Abstract
Background
New therapies are needed to shorten the time required to cure tuberculosis and to treat drug-resistant strains. The fluoroquinolone moxifloxacin is a promising new agent that may have additive activity to existing antituberculosis agents. We conducted a Phase 2 clinical trial to determine the activity and safety of moxifloxacin in the initial stage of tuberculosis treatment.
Methods
We performed a randomized, double-blind trial of a moxifloxacin-containing regimen in patients with sputum smear-positive tuberculosis in Brazil. All participants received isoniazid, rifampin and pyrazinamide at standard doses and were randomized to receive either moxifloxacin or ethambutol and matching placebos five days per week for eight weeks. The primary endpoint was the proportion of patients whose sputum culture converted to negative by Week 8. Clinical trial identifier: NCT00082173.
Results
One hundred seventy patients were enrolled, and 146 met all study eligibility criteria. In an intention to treat analysis where missing results were considered treatment failures, 59 patients (80%) assigned to moxifloxacin converted their 8-week sputum culture to negative vs. 45 (63%) of those assigned to ethambutol (p=0.03). Among patients with available cultures at Week 8, conversion rates were 92% (59/64) for moxifloxacin vs. 72% (45/61) for ethambutol (p=0.006). No differences in toxicity were observed. In a multivariate analysis, younger age (odds ratio 0.98, p=0.05), heavy baseline sputum smear positivity (OR 0.45, p <0.001) and treatment with moxifloxacin (OR 1.88, p<0.001) were significantly associated with sputum culture conversion.
Conclusion
Moxifloxacin significantly improves culture conversion in the initial phase of tuberculosis treatment. Trials to assess whether moxifloxacin can be used to shorten the duration of tuberculosis therapy are justified.
Introduction
The development of new drug regimens for tuberculosis is an urgent global health priority (1). While so-called “short-course” therapy can effectively cure drug-susceptible tuberculosis in six months, a large proportion of patients who are diagnosed with tuberculosis fail to complete a course of treatment (2). New drugs that could shorten the duration of tuberculosis therapy would considerably improve treatment completion and reduce the likelihood of recurrence and death due to inadequate therapy. In addition, 500,000 cases of tuberculosis occur each year with strains of Mycobacterium tuberculosis that are resistant to the key first-line drugs isoniazid and rifampin, and agents that are active in multidrug resistant tuberculosis are also needed (3).
Moxifloxacin is a fluoroquinolone that has potent in vitro activity against M. tuberculosis (4, 5). Early studies of moxifloxacin in murine models of tuberculosis showed that the drug had good bactericidal activity that was additive to isoniazid (6, 7). On the basis of these studies, we designed a Phase 2 trial to test the hypothesis that the substitution of moxifloxacin for ethambutol, an agent used primarily to prevent the emergence of drug resistance, would significantly increase the proportion of patients with negative sputum cultures after eight weeks of treatment. Eight-week sputum conversion has been demonstrated to be a useful surrogate marker of the sterilizing activity of tuberculosis regimens (8), and a substantial improvement in this endpoint would support evaluating shortening the duration of treatment with a moxifloxacin-containing regimen in a phase 3 trial.
Methods
The study was a randomized, double-blind, double-dummy trial for patients with sputum smear-positive tuberculosis with no prior history of treatment. Eligible patients were age 18 years or older who had clinical signs and symptoms of pulmonary tuberculosis, including an abnormal chest radiograph, and at least one sputum smear with acid fast bacilli visible by Ziehl-Neelsen staining. Potential study subjects underwent baseline screening that included blood counts, liver enzymes and kidney function testing, HIV serology, chest radiograph and culture and drug susceptibility testing for mycobacteria. Patients were excluded if they had a hemoglobin <7 g/dL, aspartate or alanine aminotransferase levels >3 times the upper limits of normal, serum creatinine >2 times the upper limit of normal, an electrocardiogram with a QTc >450 ms, were pregnant or nursing, if they had silicotuberculosis, if they had a history of severe adverse reactions to fluoroquinolones or any other study agent, or if they were HIV seropositive and had a CD4 cell count <200 mm3, as this would be an indication for antiretroviral therapy which could be affected by antituberculosis drug interactions. Initially enrolled patients were subsequently excluded if their baseline culture failed to grow M. tuberculosis or grew a strain of M. tuberculosis that was resistant to isoniazid, rifampin or ethambutol
Consenting patients were stratified by HIV serostatus and randomly assigned to treatment with one of two study regimens using permutated blocked randomization with treatment allocation concealed and allocation slips sealed in opaque envelopes opened after enrollment. Patients received either moxifloxacin 400 mg with an ethambutol placebo or moxifloxacin placebo plus ethambutol 15–20 mg/kg. The study was designed to ensure that all patients received an effective regimen for tuberculosis regardless of the contribution of the two experimental agents, moxifloxacin and ethambutol, and therefore all patients received isoniazid 300 mg, rifampicin 450 mg (weight <50 kg) or 600 mg (weight ≥50 kg), and pyrazinamide 20–25 mg/kg by directly observed therapy. Patients, study clinicians and study staff were unaware of the treatment assignments of patients, with the exception of the pharmacist who dispensed medication packets. All therapy was given by direct supervision either in the hospital clinic or in the community by study personnel, five days per week. At the end of eight weeks, all patients were placed on open-label treatment with isoniazid and rifampin three times weekly to complete another four months of therapy. Moxifloxacin and matching placebo was provided by Bayer Healthcare (West Haven, CT, USA) and ethambutol, ethambutol placebo, isoniazid, rifampin and pyrazinamide were provided by Farmanguinhos, Rio de Janeiro, Brazil. All study agents were produced under Good Manufacturing Process.
