Abstract
This study was designed to determine optimal operating conditions of a bioartificial liver (BAL) based on mass transfer of representative hepatotoxins and mediators of immune damage. A microprocessor-controlled BAL was used to study mass transfer between patient and cell compartments separated by a hollow fiber membrane. Membrane permeability (70, 150, or 400 kDa molecular weight cut-off—MWCO), membrane convection (high: 50 mL/min; medium: 25 mL/min; low: 10 mL/min; diffusion: 0 mL/min), and albumin concentration in the cell compartment (0.5 or 5 g%) were considered for a total of 24 test conditions. Initially, the patient compartment contained pig plasma supplemented with ammonia (0.017 kDa), unconjugated bilirubin (0.585 kDa), conjugated bilirubin (0.760 kDa), TNF-α (17 kDa), pig albumin (67 kDa), pig IgG (147 kDa), and pig IgM (900 kDa). Mass transfer of each substance was determined by its rate of appearance in the cell compartment. Membrane fouling was assessed by dextran polymer technique. Of the three tested variables (membrane pore size, convection, and albumin concentration), membrane permeability had the greatest impact on mass transfer (P < 0.001). Mass transfer of all toxins was greatest under high convection with a 400 kDa membrane. Transfer of IgG and IgM was insignificant under all conditions. Bilirubin transfer was increased under high albumin conditions (P = 0.055). Fouling of membranes ranged from 7% (400 kDa), 24% (150 kDa) to 62% (70 kDa) during a 2-h test interval. In conclusion, optimal toxin removal was achieved under high convection with a 400-kDa membrane, a condition which should provide adequate immunoprotection of hepatocytes in the BAL.
Keywords: bioartificial liver, mass transfer, albumin, bilirubin, TNF-α, ammonia
Introduction
The bioartificial liver (BAL) is an extracorporeal device composed of living hepatocytes for the support of patients in liver failure, and a semipermeable membrane to protect the extracorporeal hepatocytes from the patient's immune system (Matsumura et al., 1987; Strain and Neuberger, 2002). Several BAL devices have been developed and some have been tested clinically. As yet, none have shown efficacy in clinical trials to a level necessary to warrant approval by the U.S. Food and Drug Administration (FDA) (Lee et al., 2008). The reason for the apparent lack of efficacy is multifactorial. Factors such as insufficient cell mass at the start of treatment, loss of cell mass during treatment, and poor detoxification activity of cells within the device have been implicated (Brophy and Nyberg, 2008; McKenzie et al., 2008). It is also possible that prior BAL designs have insufficiently addressed the mass transfer of waste products from the patient to the device (Patzer et al., 2002). In order to increase the mass of liver cells in the device, immortalized liver cell lines that have reduced oxygen requirements have been employed (Millis and Losanoff, 2005). However, immortalized cell lines do not exhibit a full repertoire of metabolic activities, most notably a lack of complete urea cycle activity (Filippi et al., 2004; Mavri-Damelin et al., 2007, 2008; Wang et al., 1998).
The spheroid reservoir bioartificial liver (SRBAL) was developed to increase the mass of normal (not immortalized) hepatocytes available for use in an extracorporeal liver support device (Nyberg et al., 2005). The SRBAL device is able to cultivate 200–500 g of primary hepatocytes, a level believed to be sufficient to support most patients in acute liver failure (Lee et al., 2008; Sussman et al., 2009). Previous bench studies of the SRBAL have demonstrated stable viability and stable metabolic activity of primary hepatocytes isolated from rat and pig within the spheroid reservoir for up to 2 weeks (Brophy et al., 2009; Nyberg et al., 2005).
Influences of the patient's immune system and BAL membrane permeability on BAL performance are a concern. These influences are poorly studied during extracorporeal therapy (Gerlach et al., 1994; Matsushita et al., 2003; Nyberg et al., 1992). In one report, liver failure patients developed a significant xenoreactive antibody response after exposure to pig hepatocytes in a BAL employing an apheresis membrane of 200 nm permeability (Baquerizo et al., 1999). Studies involving healthy animals have indicated that a membrane permeability of 150–400 kDa (~10–20 nm) served as an effective barrier to the patient's cytotoxic immune response against hepatocytes within the BAL (Nyberg et al., 2003, 2004). Less studied is the influence of the BAL's membrane permeability on mass transfer rate, detoxification, and removal of other waste products that accumulate in liver failure (Patzer, 2006).
