Abstract
Background
Enamel matrix derivative (EMD) has been shown to enhance both soft tissue healing and regeneration of the periodontium, but the mechanisms of this action are still unknown. It is assumed that amelogenin, the most abundant protein in EMD, is the protein primarily responsible for the effects of EMD. The purpose of this study was to fractionate EMD and associate specific cellular effects of EMD with different molecular weight fractions following size exclusion chromatography.
Methods and Materials
Freshly dissolved EMD was fractionated by gel filtration and forty-five 7ml fractions collected, desalted, lyophilized, and resuspended. These fractions were analyzed for their effects on differentiation of osteoprogenitor cells (C2C12) and proliferation and differentiation of human microvascular endothelial cells (HMVEC). Alkaline phosphatase activity (C2C12) was measured as a marker for osteogenic differentiation before and after pre-incubation of the fractions with the bone morphogenetic protein (BMP) decoy receptor, noggin. Angiogenesis (HMVEC) was evaluated as a marker for endothelial cell differentiation. Enzymographic assays used polyacrylamide gels co-polymerized with denatured type I collagen to determine gelatinolytic activities in each fraction.
Results
EMD fractionated into three major protein peaks following size exclusion chromatography with Sephadex G-100. Peak I was associated with the column void volume, while peak III eluted near the salt volume. Peak II eluted between these two peaks. Proliferation and angiogenic activities were associated with both peak II and peak III for the microvascular cells. Differentiation of osteoprogenitor cells, indicated by alkaline phosphatase activity, was induced by EMD components present in peak I and the leading edge of peak II. The additional observation that this differentiation was inhibited by prior treatment of the fractions with noggin suggested the activity was induced by BMP rather than amelogenin or other unknown proteins. Gelatinolytic activities were detected in the early fractions of peaks 1 and 2 of gel fractionated EMD.
Conclusions
The cellular activities stimulated by EMD are not associated with a single molecular weight species. The fact that noggin abolishes C2C12 alkaline phosphatase activity suggests that effects on osteoprogenitor cell differentiation are the result of a BMP-like protein(s), while effects on proliferation and angiogenesis are associated with lower molecular weight species present in peak II and peak III. Finally, unheated EMD displays gelatinolytic activities that are also detectable following size exclusion separation of EMD constituents. The masses of these activities were consistent with those reported for latent and active MMP-20.
Keywords: EMD, BMP, differentiation, proliferation, angiogenesis, collagenolytic activity
INTRODUCTION
Periodontal regeneration has been approached in many ways with varying degrees of success and predictability. The lack of predictability may result from a requirement for integration of cellular and molecular effects to achieve regeneration of each different tissue component, the alveolar bone, periodontal ligament and cementum that together form the periodontal attachment apparatus. Because of the complexity of the interactions of the many cell types, growth factors and other proteins associated with periodontal regeneration, there are conflicting results from research into how these elements function individually and in concert to regenerate a diseased periodontium. A current approach to regenerative therapy includes the use of a complex mixture of proteins that is purified from enamel matrix and referred to as enamel matrix derivative (EMD). Initial clinical observations suggest that enamel matrix derivative is an effective therapeutic agent for periodontal regeneration.1–6
Unfortunately, the mechanisms underlying the positive effects of EMD on the regenerative process remain largely unknown. In vitro studies have shown that EMD has diverse effects on a variety of cell types including periodontal ligament cells, osteoblasts, and vascular cells. Depending on the cell type, EMD stimulates either proliferation or differentiation, or both.7 EMD enhances attachment and spread of periodontal ligament cells, as well as the release of transforming growth factor β (TGF-β)8 and vascular endothelial growth factor (VEGF) 9. The material may also inhibit the growth of gram-negative periodontal pathogens10.