Patients had weekly clinic visits to assess clinical status and to monitor for adverse reactions. Liver enzymes, serum creatinine levels and complete blood counts were performed monthly, and an electrocardiogram was obtained at Weeks 2, 4, 6 and 8. A sputum specimen (spontaneous or induced) was obtained weekly for smear and culture. Sputum specimens were digested and decontaminated using the N-acetyl-L-cysteine-sodium hydroxide (NALC-NaOH) method (9, 10). Pellets were resuspended in a final volume of 2 ml and used immediately for inoculation of Löwenstein-Jensen culture medium. Löwenstein-Jensen slants were prepared from a commercial powder medium base (BD, Sparks, MD, USA), according to the manufacturer’s instructions. Two hundred microliters of each decontaminated respiratory specimen was inoculated onto each of two slants. Slants were incubated at 37°C in ambient CO2 and examined visually for growth twice weekly for 8 weeks. Growth on slants was quantified by number of visible colonies, and then examined by acid-fast staining to confirm the presence of mycobacteria; isolates were speciated using standard biochemical tests (10). Drug susceptibility testing of all baseline cultures growing Mycobacterium tuberculosis was performed using the proportions method (10).
The primary endpoint of the study was the proportion of patients with negative sputum cultures after eight weeks of treatment. We assumed that the conversion rate in the control arm (ethambutol) would be 65% (8), and that moxifloxacin would increase this by 20% to 85%. To achieve a power of 0.8 with an alpha of 0.05, we needed 70 evaluable patients in each arm of the trial. We aimed to enroll 85 per arm to allow for exclusions based on negative initial culture, growth of nontuberculous mycobacteria, or baseline drug resistance. The primary endpoint of the trial, culture conversion, was assessed in a modified intention to treat analysis where those whose baseline cultures were either negative, contaminated or contained drug-resistant M. tuberculosis were excluded and where all missing week-8 results were considered to be failures (missing=failure). We also analyzed all patients with available Week 8 cultures (evaluable analysis). Multivariate logistic regression was performed using treatment allocation, selected baseline characteristics as covariates and culture conversion as the dependent variable. Time to conversion of sputum cultures to negative was estimated by the Kaplan-Meier method and the difference in median times was compared using the log-rank test. All analyses were performed using STATA Version 9 (STATA Corp., College Station, TX, USA).
Ethical review of the protocol was obtained from the Johns Hopkins Medicine Institutional Review Board and the Ethics Committee of the Federal University of Rio de Janeiro, the National Ethics Committee of the Ministry of Health (CONEP), Brasilia and the Agencia Nacional de Vigilancia Sanitaria (ANVISA), Brazil.
Role of the Funding Source
The study was funded by the Office of Orphan Product Development, United States Food and Drug Administration and was registered as a clinical trial at Clinicaltrials.gov. Additional support for training was provided by the Fogarty International Center of the National Institutes of Health, and a career development grant helped support Dr. Chaisson’s participation. None of the funding agencies was involved in the design, conduct or analysis of the study. The Investigational New Drug Office of the Food and Drug Administration reviewed the protocol and made suggestions regarding the study design prior to initiation of the trial, but this was unrelated to any funding decisions. The decision to publish was made solely by the authors and the funding agencies did not review or approve the manuscript. Bayer Healthcare donated moxifloxacin and matching placebo for the trial, but had no input into the study design, execution or analysis.