Therefore, the purpose of the current study was to optimize conditions for removal of waste products from the patient compartment during BAL therapy while minimizing the passage of harmful immune mediators into the cell (i.e., hepatocyte) compartment of the BAL device. Three membranes were tested with nominal molecular weight cutoffs (nMWCOs) of 70, 150, and 400 kDa, all permeable to albumin (molecular weight 67 kDa). The benefits of convective mass transfer were studied by pumping fluid across the BAL membrane at a range of 0–50 mL/min during testing. In addition, the influence of albumin as a transporter of nonpolar molecules was considered by supplementing medium in the hepatocyte compartment with 0.5 and 5 g% bovine albumin. Mass transfer of waste molecules (ammonia, unconjugated bilirubin, conjugated bilirubin), immune molecules (TNF-α, pig IgG, pig IgM), and pig albumin was determined from their rate of appearance in the cell compartment. Membrane fouling was determined from the difference in overall membrane permeability measured by dextran polymer technique before and after the mass transfer testing. Our findings suggest that a 400 kDa membrane with high convection in the SRBAL device should provide adequate immunoprotection of hepatocytes and optimal toxin removal for biochemical support of patients in acute liver failure.
Materials and Methods
Materials
Chemicals were obtained from Sigma–Aldrich (St. Louis, MO) unless stated otherwise. Bilirubin (B-4126) contained three different unconjugated α-isomers. Recombinant human TNF-α (T0157) was expressed in yeast. Polydisperse dextran polymer fractions (MWs 20, 70, 150, 250, 500, and 2,000 kDa) were obtained from Pharmacosmos AS (Holbaek, Denmark). Bovine serum albumin was obtained as a 35% liquid. High permeability polysulfone-based hollow fiber modules with 0.3 m2 surface area and nMWCO values of 70, 150, and 400 kDa were purchased from Minntech Corporation (Minneapolis, MN). Exeltra 150 ultrafiltration module with a cellulose triacetate membrane (surface area 1.5 m2 and a priming volume of 95 mL) was obtained from Baxter (Deerfield, IL). Plasma was pooled from pig blood and collected in blood-pack units with citrate (1 mg/mL blood; Baxter). Pen-Strep 100 U/mL was added to the pig plasma. The roller pumps were obtained from MasterFlex rollerpumps (Cole-Parmer, Vernon Hills, IL).
Assays
Measurements of total and direct (conjugated) bilirubin were performed with a QuantiChrom™ Bilirubin Assay Kit (DIBR-180; BioAssay System, Hayward, CA). Human TNF-α ELISA kit was obtained from eBioscience (San Diego, CA). Pig IgG and pig IgG ELISA quantitation kits were obtained from Bethyl Laboratories, Inc. (Montgomery, TX). Ammonia was analyzed in the Central Clinical Laboratory (Mayo Clinic, Rochester, MN). All assays were done in triplicates.
Outline of the Study
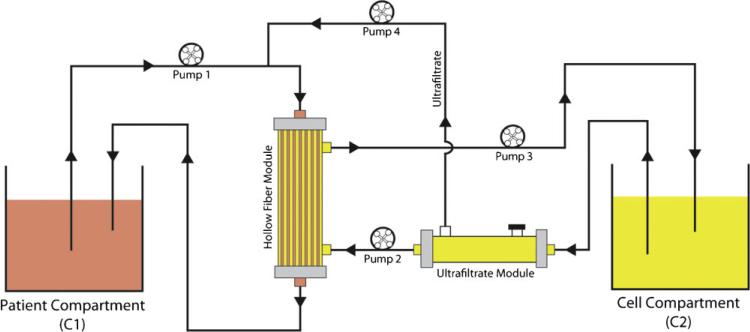
A schematic of the apparatus used to measure mass transfer from patient compartment (C1) to cell compartment (C2) was depicted in Figure 1. The patient compartment was initially filled with pig plasma supplemented with test substances which included 30 mg/dL mixture of conjugated and unconjugated bilirubin, 260 μM ammonia, 100 pg/mL TNF-α, along with the pig albumin, pig IgG, and pig IgM naturally present in pig plasma. A single large batch of pig plasma plus test supplements was prepared and refrigerated at 4°C prior to use to avoid batch-to-batch variability. The cell compartment was initially filled with phosphate-buffered saline (PBS) supplemented with bovine albumin (0.5 or 5.0 g%), but no test substances.
Figure 1.
Outline of the experiment. The patient compartment (C1) was filled with pig plasma supplemented with ammonia, bilirubin, and human TNF-α. The cell compartment (C2) was filled with PBS and supplemented with 0.5% or 5% bovine serum albumin. Flow rates in each compartment were controlled with roller pumps. An ultrafiltration module was included in the fluid circuit of compartment 2 to allow predilution of the inflow to the hollow fiber module, which in turn allowed determination of mass transfer by convective flow across the hollow fibers (see text).