Investigators propose that the cellular effects associated with EMD are due primarily to the protein amelogenin which is the predominant component of EMD, making up approximately 90% of the material. One study reported that the proliferative activity observed when cells were exposed to EMD was not due to amelogenin, but some other component in the protein mixture.11 Analysis of gene expression profiles of periodontal ligament cells treated with EMD demonstrated that inflammatory genes were down regulated, while other genes coding for growth factors and their receptors were up-regulated.12 By contrast, other reports confirm that amelogenin contains integrin binding sites required for cellular attachment13,14 and that the protein promotes both cell attachment and spreading.15 A controversial suggestion is that amelogenin has the capacity to stimulate immature mesenchymal cells to change their phenotype and enter tissue specific maturation pathways.16,17 By contrast, other studies suggest that bone morphogenetic proteins (BMP)18,19 or transforming growth factors20 are the factors responsible for the effects of EMD on cell differentiation.
Thus, the extent to which the observed beneficial effects of EMD on periodontal regeneration result from the action of amelogenin remains to be resolved. Therefore, the goal of this study was to fractionate the various proteins contained in EMD in order to characterize their effects on differentiation, proliferation, angiogenesis, and collagenolytic activities.
Materials and Methods
Materials
Multiple 30 mg vials of unheated, lyophilized enamel matrix derivative* (EMD) from the same lot number, 094093 2002–09, were used in this study. This material was not heat-treated and therefore, differs from the material that is currently available commercially. A fresh stock solution was prepared by dissolving EMD in 10 mM acetic acid and then allowing it to stand at 4°C for at least 1 hour prior to use in order to solubilize the material. Control solutions were prepared similarly from 10 mM acetic acid vehicle. For column separations, EMD was dissolved in 0.05 M sodium bicarbonate buffer, pH 10.8. Freshly dissolved material was allowed to stand at 4°C for at least 1 hour prior to column application in order to solublize the material. It has been shown that while enamel matrix proteins tend to aggregate and become insoluble at physiological pH and temperature, the solubility increases at acid or alkaline pH and low temperature.21,22
Porcine amelogenin, prepared as described by Ryu et al.23, was the generous gift of Dr. James Simmer, University of Michigan School of Dentistry (Ann Arbor, MI). Working solutions of amelogenin for cell culture studies were prepared by diluting a stock solution of 15 mg/ml in 10 mM acetic acid with cell culture media appropriate for the experiment and cell type studied.
C2C12 cells (CRL-1772) served as an in vitro model cell system for evaluating the differentiation potential of EMD on osteoprogenitor cells†. The C2C12 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 4 mM L-glutamine and adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum (FBS).
Human microvascular endothelial cells (HMVEC) served as a model vascular cell‡. Three different lot numbers derived from dermal neonatal tissue and pooled from multiple donors were used over the course of the project: 3F1066, 3F1489, and 4F1613. The cells were maintained in EGM2 MV medium. This medium was prepared from EBM2 medium‡ by adding components of a growth factor supplement kit‡. Cells were used as long as they maintained the same endothelial cell morphology evident in the parent culture, but usually no more than 4 to 5 passages.
Methods
EMD Fractionation
EMD was fractionated at 4° C using a 2.5 × 100 cm column of Sephadex G-100§, equilibrated with 0.05 M sodium bicarbonate buffer, pH 10.8 (column buffer). The column flow rate was 21 ml/hr. The elution profile of the column and a molecular weight calibration curve were determined prior to EMD fractionation. EMD (30mg) dissolved in 5 ml of column buffer was applied to the column and the eluate collected in 20 minute (7ml) fractions. Aliquots from each fraction were assayed for protein content using the BCA protein assay". The protein elution pattern was determined by plotting the protein content of each fraction aliquot versus the fraction number. Fractions were desalted over PD-10 columns¶, lyophilized, and the protein concentration confirmed after resuspension in 10 mM acetic acid. Samples were then stored at −20°C. At the time of assay, the protein concentration of each fraction was adjusted to 50 μg/ml using DMEM cell culture medium. Some fractions did not contain detectable protein and were not tested.