Results
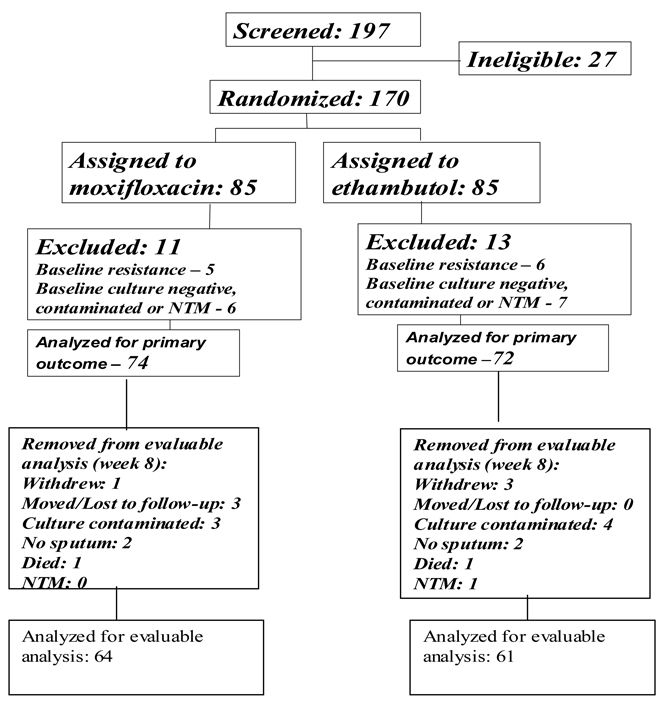
From October 2004 through March 2007, one-hundred-ninety seven patients were screened for participation, 27 of whom were found to ineligible. One-hundred-seventy patients were entered into the trial and evenly randomized to the two treatment arms (Figure 1). Eleven patients in the moxifloxacin arm and 13 in the ethambutol arm were excluded from analysis because of baseline resistance to study drugs, growth of nontuberculous mycobacteria or contaminated cultures. Baseline characteristics of the patients are shown in Table 1. Most patients were young adults, with a mean age of 32 years, and 55 (38%) were female. HIV serology was obtained for all patients, and only five were positive, three in the moxifloxacin arm and two in the ethambutol arm. All patients had pulmonary infiltrates on their baseline chest radiograph, and 65 (45%) had bilateral pulmonary disease. A higher proportion of patients assigned to moxifloxacin had cavitation on their chest x-ray than those assigned to ethambutol (81% vs. 57%). Baseline sputum smears were graded according to the number of acid fast bacilli seen per high power field (Table 1): 30% of patients (44) had >10 bacilli per high power field, with no differences between study arms. Patients assigned to moxifloxacin also had an approximately 4 kg lower median baseline weight than those assigned to ethambutol. There were no other differences in baseline clinical characteristics between the two treatment arms.
Figure 1.
Summary of recruitment, allocation and disposition of study participants.
Table 1.
Baseline characteristics of study participants
| Variable | Moxifloxacin | Ethambutol | All | |
|---|---|---|---|---|
| N | 74 | 72 | 146 | |
| Age (years) – mean(SD) | 32.5 (11.7) | 35.7 (12.0) | 34.1 (11.9) | |
| Sex | ||||
| Male | 41 (55%) | 50 (69%) | 91 (62%) | |
| Female | 33 (45%) | 22 (31%) | 55 (38%) | |
| Race | ||||
| Non-white | 44 (59%) | 40 (56%) | 84 (58%) | |
| White | 30 (41%) | 32 (44%) | 62 (42%) | |
| HIV Seropositive | 3 (4%) | 2 (3%) | 5 (3%) | |
| Socio-economic status | ||||
| Mean income (SD) (reais/month) |
636 (550) | 847 (724) | 738 (647) | |
| Formal employment | 35 (47%) | 41 (57%) | 76 (52%) | |
| Weight (kg) – mean(SD) | 54.3 (9.6) | 58.2 (9.9) | 56.2 (9.9) | |
|
Baseline Sputum Acid Fast Bacilli Smear |
||||
| < 1 per hpf* | 42 (57%) | 33 (46%) | 75 (51%) | |
| 1–10 per hpf | 10 (14%) | 17 (24%) | 27 (18%) | |
| >10 per hpf | 22 (30%) | 22 (31%) | 44 (30%) | |
| Baseline x-ray | ||||
| Infiltrate | 74 (100%) | 72 (100%) | 146 (100%) | |
| Bilateral disease | 34 (46%) | 31 (43%) | 65 (44.5%) | |