Mass Transfer Testing
The rate of mass transfer (i.e., the permeability) of test substances from patient compartment to cell compartment was measured with the hollow fiber module located at the intersection of the two flow circuits as shown in Figure 1. Priming volumes of the hollow fiber modules were approximately 30 mL on the intracapillary side (patient compartment) and approximately 130 mL on the extracapillary side (cell compartment). Flow rates in the patient circuit were maintained at 100 mL/min by pump 1. Flow rates in the cell compartment were also maintained at 100 mL/min by pump 3, while rates of pumps 2 and 4 were adjusted to achieve convective flow rates of 0–50 mL/min across the hollow fibers. All combinations of membrane permeability (i.e., 70, 150, or 400 kDa MWCO), convective flow (0 mL/min—pure diffusion; 10 mL/min—low convection; 25 mL/min—medium convection; 50 mL/min—high convection), and both albumin concentrations (0.5 or 5 g%) in the cell compartment were tested for a total of 24 test conditions. Mass transfer of each test substance was determined by its rate of appearance in the cell compartment (one complete run per condition). Concentration data collected during 120 min of perfusion or until the test conditions approached steady-state equilibrium were fit to a simple linear regression model. A retention time was estimated for each test substance using its initial concentration in C1 multiplied by volume of C1 and divided by the mass transfer rate determined by the linear regression model.
Dextran Polymer Technique
The overall permeability of each membrane was measured before and after mass transfer testing by dextran technique (Modification of ASTM: E1343-90). The filtrate sample used for the determination of overall permeability was collected at system equilibration. Briefly, a solution of a mixture of polydispersed dextran fractions each with a concentration of 2 mg/mL in PBS (pH 7) was used to standardize the measure of overall membrane permeability. The concentration of the individual dextran fractions in the mixture was selected to obtain a uniformly high concentration of dextrans over a wide MW range. The dextran composition of the sample was analyzed with size exclusion chromatography using a Beckman Coulter instrument (Fullerton, CA) with a Jordi Gel GBR mixed bed column and a Jordi RI2000 refractive index detector (Bellingham, MA). Sieving curves were generated from integrated chromatograms of the filtrate standardized to a known challenge solution. Percent fouling was determined for each the hollow fiber modules from the ratio of (peak height pre – peak height post) divided by peak height pre of the integrated chromatograms.
Statistics
The SPSS 11.0 statistical package was used to establish significance between the groups (Chicago, Il). Rank sum analysis was used to identify an optimal operating condition from the 24 test conditions under evaluation. Data are reported as means of groups tested. P-value of <0.05 was considered statistically significant.
Results
Overview
Mass transfer rates for seven test substances important to the operation of a BAL were measured using a bench top, computer controlled, four-pump system outlined in Figure 1. This system employed a two-compartment configuration analogous to a patient in liver failure (C1) under hepatocyte treatment with the spheroid reservoir BAL (C2). Our test system included a hollow fiber module to separate the two compartments. Of note, there were no cells present in the compartment referred to as the cell compartment during our studies. An ultrafiltration module was incorporated into the C2 fluid circuit to provide predilution of the inflow to the hollow fiber module as illustrated in Figure 1. Four pumps defined the operating conditions of the system. All seven substances were present in C1 at t = 0 min, and mass transfer rates for each substance were determined from their rate of appearance in C2.
Mass Transfer Rates
Mass transfer rates for each of these seven representative substances are summarized in Table I. These measurements were obtained under conditions of pure diffusion (0 mL/min), low convection (10 mL/min), medium convection (25 mL/min), and high convection (50 mL/min). The table was further divided into mean mass transport rates for each of the three membranes tested. We observed no significant effect of albumin concentration when concentrations of 0.5% BSA and 5% BSA were tested in the C2 circuit. The only molecule to show a trend toward increased mass transfer was bilirubin (P = 0.055, data not shown) under high albumin conditions. Therefore, the results of the low and high albumin conditions were combined for reporting in Table I.
Table I.
Overall summary of mass transfer rates.
| Molecule | Protein polarity | Units | Diffusion |
Low convection |
Medium convection |
High convection |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 kDa | 150 kDa | 400 kDa | 70 kDa | 150 kDa | 400 kDa | 70 kDa | 150 kDa | 400 kDa | 70 kDa | 150 kDa | 400 kDa | |||
| Ammonia | Polar/nonpolar | μmol/h/m2 | 75.2 | 101.4 | 135.2 | 96.2 | 96.8 | 104.4 | 87.2 | 131.2 | 211.0 | 126.8 | 161.0 | 180.6 |
| Total bilirubin | Nonpolar | μmol/h/m2 | 50.2 | 81.0 | 141.6 | 47.4 | 87.4 | 132.8 | 23.6 | 137.4 | 192.8 | 37.6 | 152.8 | 247.6 |
| Direct bilirubin | Polar | μmol/h/m2 | 50.0 | 54.8 | 80.0 | 46.8 | 73.8 | 79.4 | 14.4 | 55.4 | 115.2 | 8.6 | 97.2 | 115.2 |
| Indirect bilirubin | Nonpolar | μmol/h/m2 | 0.2 | 26.2 | 61.6 | 0.6 | 13.6 | 53.4 | 9.4 | 82.0 | 77.4 | 29.2 | 55.8 | 132.4 |
| TNF-α | Polar | fmol/h/m2 | 0.2 | 0.7 | 1.1 | 0.3 | 1.5 | 1.6 | 0.2 | 1.7 | 1.9 | 0.1 | 1.9 | 2.3 |
| Albumin | Polar | μmol/h/m2 | 0.0 | 32.0 | 52.8 | 0.2 | 46.2 | 77.4 | 1.2 | 47.2 | 67.0 | 0.0 | 41.2 | 50.0 |
| IgG | Polar | μmol/h/m2 | 0.0 | 3.0 | 3.2 | 0.0 | 2.2 | 3.6 | 0.0 | 4.6 | 6.4 | 0.0 | 5.6 | 7.8 |
| IgM | Polar | μmol/h/m2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Unit: mol/h/m2.
