Abstract
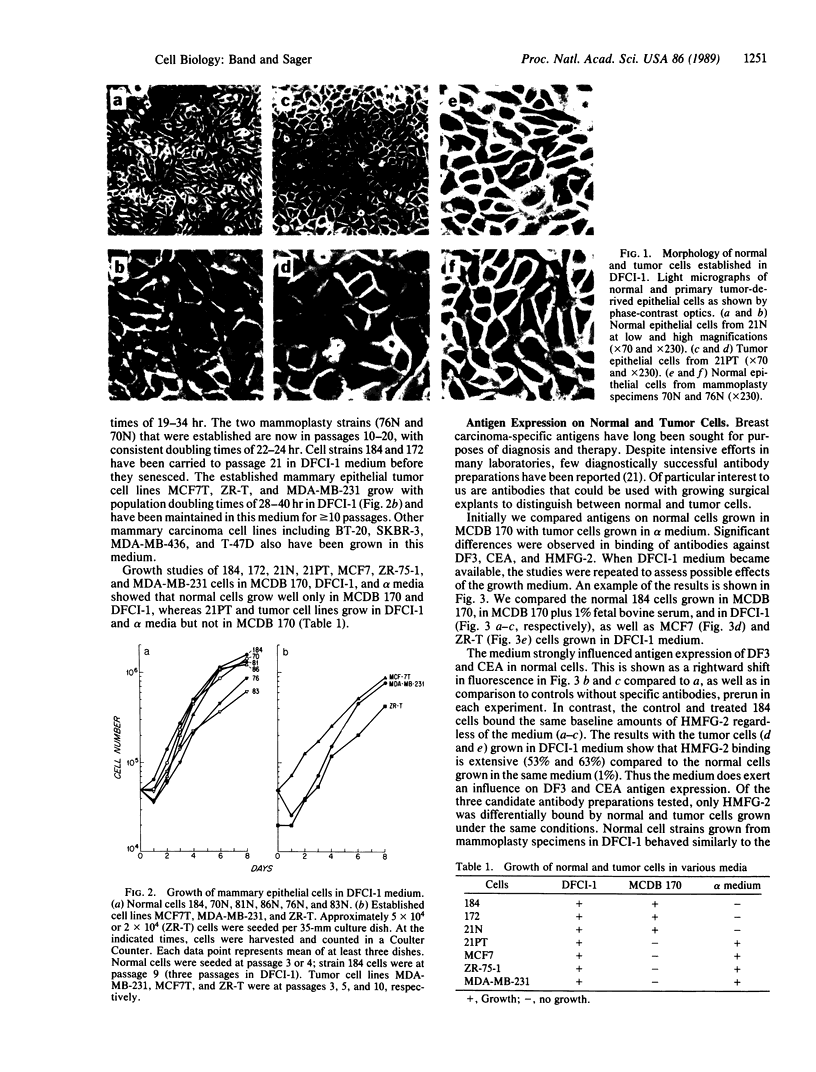
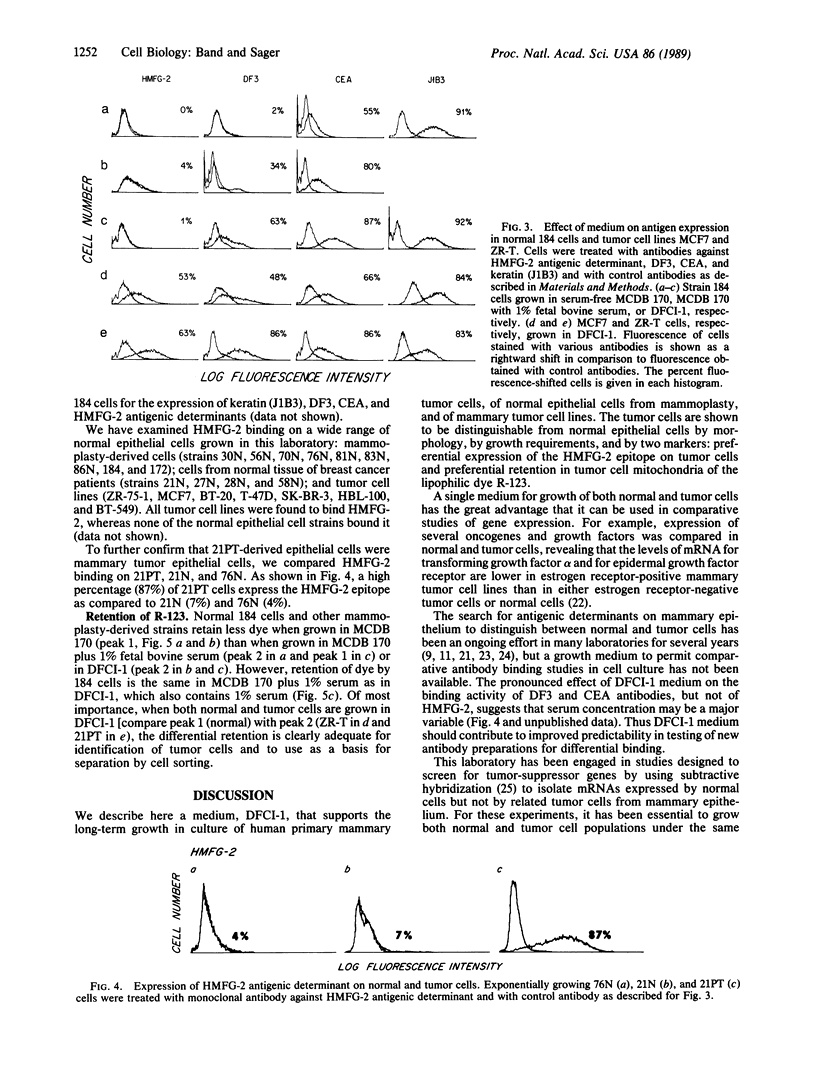
A medium is described that supports long-term growth in culture of human primary mammary tumor cells, of normal epithelial cells from mammoplasty, and of mammary tumor cell lines. Tumor cells are shown to be distinguishable from normal mammary epithelial cells by morphology, by growth requirements, and by two markers: preferential expression of the HMFG-2 epitope on tumor cells and preferential retention in tumor cell mitochondria of the lipophilic fluorescent dye rhodamine 123. Differential fluorescence of HMFG-2 fluorescein-conjugated antibodies can be used as a basis for separation of normal and tumor cells in a fluorescence-activated cell sorter, as can differential retention of rhodamine 123.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Zajchowski D., Stenman G., Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Crissman H. A., Stevenson A. P., Orlicky D. J., Kissane R. J. Detailed studies on the application of three fluorescent antibiotics for DNA staining in flow cytometry. Stain Technol. 1978 Nov;53(6):321–330. doi: 10.3109/10520297809111954. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Hammond S. L., Ham R. G., Stampfer M. R. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D. F., Sekine H., Ohno T., Abe M., Keefe K., Kufe D. W. Use of a murine monoclonal antibody for detection of circulating plasma DF3 antigen levels in breast cancer patients. J Clin Invest. 1985 May;75(5):1671–1678. doi: 10.1172/JCI111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D., Inghirami G., Abe M., Hayes D., Justi-Wheeler H., Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984 Fall;3(3):223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lundy J., Thor A., Maenza R., Schlom J., Forouhar F., Testa M., Kufe D. Monoclonal antibody DF3 correlates with tumor differentiation and hormone receptor status in breast cancer patients. Breast Cancer Res Treat. 1985;5(3):269–276. doi: 10.1007/BF01806021. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Feola J. M., Beach J. L. HeLa cell tumor response to 60Co, Cs-137, Cf-252 radiations and cisplatin chemotherapy in nude mice. Cancer. 