| Cavitation | 60 (81%) | 41 (57%) | 101 (69%) | |
| Adenopathy | 8 (11%) | 11 (15%) | 19 (13%) | |
| Pleural effusion | 4 (5%) | 7 (10%) | 11 (8%) | |
| Baseline Symptoms | ||||
| Productive Cough | 59 (80%) | 57 (79%) | 116 (79%) | |
| Hemoptysis | 25 (34%) | 20 (28%) | 45 (31%) | |
| Fever | 59 (80%) | 58 (81%) | 117 (80%) | |
| Night Sweats | 61 (82%) | 57 (79%) | 118 (81%) | |
| Weight loss | 66 (89%) | 70 (97%) | 136 (93%) | |
| Current or past smoker | 29 (39 %) | 37 (51%) | 66 (45%) | |
|
Mean days of treatment before enrollment (SD) |
6.4 (3.2) | 5.6 (2.5) | 6.0 (2.9) | |
|
Baseline Blood results – mean(SD) |
||||
| White Blood Cells (/ml) | 9324 (2961) | 9728 (3921) | 9523 (3462) | |
| Hematocrit (%) | 36.4 (5.7) | 36.9 (5.1) | 36.7 (5.4) | |
| Hemoglobin (g/dL) | 12.3 (1.8) | 12.3 (1.8) | 12.3 (1.8) | |
| Neutrophils (/ml) | 6784 (2530) | 7072 (3398) | 6926 (2983) | |
| Alkaline Phosphatase (U/L) |
116.4 (62.3) | 121.2 (49.2) | 118.7 (56.1) | |
| ALT (U/L) | 35.8 (22.3) | 35.6 (18.9) | 35.7 (20.6) | |
| AST (U/L) | 27.8 (16.3) | 26.8 (14.4) | 27.3 (15.4) | |
| Total Bilirubin (mg/dL) | 0.55 (0.35) | 0.49 (0.26) | 0.5 (0.3) | |
| GGT (U/L) | 72.6 (50.5) | 96.1 (89.3) | 84.2 (73.0) | |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.8 (0.1) | 0.8 (0.2) | |
hpf = high power field
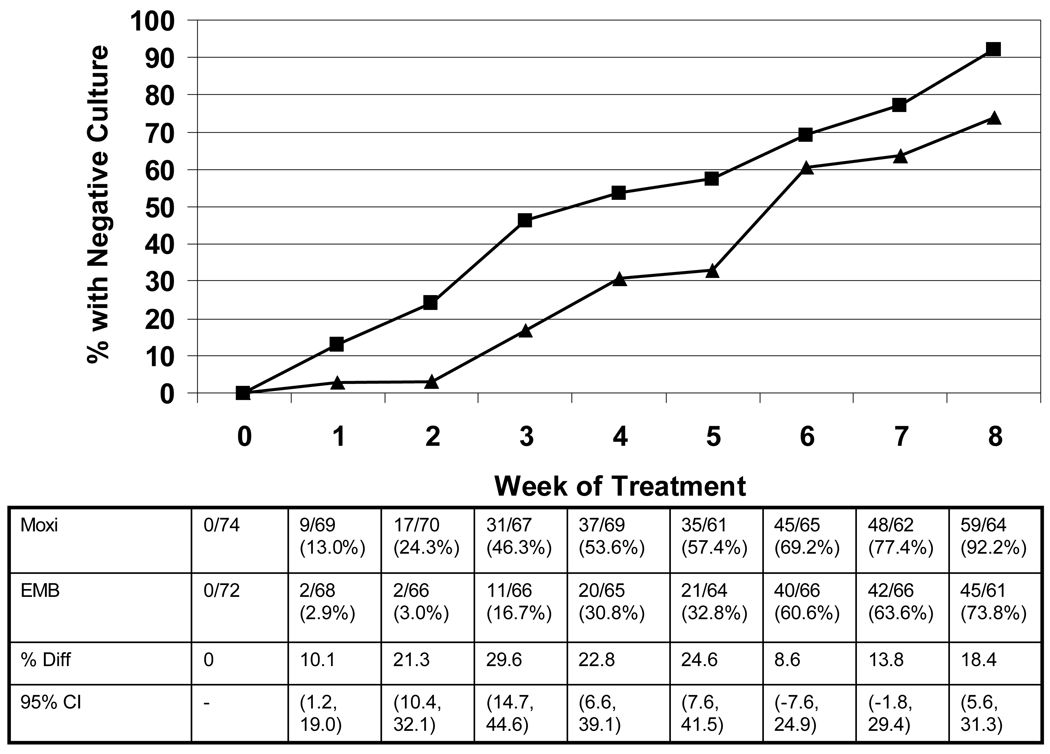
The primary outcomes of the study are shown in Table 2. By modified intention to treat analysis considering missing values as treatment failures, the proportions converting were 80% for moxifloxacin (59/74) and 63% for ethambutol (45/72; p=0.03). Of patients who had evaluable culture data at Week 8 (evaluable analysis), 92% of those treated with moxifloxacin (59/64) converted to negative vs. 74% of those receiving ethambutol (45/61; p <0.01). Patients treated with moxifloxacin became culture-negative more rapidly than those treated with ethambutol. After only one week, 9 of 69 patients receiving moxifloxacin (13%) had negative sputum cultures compared with 2/68 patients receiving ethambutol (3%; p=0.03). As shown in Figure 2, at every weekly interval after enrollment patients treated with moxifloxacin had a higher rate of culture conversion than those randomized to ethambutol, and this difference was significant at each time point except Weeks 6 and 7. Patients in the moxifloxacin arm converted sputum cultures to negative more rapidly than those treated with ethambutol, as well. The median time to consistently negative cultures was 35.5 days for moxifloxacin patients vs. 48.5 days for ethambutol patients (log-rank p = 0.005).