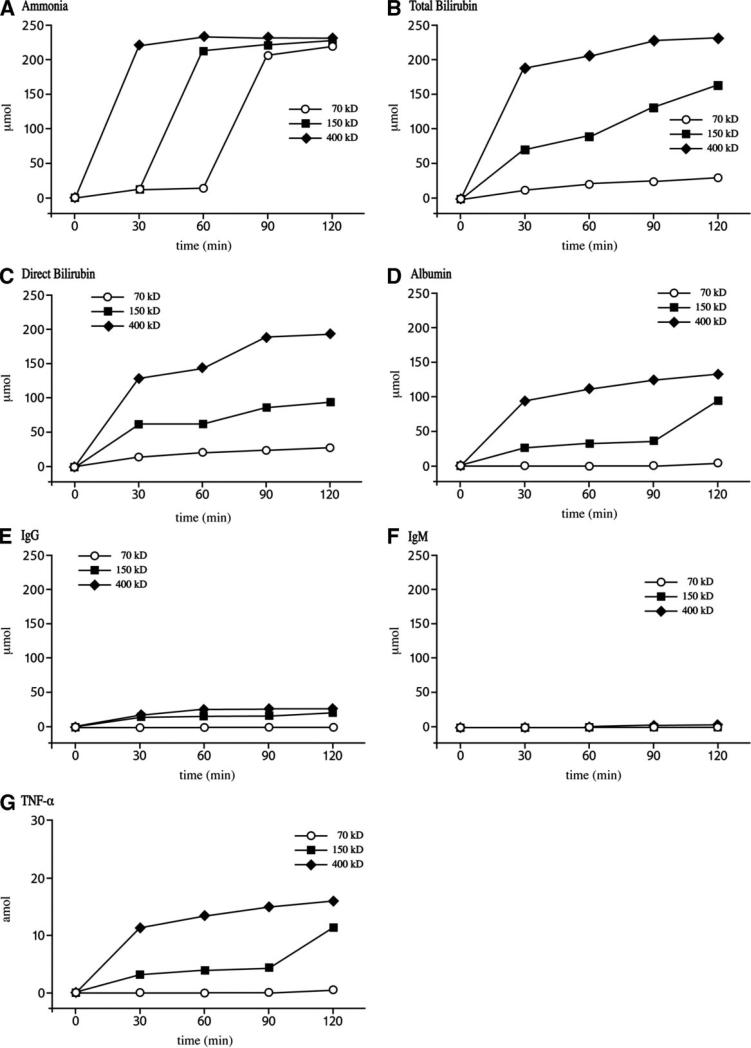
Of the three variables tested, which included membrane pore size, convection rate, and albumin concentration, membrane permeability had the greatest impact on mass transfer ( P < 0.001). In fact, under all conditions of convection and for each of the seven test substances, mass transfer was greatest when the 400 kDa membrane was utilized. Raw data for mass transfer under high convection conditions are provided in Figure 2. In each case, mass transfer of the test substance was fastest and of greatest magnitude using the 400 kDa membrane followed by 150 and 70 kDa membrane. With regard to ammonia (Fig. 2A), equilibrium was established within 30 min of testing the 400 kDa membrane followed by within 60 min with the 150 kDa membrane and 90 min with the 70 kDa membrane. The equilibrium of total bilirubin transport was also reached within approximately 30 min using the 400 kDa membrane (Fig. 2B). Consistent with the total bilirubin result was leveling off of the albumin transport within 30 min under high convection conditions using the 400 kDa membrane (Fig. 2D). The benefit of increased permeability was most apparent as test molecules approached the nMWCO of the membrane. For example, mass transfer of TNF-α (molecular weight 17 kDa) was significantly lower when tested using the 70 kDa membrane compared to the 150 and 400 kDa membranes under each of the four convection conditions. A similar pattern was observed with albumin (molecular weight 67 kDa) and IgG (molecular weight 147 kDa). The passage of pig IgG and pig IgM was negligible across all three membranes under evaluation (Table I, Fig. 2E and F).