Determination of Alkaline Phosphatase Activity
C2C12 cells were plated into 96 well plates at 2 × 104 cells/well in DMEM containing 4 mM L-glutamine and adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% FBS (growth medium). After 18 hours, the medium was removed and replaced with fresh growth medium containing 5% FBS and EMD or amelogenin at concentrations of 0, 6.25, 12.5, 25, 50, and 100 μg/ml. Fractionated EMD samples were tested at a final protein concentration of 25 μg/ml. Fractionated EMD samples were also preincubated with 100 ng/ml noggin# for 1 hour prior to addition to the cells. At the appropriate time (0, 2, 5, or 9 days), the medium was removed and the cell layer washed twice with phosphate buffered saline (PBS). To measure alkaline phosphatase activity, 50 μl PBS and 50 μl of a 1:1:1 (vol:vol:vol) mixture of 1.5 M 2-amino2-methyl-1-propanol, pH 10.25, 20 mM p-nitro-phenyl phosphate, and 10 mM MgCl2 were added to each well and incubated with the cells for 40 minutes at 37°C. The reaction was stopped by addition of 100 μl of 1 N sodium hydroxide and the absorbance read at 405 nm. A standard curve was prepared from known concentrations of p-nitrophenol. The alkaline phosphatase activity was expressed as the amount of p-nitrophenol formed/well/40 minutes.
Analysis of Cell Proliferation
HMVEC cells (5 × 103 cells/well) were plated into 96 well plates in 200 μl EGM2 MV medium. After 18 hours, the medium was removed and replaced with EBM2 medium containing 2% serum and EMD or amelogenin at concentrations of 0, 6.25, 12.5, 25, 50, or 100 μg/ml. Fractionated EMD samples were tested at a final protein concentration of 25 μg/ml. After incubation for 72 hours at 37°C (5% CO2: 95 % air), the medium was removed and replaced with fresh serum free culture medium containing 10% (vol:vol) WST-1 reagent**. Incubation was continued for 2 hours at 37°C prior to determining the absorbance of the reaction products at 450 nm.
In vitro Angiogenesis Assay
The BD Biocoat® 96 well angiogenesis system was used†† to determine the angiogenic activity of EMD, amelogenin, and fractionated EMD samples in vitro. Twenty thousand HMVEC cells in 50 μl of EBM2 medium containing 5% serum but no endothelial cell growth factors (control -) were plated per well. An additional 50 μl of medium containing EMD or amelogenin at 50 μg/ml, or EMD fractions containing 50 μg protein/ml, was added to each well. Control wells contained the same number of cells in EBM2 medium containing 5% serum and the endothelial cell growth factors (control +). The plates were incubated at 37 °C in humidified air containing 5% CO2 and 95% air. At time periods from 0 to 4 hrs, the plates were removed from the incubator and digital images obtained using brightfield microscopy at 100X. The images were scored blindly for the extent of angiogenesis using a previously defined (0–5) scoring system‡‡ based on that reported by Malinda et al.24
Polyacrylamide Gel Electrophoresis
EMD and amelogenin were resolved on 15% polyacrylamide gels using the SDS-PAGE procedure described by Laemmli25. Aliquots of 5 or 2.5μg of unfractionated EMD or amelogenin, or 2μg of fractionated EMD were applied to the gel. Resolved bands were stained with Coomassie Brilliant Blue R-250 and photographed. For determination of enzyme activity, the gels were co-polymerized with 150 μg/ml denatured type I collagen as described by Overall and Limeback26 and modified by Steffensen et al.27
Data Analysis
Data were analyzed by ANOVA with Tukey’s method as the post hoc test using the Prism statistical software program v 2.01§§. Angiogenesis scoring was analyzed using the Kruskal-Wallis test.
Results
Before separating EMD into fractions for testing, baseline activities for unfractioned EMD and purified amelogenin were determined for both C2C12 and HMVEC cell lines.