Table 2.
Primary efficacy outcome of the trial.
| Outcome | Moxifloxacin Culture -/N (%) |
Ethambutol Cluture-/N (%) |
Difference (95% CI) | P Value |
|---|---|---|---|---|
| Modified Intent To Treat (Missing=Failure) |
59/74 (80%) | 45/72 (63%) | 17.2 (2.8 – 31.7) | 0.03 |
| Evaluable patients (Culture done and evaluable) |
59/64 (92%) |
45/61 (74%) |
18.4 (5.6, 31.3) |
0.006 |
Figure 2.
Proportions of patients in each treatment arm with negative cultures by week of treatment.
Univariate and multivariate logistic regression modeling was done using baseline characteristics and treatment group using sputum conversion as the dependent variable. Using the as-treated and the modified intention-to-treat definitions showed extremely similar results, and the modified intention to treat analysis is shown in Table 3. Treatment with moxifloxacin was associated with a 1.88-fold increase in the odds of being culture negative at Week 8 in multivariate analysis (p<0.001). Sex and baseline weight were not associated with culture conversion, but age was significantly associated with conversion, as patients with positive cultures were significantly older than those who converted to culture-negative (39.3 years vs. 32.6 years, adjusted P=0.03).. Cavitation on chest x-ray was not significantly associated with the likelihood of conversion. Patients who remained culture positive at 8 weeks were more likely than those who converted to negative to have had baseline sputum smears with >10 bacilli per high power field (11/27, 40.7% vs. 36/119, 30.3%; multivariate OR 0.45, p <0.001).
Table 3.
Univariate and multivariate logistic regression analysis for culture conversion at Week 8 using the modified intention to treat approach.
| Baseline Covariate | Univariate Analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Patients with positive culture at 8 weeks |
Patients with negative culture at 8 weeks |
OR (95% CI) | p-value | Odds Ratio (95% CI) |
p-value | |
| Mean (95% CI) |
Mean (95% CI) |
|||||
| Mean Age (Years) | 39.3 (34.8, 43.8) |
32.6 (30.5, 34.6) |
0.98 (0.96 – 1.00) | 0.007 | 0.98 (0.96 – 1.00) |
0.03 |
| Mean Weight (Kgs) |
56.2 (52.1, 60.2) |
56.0 (54.3, 57.7) |
1.00 (0,98 −1.01) | 0.566 | 1.00 (0.99 – 1.02) |
0.756 |
| No. with covariate/All Culture Positive Patients (%) |
No. with covariate/All Culture Negative Patients (%) |
|||||
| Female Sex | 9/27 (44.3) | 47/119 (39.5) | 1.17 (0.82 – 1.65) | 0.385 | 1.10 (0.77 – 1.57) |
0.611 |
| Cavitation on X- ray |
18/27 (66.7) | 82/119 (68.9) | 1.25 (0.86 – 1.81) | 0.245 | 1.11 (0.78 – 1.57) |
0.556 |
| AFB per hpf** on smear |
||||||
| <1 | 7 (26%) | 66 (56%) | 0.831 | |||
| 1–10 | 9 (33%) | 17 (14%) | 0.77 (0.45 – 1.33) | 0.348 | 0.95 (0.56, 1.60) |
<0.0001 |
| >10 | 11(41%) | 36 (30%) | 0.46 (0.32 – 0.67) | < 0.0001 | ||
| 0.45 (0.31, 0.65) |
||||||
| Moxifloxacin arm | 7/27 (25.9) | 67/119 (56.3) | 1.98 (1.41 – 2.76) |
< 0.0001 | 1.86 (1.32 – 2.62) |
< 0.001 |
moxifloxacin vs. ethambutol
acid-fast bacilli per high power field
Toxicities in the study did not differ by treatment arm. There were 16 serious adverse events in 12 patients, 8 in the moxifloxacin arm and 8 in the ethambutol arm; one grade 3 cutaneous reaction requiring hospitalization in the ethambutol arm was judged to be related to study drugs by the treating physicians who were blinded to treatment assignment. All other serious adverse events were considered not related to medications. A listing of serious adverse events is shown in Table 4. Eight patients died during the study, three in the moxifloxacin arm and five in the ethambutol arm, including one in each arm who were still receiving study phase treatment. No death was attributed to study treatment. Only five patients discontinued treatment due to toxicities; two patients in the moxifloxacin arm stopped due to Grade 2 nausea and vomiting and one due to Grade 2 parasthesias and ataxia. Three patients in the ethambutol arm stopped due to Grade 2 rash and pruritis (2) and Grade 3 peripheral neuropathy (1). No clinically or statistically significant changes in the QTc interval were observed in patients on either arm of the trial.