Figure 2.
Raw mass transfer data for each test substance under high convection conditions. A: Ammonia, (B) total bilirubin, (C) direct bilirubin, (D) pig albumin, (E) pig IgG, (F) pig IgM, and (G) human TNF-α.
Rank Sum Analysis
A rank sum analysis of mass transfer rates was used to determine the optimal conditions for operation of the two-compartment apparatus. Based on rank sum analysis of waste-related molecules alone (ammonia, bilirubin, TNF-α, albumin), the two best operating conditions were medium convection and high convection both using the 400 kDa membrane ( P < 0.001). Results were unchanged when the adverse effects of IgG and IgM permeability were factored into the rank sum, presumably since the passage of IgG and IgM were negligible across the 400 kDa membrane.
Retention Time of Test Substances
Table II summarizes the molecular weight of each of the test substances as well as estimated retention time for each molecule within C1 under conditions of high convection with the 400 kDa membrane. As expected, the smallest molecules such as ammonia, bilirubin, and TNF-α had the shortest retention times while IgM, the largest molecule, had a significantly prolonged retention time.
Table II.
Influence of molecular weight on retention time under high convection, 400 kDa conditions.
| Molecule | MW (Da) | Minutes |
|---|---|---|
| Ammonia | 15 | 338 |
| Direct bilirubin | 760 | 382 |
| Indirect bilirubin | 585 | 393 |
| TNF-α | 17,000 | 347 |
| Albumin | 67,000 | 420 |
| IgG | 147,000 | 446 |
| IgM | 900,000 | 2,372 |
The retention time of each test substance is expressed in minutes; retention time is a function of the initial concentration of the test substance multiplied by compartment volume and divided by the mass transfer rate of the test substance from Table I.
Membrane Fouling by Dextran Technique
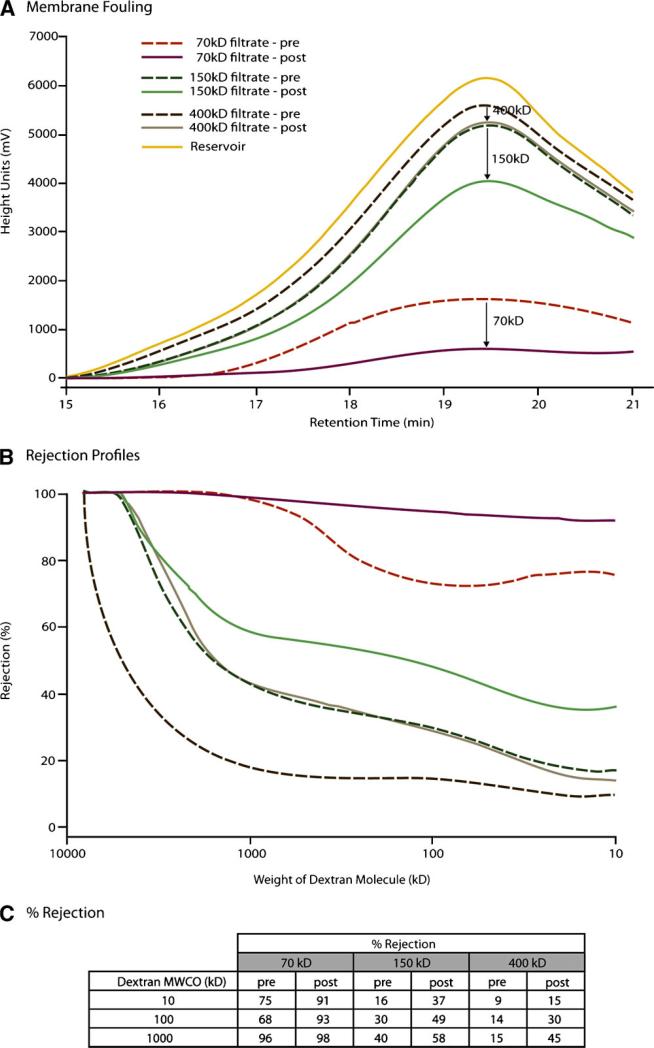
Permeability of each membrane was tested by dextran technique prior to and after mass transfer testing. The results of the dextran studies are summarized in Figure 3. Figure 3A illustrates chromatograms for each of the membranes when tested before and after mass transfer studies. As expected, the greatest magnitude of transport was seen with the 400 kDa membrane followed by 150 kDa and finally the 70 kDa membrane. We utilized the ratio of peak heights before and after to estimate a percent reduction due to membrane fouling. The magnitude in height units was as follows: 70 kDa membrane showed a 62.5% reduction (1,505 – 565) ÷ 1,505, the 150 kDa membrane showed a 23.7% reduction (5,157 – 3,936) ÷ 5,157, and the 400 kDa membrane showed a 7.1% reduction (5,576 – 5,181) ÷ 5,576. These data suggested that exposure of the BAL membrane to plasma would have a most adverse effect on permeability of a 70 kDa membrane (i.e., 62.5% reduction), while the 400 kDa membrane would have the least amount of fouling (7.1% reduction). Rejection profiles were generated with a standard curve to convert retention time to molecular weight of dextran as illustrated in Figure 3B. Rejection profiles, derived from the relationship that percent rejection = 100% × (C1 – C2)/C1, are illustrated for each of the three membranes using data collected prior to and after mass transfer testing. For each of the three membranes, an increase in rejection profile was observed; as expected the 400 kDa membrane had the lowest percent rejection. Figure 3C summarizes the intersections of percent rejection profiles with 10, 100, and 1,000 kDa dextran molecular weights from Figure 3B. Of note, dextran permeability differed from the nMWCO for each of the three membranes. This difference suggests that dextran molecules, which are linear in conformation, exhibit a much greater permeability than a globular protein of similar molecular weight. Of note, solutions of globular protein were used by the manufacturer to estimate nominal molecular cut-off for each membrane. As a result, the molecular weight values on Figure 3B do not match those reported by the manufacturer for each membrane. In summary, Figure 3 illustrates that the 70 kDa membrane is most adversely affected by exposure to a plasma solution with regard to its rejection profile. Greatest permeability was observed with the 400 kDa membrane at each of the molecular weights tested.