1984 Jul 15;54(2):247–252. doi: 10.1002/1097-0142(19840715)54:2<247::aid-cncr2820540211>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Muirhead K. A., Schmitt T. C., Muirhead A. R. Determination of linear fluorescence intensities from flow cytometric data accumulated with logarithmic amplifiers. Cytometry. 1983 Jan;3(4):251–256. doi: 10.1002/cyto.990030404. [DOI] [PubMed] [Google Scholar]
- Nadakavukaren K. K., Summerhayes I. C., Salcedo B. F., Rheinwald J. G., Chen L. B. A monoclonal antibody recognizing a keratin filament protein in a subset of transitional and glandular epithelia. Differentiation. 1984;27(3):209–220. doi: 10.1111/j.1432-0436.1984.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Preservation of defined phenotypic traits in short-term cultured human breast carcinoma derived epithelial cells. Cancer Res. 1987 Feb 1;47(3):856–866. [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Lan S., Ceriani R., Hackett A. J., Stampfer M. R. Clonal proliferation of cultured nonmalignant and malignant human breast epithelia. Cancer Res. 1981 Nov;41(11 Pt 1):4637–4643. [PubMed] [Google Scholar]
- Smith H. S., Wolman S. R., Dairkee S. H., Hancock M. C., Lippman M., Leff A., Hackett A. J. Immortalization in culture: occurrence at a late stage in the progression of breast cancer. J Natl Cancer Inst. 1987 Apr;78(4):611–615. [PubMed] [Google Scholar]
- Smith H. S., Wolman S. R., Hackett A. J. The biology of breast cancer at the cellular level. Biochim Biophys Acta. 1984;738(3):103–123. doi: 10.1016/0304-419x(84)90009-x. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Summerhayes I. C., Lampidis T. J., Bernal S. D., Nadakavukaren J. J., Nadakavukaren K. K., Shepherd E. L., Chen L. B. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Stoker M. G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977 Dec 15;20(6):903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Zajchowski D., Band V., Pauzie N., Tager A., Stampfer M., Sager R. Expression of growth factors and oncogenes in normal and tumor-derived human mammary epithelial cells. Cancer Res. 1988 Dec 15;48(24 Pt 1):7041–7047. [PubMed] [Google Scholar]