Table 4.
Serious Adverse Events by Study Arm.
| Arm | Patient | Event | Days from Enrollment to Event |
Primary Diagnosis | Attribution to Study Drugs |
Grade |
|---|---|---|---|---|---|---|
| EMB | P1 | Hospitalization | 68 | Cutaneous reaction | Probably Related |
3 |
| P2 | Death | 268 | Gunshot wound | Not related | 5 | |
| P3 | Death | 65 | Gunshot wound | Not related | 5 | |
| P4 | Death | 31 | Tuberculosis | Not related | 5 | |
| P5 | Hospitalization | 171 | Polyneuropathy | Not related | 3 | |
| Death | 240 | Unknown | Not related | 5 | ||
| P6 | Hospitalization | 84 | Unilateral leg edema | Not related | 2 | |
| Death | 95 | Subdural hemorrhage | Not related | 5 | ||
| MOX | P7 | Hospitalization | 31 | Community acquired pneumonia |
Not related | 3 |
| Hospitalization | 377 | Pulmonary abscess | Not related | 2 | ||
| P8 | Death | 242 | Urinary sepsis | Not related | 5 | |
| P9 | Hospitalization | 57 | Spontaneous abortion | Not related | N/A | |
| P10 | Death | 372 | Gunshot wound | Not related | 5 | |
| P11 | Hospitalization | 19 | Dysphasia | Not related | 4 | |
| Death | 61 | Esophageal neoplasm | Not related | 5 | ||
| P12 | Grade 4 Toxicity |
69 | Proteinuria | Not related | 4 |
Patients were followed for at least 12 months after completion of their 6-month course of treatment, consisting of the 2-month experimental arms and a further 4 months of isoniazid and rifampin. A total of 7 patients (5%) experienced a recurrence of tuberculosis confirmed by positive culture and compatible clinical symptoms; 4 patients in the ethambutol arm (at 6, 7, 22 and 32 months after completing treatment) and 3 patients in the moxifloxacin arm (at 11, 16 and 27 months after completion). Six of seven isolates were tested for drug resistance, and all remained susceptible to isoniazid and rifampin. DNA fingerprinting data on these isolates, to distinguish relapse from reinfection, is not available.
Discussion
We found that moxifloxacin significantly increased the conversion of sputum cultures to negative in patients with pulmonary tuberculosis in this Phase 2 trial. Patients who received moxifloxacin instead of ethambutol had a greater likelihood of being culture negative after only one week of treatment, and this difference continued through the eight weeks of intensive phase therapy. Whether analyzed by intention-to-treat or as-treated, patients randomized to moxifloxacin had an absolute difference in culture conversion after eight weeks of just under 20%. By comparison, the addition of rifampin to tuberculosis regimens in the 1970s increased culture conversion rates at two months by 15–20% and allowed the duration of treatment to be reduced from 18 months to six-to-nine months (11, 12). In subsequent years, the addition of pyrazinamide to regimens containing isoniazid, rifampin and ethambutol or streptomycin increased the 8-week sputum conversion rate by 13% and permitted treatment to be shortened from nine to six months (13). Our data suggest that the improvement in sterilization provided by moxifloxacin could permit the shortening of tuberculosis treatment by one or several months, based on reductions in treatment duration achieved by similar improvements in 2-month culture conversion with pyrazinamide (13). In addition, treatment shortening to three or four months using moxifloxacin has been possible in murine models of tuberculosis therapy in studies published since this trial was initiated (14, 15).
Moxifloxacin has been shown to have potency in two other trials carried out during the same time period as this study. The Tuberculosis Trials Consortium Study 27 also substituted moxifloxacin for ethambutol and found that culture conversion occurred more rapidly with moxifloxacin, but the proportions with negative cultures were equivalent after eight weeks (16). Study 27 was a multicenter trial and had a factorial design wherein half of all patients where treated with a thrice-weekly regimen and the other half with daily treatment. In addition, the culture methods used were not standardized in the participating laboratories, and more sensitive liquid culture media were used. In our study, the well-validated Löwenstein-Jensen culture method was used, and all cultures were performed in a single laboratory using a standard protocol. While a single center study is more efficient and ensures standardization of laboratory methods in a Phase 2 trial, the results from a single population may not be generalizable to all populations.