Figure 3.
A Chromatograms for evaluation of membrane fouling during exposure to pig plasma by dextran technique. B: Rejection profiles determined by dextran technique for the three membranes under evaluation. C: Summary of % rejection for 10, 100, and 1,000 kDa molecules of dextran.
Discussion
The most important observation from our studies was that membrane permeability had the greatest impact on mass transfer and the influence of membrane permeability could not be overcome by increased transmembrane convective flow under the current experimental conditions. The combination of increased membrane permeability with increased convective flow was additive such that the greatest mass transport for each of the test substances was achieved with either medium or high convective flow and the 400 kDa membrane. The benefit of convective flow appeared to plateau with a medium convection suggesting a build up of plasma proteins on the membrane at an ultrafiltration rate of 25 mL/min. This possibility is supported by the observation that, in some cases (albumin, 400 kDa membrane); a slightly higher rate of mass transport was observed using medium convection compared to high convection with the same membrane permeability.
A somewhat unexpected pattern of mass transport was observed with ammonia at the 70 and 150 kDa high convection conditions as illustrated in Figure 2. This pattern of delayed transport suggests that ammonia exhibited breakthrough characteristics rather than an approach to equilibrium as observed with all other molecules. This pattern suggested binding to and saturation of the 70 and 150 kDa membranes followed by eventual elution. As expected, the 400 kDa membrane delivered the highest rate of elimination of ammonia.
It should be noted that the transfer of cytotoxic molecules such as IgG and IgM was negligible under all of the conditions tested. We have previously demonstrated that the use of a membrane with permeability 150–400 kDa was immunoprotective and did not lead to death of hepatocytes within a xenogeneic BAL during extracorporeal perfusions of 2–4 h in duration (Nyberg et al., 2004). It is unclear whether prolonged exposure will have an adverse effect when membranes of this permeability are tested. However, we have shown that a sensitization effect could be avoided when a 400 kDa was tested in normal animals 2 weeks following a primary exposure (Nyberg et al., 2004). Therefore, we believe that the conditions of high convective flow using a 400 kDa membrane will provide the maximum mass transfer for extracorporeal application of a BAL without leading to excessive cell death from damage mediated by the patient's immune system.
It remains to be seen whether mass transfer under high convection with 400 kDa membrane will be sufficient to improve survival outcome of patients with acute liver failure. Prior systems such as the HepatAssist2000 device by Circe Biomedical utilized a much larger membrane of 0.2 μm permeability to protect porcine hepatocytes inside the BAL device during clinical application (Demetriou et al., 2004). It was reported that a significant anti-Gal response developed in patients who were treated with the Circe device and its 0.2 μm membrane after repeat exposures (Baquerizo et al., 1999). Such an immune response appears to be inhibited when a tighter membrane of 400 kDa was utilized (Nyberg et al., 2004). The ELAD therapy system which utilizes a human C3a hepatoblastoma cell line for its source of hepatic cell function also utilizes a membrane with 150–400 kDa cut-off (Millis and Losanoff, 2005). Clinical trials of the ELAD system have suggested efficacy (Millis et al., 2002). The mechanism of benefit remains uncertain, since C3A cells lack normal urea cycle activity when tested under in vitro conditions (Mavri-Damelin et al., 2008).
