience. 2007;147:974–985. doi: 10.1016/j.neuroscience.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Greenwood PM, Reinvang I, Fjell AM, Walhovd KB, Westlye LT, et al. Interactive effects of APOE and CHRNA4 on attention and white matter volume in healthy middle-aged and older adults. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:31–43. doi: 10.3758/cabn.6.1.31. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, et al. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. American Journal of Human Genetics. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, DeCamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: Demonstration of alpha3beta4, a novel subtype in the mammalian nervous system. Journal of Neuroscience. 1996;16:7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology. 1992;41:31–37. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”—A practical method for grading the cognitive state of patients for the clinician. Journal of Current Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella JA, Casey BJ. Genes, brain, and behavior: Bridging disciplines. Cognitive, Affective & Behavioral Neuroscience. 2006;6:1–8. doi: 10.3758/cabn.6.1.1. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. Actions of alpha-2 noradrenergic agonists on spatial working memory and blood pressure in rhesus monkeys appear to be mediated by the same receptor subtype. Psychopharmacology (Berlin) 2002;162:304–312. doi: 10.1007/s00213-002-1110-6. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends in Cognitive Sciences. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Greenwood P, Kumar R, Sundararajan R, Fryxell KJ, Lin M, Parasuraman R. Effects of attention on working memory are modulated by a nicotinic SNP (CHRNA4) but not a noradrenergic SNP (DBH); Paper presented at the Society for Neuroscience; Atlanta, Georgia. 2006. [Google Scholar]
- Greenwood PM, Fossella JA, Parasuraman R. Specificity of the effect of a nicotinic receptor polymorphism on individual differences in visuospatial attention. Journal of Cognitive Neuroscience. 2005;17:1611–1620. doi: 10.1162/089892905774597281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Scale of attentional focus in visual search. Perception & Psychophysics. 1999;61:837–859. doi: 10.3758/bf03206901. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behavioral and Cognitive Neuroscience Reviews. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. The scaling of spatial attention in visual search and its modification in healthy aging. Perception & Psychophysics. 2004;66:3–22. doi: 10.3758/bf03194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Putnam K, Levy J, Parasuraman R. Scaling of visuospatial attention undergoes differential longitudinal change as a function of APOE genotype prior to old age: Results from the NIMH BIOCARD study. Neuropsychology. 2005;19:830–840. doi: 10.1037/0894-4105.19.6.830. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Nobre AC. Orienting attention to locations in internal representations. Journal of Cognitive Neuroscience. 2003;15:1176–1194. doi: 10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Hare MP, Palumbi SR. The accuracy of heterozygous base calling from diploid sequence and resolution of haplotypes using allele-specific sequencing. Molecular Ecology. 1999;8:1750–1752. doi: 10.1046/j.1365-294x.1999.00738-1.x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. Journal of Neurophysiology. 1997;77:3326–3339. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, et al. Nicotinic acetylcholine receptor alpha4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatric Genetics. 2001;11:37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kaplan S, Boardman A. Acute nicotine interactions with nicotinic and muscarinic antagonists: Working and reference memory effects in the 16-arm radial maze. Behavioural Pharmacology. 1997;8:236–242. [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berlin) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berlin) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biological Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: Protection or prioritization? Perception & Psychophysics. 2007;69:1422–1434. doi: 10.3758/bf03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, et al. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM. Molecular genetics of visuospatial attention and working memory. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford; 2004. pp. 245–259. [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Kumar R, Fossella J. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychological Science. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berlin) 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Developmental Science. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacology (Berlin) 2002;164:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: Further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behavioural Brain Research. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, et al. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: Comparison with diazepam and implications of dementia. Psychopharmacology (Berlin) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. British Journal of Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Noradrenergic–cholinergic interaction: Its possible role in memory dysfunction associated with senile dementia. Archives of Gerontology and Geriatrics: Supplement. 1989;1:99–108. [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiology of Aging. 2004;25:1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Marrocco RT. Salivary cotinine levels in human tobacco smokers predict the attentional validity effect size during smoking abstinence. Psychopharmacology (Berlin) 2003;166:11–18. doi: 10.1007/s00213-002-1293-x. [DOI] [PubMed] [Google Scholar]
- Steinlein OK, Deckert J, Nothen MM, Franke P, Maier W, Beckmann H, et al. Neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) and panic disorder: An association study. American Journal of Medical Genetics. 1997;74:199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293:120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: Memory or attention? Behavioural Brain Research. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. Journal of Neuroscience. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chuang K, Ahluwalia M, Patel S, Umblas N, Mirel D, et al. High-throughput SNP genotyping by single-tube PCR with Tm-shift primers. BioTechniques. 2005;39:885–893. doi: 10.2144/000112028. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—revised: Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—revised: Manual. New York: Psychological Corporation; 1987. [Google Scholar]
- White HK, Levin ED. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berlin) 2004;171:465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Konrad A, Vucurevic G, Stoeter P, Sander T, et al. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Human Molecular Genetics. 2007;16:2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: Comparison of monkey and human performance. Psychopharmacology (Berlin) 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology (Berlin) 1997;132:315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- Wood JG, Joyce PR, Miller AL, Mulder RT, Kennedy MA. A polymorphism in the dopamine beta-hydroxylase gene is associated with “paranoid ideation” in patients with major depression. Biological Psychiatry. 2002;51:365–369. doi: 10.1016/s0006-3223(01)01367-1. [DOI] [PubMed] [Google Scholar]
- Yago E, Duarte A, Wong T, Barcelo F, Knight RT. Temporal kinetics of prefrontal modulation of the extrastriate cortex during visual attention. Cognitive, Affective & Behavioral Neuroscience. 2004;4:609–617. doi: 10.3758/cabn.4.4.609. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]