The OFLOTUB study, carried out at several sites in South Africa, compared several fluoroquinolones to ethambutol and found that moxifloxacin and gatifloxacin resulted in higher culture conversion rates on solid media after eight weeks, whereas ofloxacin did not (17). Modeled data from quantitative cultures also suggested that moxifloxacin resulted in more rapid culture conversion, although solid media data were not presented for earlier time points. Our results confirm that moxifloxacin significantly improves culture conversion compared to ethambutol and document that the effect of moxifloxacin occurs early in treatment, after only one week.
In multivariate analysis, assignment to moxifloxacin, heavy baseline sputum smear positivity (>10 bacilli per high power field) and age were associated with culture conversion. Older age has been associated with poorer treatment responses in several trials of tuberculosis therapy (16, 18). Significantly more patients assigned to moxifloxacin had cavities on chest radiographs, but this was not associated with reduced efficacy. By contrast, several recent studies have reported lower culture conversion rates in patients with cavitary lesions on chest x-rays, though another large clinical trial did not find such a difference (16, 18–20). If the presence of cavities is truly associated with slower time to culture conversion, then the results of this study might underestimate the potency of moxifloxacin, given the higher proportion of cavities (81% vs. 57%) in patients assigned to receive the drug. Interestingly, baseline sputum smear grade was significantly associated with culture conversion, and heavy smear positivity (>10 organisms per high power field) was equally distributed in the two study arms. Both cavitation on x-ray and sputum smear positivity are semi-quantitative measures that are subject to observer bias and may have inter-observer variability. The number of patients with HIV infection, found to be associated with poorer outcomes in the Tuberculosis Trials Consortium trial (16), was too small for meaningful analysis in this study.
Moxifloxacin was well tolerated and not associated with increased risk of adverse reactions or clinical complications compared with ethambutol, a drug generally considered to be very safe. In particular, we found no change in the QTc interval on serial electrocardiograms taken during the trial (data not shown). QTc prolongation is a reported toxicity of other fluoroquinolones, but has not been noted with moxifloxacin during shorter durations of treatment. Because tuberculosis treatment is lengthier than treatment of community acquired pneumonia, the primary clinical usage for moxifloxacin, the finding of no important adverse events is reassuring and suggests that moxifloxacin could be safely used for even longer periods of time, though this needs to be confirmed in additional studies that are large enough to detect small but important rates of toxicity.
The results of our trial have considerable implications for future trials. Firstly, the significantly improved culture conversion rates found after eight weeks indicate that moxifloxacin, in combination with other first line antituberculosis drugs, could shorten the time required to cure tuberculosis by several months. Reducing the duration of tuberculosis therapy would substantially improve treatment outcomes, as treatment default is directly related to the length of therapy. In addition, shorter regimens for treating tuberculosis would substantially reduce workloads for overburdened tuberculosis control programs, particularly in high-incidence countries. Secondly, the demonstration of marked microbiological activity with moxifloxacin treatment indicate that this agent can be used to treat tuberculosis caused by organisms with resistance to first line antituberculosis agents, including isoniazid and rifampin. Given its clinical effectiveness and superior in vitro potency against M. tuberculosis compared with other fluoroquinolones (4), moxifloxacin might be considered the preferred fluoroquinolone for treating multidrug resistant tuberculosis; gatifloxacin may be as active but is associated with a risk of dysglycemia when used for community-acquired pneumonia in the elderly (21).
Our data add to a growing body of evidence suggesting that moxifloxacin could substantially shorten tuberculosis therapy by increasing the initial killing of organisms and improving the sterilizing activity of combination drug regimens (14–16, 22–25). Animal models also suggest that when moxifloxacin is substituted for isoniazid it is even more potent than when substituted for ethambutol (14), but human studies have not borne this out to date (26). The results of our trial support the undertaking of clinical trials evaluating whether shorter courses of moxifloxacin-containing regimens can cure tuberculosis as well or better than the current six-month regimen. Such trials are currently underway and their results are eagerly awaited.
Acknowledgements
Grant support: Food and Drug Administration Office of Orphan Products Development Grant No. R01FD002135. Additional support from NIH Fogarty International Center Grant TW006885 and NIH Grant 01607. Moxifloxacin and matching placebo were supplied by Bayer Healthcare, which had no role in the design, conduct or analysis of the study. We thank the members of our Data Safety and Monitoring Board, Drs. Reynaldo Dietze (Chair), Leda Jamal and Ronir Raggio Luiz, for their valuable assistance. We also thank Drs. Jacques Grosset, Eric Nuermberger, Andrew Vernon, Fernanda Mello, Susan Dorman and Richard O’Brien for encouragement and advice. Finally, we are deeply indebted to the patients who volunteered to participate in this trial.