Therefore, we have continued to utilize primary (non-transformed) hepatocytes from a porcine source in our new spheroid reservoir BAL design (Nyberg et al., 2005). The number of hepatocytes required for a successful outcome remains unknown though we suspect that a hepatocyte mass in the range of 20–40% of normal liver mass should be sufficient to prolong survival provided these cells remain viable and functional, and they are not limited by mass transfer of waste products between the patient and the hepatocyte reservoir.
Now, after determination of optimal operating condition for mass transfer in the BAL, it is appropriate to ask the question, does this information influence further design of the device or its likelihood of success during clinical application? In order to answer this question, it is appropriate to estimate the metabolic demands of the patient in liver failure along with the metabolic activity that can be provided by hepatocytes within the BAL. A scale-up analysis of considerations appropriate for clinical application of a BAL is provided in Table III. Two representative essential hepatic functions, ammonia detoxification and bilirubin conjugation, were considered in this analysis along with the synthesis of albumin. For the purpose of this analysis, we utilized the high convection 400 kDa membrane condition reported in Table I. Clinical rates of ammonia detoxification, bilirubin conjugation, and albumin synthesis were estimated from clinical parameters based on a 70 kg man with 5 L blood volume. Experimental rates for ammonia detoxification, bilirubin conjugation, and albumin synthesis were based on those previously determined by our laboratory for primary hepatocytes grown in the spheroid configuration.
Table III.
Summary of scale-up considerations for clinical application of a bioartificial liver.
| Hepatic function | Clinical rate (μmol/h) | Hollow fiber surface area (m2) | Hepatocyte rate (μmol/h/g) | Scale-up (grams of hepatocytes) |
|---|---|---|---|---|
| Ammonia detoxification | 400 (Elin, 2007) | 0.3 | 2.6 (Nyberg et al., 2005) | 153 |
| Bilirubin conjugation | 12a | 0.3 | 0.22 (Nyberg, unpublished work) | 55 |
| Albumin synthesis | 6b | 0.3 | 2.0 (Brophy et al., 2009) | 3 |
Based on the assumption: 70 kg man, 5 L blood volume, [Hgb] = 10g/dL, 40 mg bili/g Hgb, 120-day life span of red blood cell.
Based on the assumption: 70 kg man, 5 L blood volume, [albumin] = 4 g/dL, 15-day life span of albumin.
Based on scale-up considerations outlined in Table III, it appears that ammonia detoxification will be the most difficult hepatic function to support with a BAL. A clinical rate of 400 μmol/h could be achieved with a spheroid reservoir BAL loaded with 153 g of hepatocytes. However, in order to avoid mass transfer limitations in such system, surface area of the hollow fiber module would need to be increased 8-fold—from 0.3 to 2.4 m2. Of note, hollow fiber modules are commercially available with a membrane permeability of 400 kDa and 2.5 m2 surface area.
With regard to bilirubin conjugation, our analysis suggests that 55 g of hepatocyte spheroids would provide a clinically acceptable rate of bilirubin conjugation. The 400 kDa, 0.3 m2 hollow fiber membrane should easily provide this level of bilirubin transfer. Similarly, the hollow fiber module used in our studies was sufficient to meet clinical levels of albumin production. Albumin production is not an essential clinical feature of a BAL since albumin is a commercially available intravenous product. Nonetheless, the hepatocyte BAL should provide sufficient albumin to meet patient needs.
In summary, we have performed a mass transfer analysis appropriate for clinical application of a spheroid reservoir BAL. The results suggest that a 400-kDa hollow fiber module operated under high convection should not experience mass transfer limitations. However, with regard to ammonia detoxification, it is recommended that a larger membrane with 2.5 m2 surface area or greater be utilized. Under clinical application, this membrane exposed to blood plasma could experience some level of fouling. Under our current experimental conditions, a reduction in permeability of approximately 7% was observed over a 2-h exposure. We conclude that the operating conditions identified in the current study would be appropriate for the next phases of development of the spheroid reservoir BAL.
Acknowledgments
Special thanks to Pamela Dahle for assistance with the preparation of the manuscript. Thanks to Walter Kremers and Ross Dierkhising for statistical analysis.