Funding: United States Food and Drug Administration Office of Orphan Product Development, with additional support from the National Institutes of Health.
Footnotes
Clinical Trial Identifier: NCT00082173
Conflict of Interest
None of the authors reports any conflicts of interest with respect to this study.
References
- 1.Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C, Watt CJ, Bleed D. Low access to a highly effective therapy: a challenge for international tuberculosis control. Bull World Health Organ. 2002;80(6):437–444. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: Global tuberculosis control 2008 - surveillance, planning, financing. WHO/HTM/TB/2008.393. 2008
- 4.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie SH, Billington O. Activity of moxifloxacin against mycobacteria. J Antimicrob Chemother. 1999;44(3):393–395. doi: 10.1093/jac/44.3.393. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki E, Miyazaki M, Chen JM, Chaisson RE, Bishai WR. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43(1):85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lounis N, Bentoucha A, Truffot-Pernot C, Ji B, O'Brien RJ, Vernon A, Roscigno G, Grosset J. Effectiveness of once-weekly rifapentine and moxifloxacin regimens against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother. 2001;45:3482–3486. doi: 10.1128/AAC.45.12.3482-3486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis. 1993;147:1062–1063. doi: 10.1164/ajrccm/147.4.1062. [DOI] [PubMed] [Google Scholar]
- 9.Kubica GP, Dye WE, Cohn ML, Middlebrook G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963;87:775–779. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- 10.Kent Patricia T, Kubica, George P. Public Health Mycobacteriology; A Guide For The Level III Laboratory. Atlanta, GA: U.S. Department of Health and Human Services, Center for Disease Control; 1985. [Google Scholar]
- 11.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10 Suppl 2):S231–S279. [PubMed] [Google Scholar]
- 12.East African / British Medical Research Council. Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972:1079–1085. [PubMed] [Google Scholar]
- 13.Hong Kong Chest Service / British Medical Research Council. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis: the results up to 24 months. Am Rev Respir Dis. 1978;118:219–227. doi: 10.1164/arrd.1978.118.2.219. [DOI] [PubMed] [Google Scholar]
- 14.Nuermberger EL, Yoshimatsu T, Tyagi S, O'Brien RJ, Vernon AN, Chaisson RE, Bishai WR, Grosset JH. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169:421–426. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 15.Nuermberger EL, Yoshimatsu T, Tyagi S, Williams K, Rosenthal I, O'Brien RJ, Vernon AA, Chaisson RE, Bishai WR, Grosset JH. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170:1131–1134. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- 16.Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 17.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–138. [PubMed] [Google Scholar]
- 18.Tuberculosis Trials Consortium. Once-weekly rifapentine and isoniazid versus twice-weekly rifampin and isoniazid in the continuation phase of therapy for drug-susceptible pulmonary tuberculosis: a prospective, randomized clinical trial among HIV-negative persons. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 19.Chang KC, Leung CC, Yew WW, Ho SC, Tam CM. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med. 2004;170(10):1124–1130. doi: 10.1164/rccm.200407-905OC. [DOI] [PubMed] [Google Scholar]
- 20.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364:1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 21.Park-Wyllie LY, Juurlink DN, Kopp A, Shah BR, Stukel TA, Stumpo C, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 22.Pletz MW, De Roux A, Roth A, Neumann KH, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother. 2004;48(3):780–782. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosling RD, Uiso LO, Sam NE, Bongard E, Kanduma EG, Nyindo M, Morris RW, Gillespie SH. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168(11):1342–1345. doi: 10.1164/rccm.200305-682OC. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J, Hadad D, Boom W, Daley C, Peloquin C, Eisenach K, Jankus D, Debanne S, Charlebois E, Maciel E, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006 Jun;10(6):605–612. [PubMed] [Google Scholar]
- 25.Gillespie SH, Gosling RD, Uiso L, Sam NE, Kanduma EG, McHugh TD. Early bactericidal activity of a moxifloxacin and isoniazid combination in smear-positive pulmonary tuberculosis. J Antimicrob Chemother. 2005;56(6):1169–1171. doi: 10.1093/jac/dki376. [DOI] [PubMed] [Google Scholar]
- 26.Dorman SE, Johnson JJ, Goldberg S, et al. Moxifloxacin vs. isoniazid in the first 2 months of treatment for pulmonary tuberculosis; 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago. 2007. abstract L-736-B. [Google Scholar]