References
- Baquerizo A, Mhoya A, Kearns-Jonker M, Arnaout W, Shackleton C, Busuttil R, Demetriou A, Cramer D. Characterization of human xenoreactive antibodies in liver failure patients exposed to pig hepatocytes after bioartificial liver treatment. Transplantation. 1999;67:5–18. doi: 10.1097/00007890-199901150-00003. [DOI] [PubMed] [Google Scholar]
- Brophy C, Nyberg S. Extracorporeal treatment of acute liver failure. Hepatol Res. 2008;38(Suppl 1):S34–S40. doi: 10.1111/j.1872-034X.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, Nyberg SL. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49(2):578–586. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou A, Brown R, Busuttil R, Fair J, McGuire B, Rosenthal P, Schulte J, Lerut J, Nyberg S, Salizzoni M, Fagan E, de Hemptinne B, Broelsch C, Muraca M, Salmeron J, Rabkin J, Metselaar H, Pratt D, De La Mata M, McChesney L, Everson G, Lavin P, Stevens A, Pitkin Z, Solomon B. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–670. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. Reference intervals and laboratory values. In: Goldman JK, editor. Cecil medicine. 23rd edn. Saunders; St. Louis, MO: 2007. [Google Scholar]
- Filippi C, Keatch S, Rangar D. Improvement of C3A metabolism for usage in bioartificial liver support systems. J Hepatol. 2004;41:499–505. doi: 10.1016/j.jhep.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Gerlach J, Trost T, Ryan CJ, Meissler M, Hole O, Muller C, Neuhaus P. Hybrid liver support system in a short term application on hepatectomized pigs. Int J Artif Organs. 1994;17(10):549–553. [PubMed] [Google Scholar]
- Lee W, Squires RJ, Nyberg S, Doo E, Hoofnagle J. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Guevara G, Huston H, Hamilton W, Rikimaru M, Yamasaki G, Matsumura M. Hybrid bioartificial liver in hepatic failure: Preliminary clinical report. Surgery. 1987;101:99–103. [PubMed] [Google Scholar]
- Matsushita T, Amiot B, Hardin J, Platt J, Nyberg S. Membrane pore size impacts performance of a xenogeneic bioartificial liver. Transplantation. 2003;76:1299–1305. doi: 10.1097/01.TP.0000080067.79190.3C. [DOI] [PubMed] [Google Scholar]
- Mavri-Damelin D, Eaton S, Damelin LH, Rees M, Hodgson HJ, Selden C. Ornithine transcarbamylase and arginase I deficiency are responsible for diminished urea cycle function in the human hepatoblastoma cell line HepG2. Int J Biochem Cell Biol. 2007;39(3):555–564. doi: 10.1016/j.biocel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Mavri-Damelin D, Damelin LH, Eaton S, Rees M, Selden C, Hodgson HJ. Cells for bioartificial liver devices: The human hepatoma-derived cell line C3A produces urea but does not detoxify ammonia. Biotechnol Bioeng. 2008;99:644–651. doi: 10.1002/bit.21599. [DOI] [PubMed] [Google Scholar]
- McKenzie T, Lillegard J, Nyberg S. Artificial and bioartificial liver support. Semin Liver Dis. 2008;28:210–217. doi: 10.1055/s-2008-1073120. [DOI] [PubMed] [Google Scholar]
- Millis J, Losanoff J. Technology insight: Liver support systems. Nat Clin Pract Gastroenterol Hepatol. 2005;2:398–405. doi: 10.1038/ncpgasthep0254. [DOI] [PubMed] [Google Scholar]
- Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, Maguire P. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: System modifications and clinical impact. Transplantation. 2002;74(12):1735–1746. doi: 10.1097/00007890-200212270-00016. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Platt J, Shirabe K, Payne W, Hu W-S, Cerra F. Immuno-protection of xenocytes in a hollow fiber bioartificial liver. ASAIO J. 1992;38:M463–M467. doi: 10.1097/00002480-199207000-00077. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Yagi T, Matsushita T, Hardin J, Grande J, Gibson L, Platt J. Membrane barrier of a porcine hepatocyte bioartificial liver. Liver Transplant. 2003;9:298–305. doi: 10.1053/jlts.2003.50024. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Amiot B, Hardin J, Baskin Bey E, Platt J. Cytotoxic immune response to a xenogeneic bioartificial liver. Cell Transplant. 2004;13:783–791. doi: 10.3727/000000004783983378. [DOI] [PubMed] [Google Scholar]
- Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplant. 2005;11(8):901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- Patzer J. Principles of bound solute dialysis. Ther Apher Dial. 2006;10:118–124. doi: 10.1111/j.1744-9987.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- Patzer JF II, Campbell B, Miller R. Plasma versus whole blood perfusion in a bioartificial liver assist device. ASAIO J. 2002;48(3):226–233. doi: 10.1097/00002480-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Strain A, Neuberger J. A bioartificial liver—State of the art. Science. 2002;295:1005–1009. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- Sussman NL, McGuire BM, Kelly JH. Hepatic assist devices: Will they ever be successful? Curr Gastroenterol Rep. 2009;11(1):64–68. doi: 10.1007/s11894-009-0010-x. [DOI] [PubMed] [Google Scholar]
- Wang L, Sun J, Li L, Mears D, Horvat M, Sheil A. Comparison of porcine hepatocytes with human hepatoma (C3A) cells for use in a bioartificial liver support system. Cell Transplant. 1998;7:459–468. doi: 10.1177/096368979800700505. [DOI] [PubMed] [Google Scholar]