Alkaline Phosphatase Activity
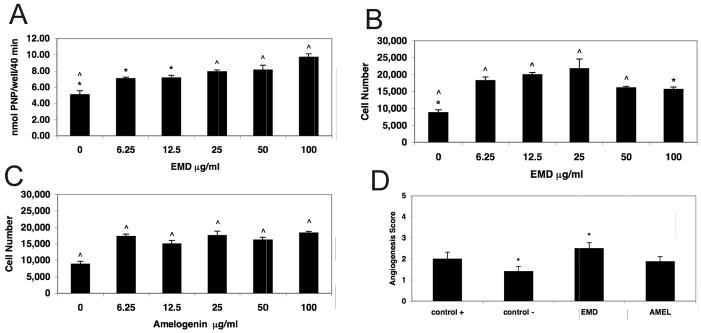
Differentiation of C2C12 was evaluated on days 2, 5, and 9 by determining alkaline phosphatase activity following stimulation by EMD or amelogenin at concentrations between 0 and 100μg/ml. On day 2 there was no increase in alkaline phosphatase activity for any of the EMD concentrations tested compared to the unstimulated control (data not shown). On day 5, the increase in alkaline phosphatase activity was statistically significant at each concentration of EMD tested when compared to unstimulated control cells (Fig. 1a). The increase of alkaline phosphatase activity on day 9 was not significant because of an increase in the baseline activity of the unstimulated control cells (data not shown). These results are consistent with those reported by Ohyama et al.28 who reported that EMD-stimulated alkaline phosphatase activity of C2C12 cells was maximal on day 7, and that baseline activity gradually increased during culture in the absence of EMD. Thus, the results showed that EMD stimulated C2C12 cell differentiation, in both a time- and dose-dependent manner. By contrast, there were no significant increases in alkaline phosphatase activity at any of the amelogenin concentrations tested on days 2, 5 or 9, indicating that differentiation did not occur in response to stimulation by this factor (data not shown).
Figure 1.
Figure 1a. Effect of EMD on Alkaline Phosphatase Activity of C2C12 Cells. At day 5, there was a statistically significant increase at each concentration of EMD tested when compared to unstimulated control. Alkaline phosphatase activity is expressed as the amount of p-nitrophenol (PNP) formed/well/40minutes. The data are the mean ± s.e.m. (n = 8). * = p < 0.05, ^ = p < 0.001.
Figure 1b. Effect of EMD on HMVEC Cell Proliferation. EMD in medium containing 2% serum stimulated significant increases in HMVEC cell proliferation at all concentrations tested compared to the 2% serum control. The greatest increase in EMD stimulated HMVEC cell proliferation occurred at 25μg/ml. The data are the mean ± s.e.m. (n = 8). *= p < 0.01, ^ = p < 0.001.
Figure 1c. Effect of Amelogenin on HMVEC Cell Proliferation. When stimulated with amelogenin in medium containing 2% serum, proliferation was significantly increased at all concentrations tested compared to the 2% serum control. Note: For any concentration tested, amelogenin stimulated proliferation was not significantly different from 6.25μg/ml, the lowest concentration of amelogenin tested. The data are the mean ± s.e.m. (n = 8). ^ = p < 0.001.
Figure 1d. Effect of EMD and Amelogenin on HMVEC Cell Angiogenesis after Four Hours. At four hours following stimulation by EMD (25 μg/ml) there was a significant increase (p < 0.015) in the angiogenesis score to 2.5 indicating that capillary tubes were now visible. Although not statistically significant, stimulation by EMD was greater than the stimulation by amelogenin (25 μg/ml) and the positive control. By contrast, the effect of amelogenin on angiogenesis at this time was not significantly different from either the positive or negative control media. Data were analyzed by the Kruskal-Wallis test (N = 8).
Proliferation Activity
When stimulated with either EMD or amelogenin in 2% serum-containing medium, HMVEC cell proliferation was significantly increased at all concentrations tested compared to the 2% serum control. The greatest increase in EMD stimulated HMVEC cell proliferation occurred at 25 μg/ml (p < 0.001) (Fig. 1b). Although proliferation was increased compared to unstimulated control at EMD concentrations of 50 and 100 μl/ml, the magnitude of the increase was significantly less than the peak stimulation at 25 μl/ml (p< 0.01). By contrast, amelogenin stimulated proliferation peaked at 6.25 μg/ml (p < 0.001), the lowest concentration of amelogenin tested (Fig. 1c). Amelogenin concentrations ranging from 12.5 to 100 μg/ml stimulated proliferation to levels that were not significantly different from that seen following stimulation with 6.25 μg/ml of the factor.
Angiogenesis
At 4 hours following stimulation by 25 μg/ml EMD, there was a significant increase (p < 0.015) in the angiogenesis score to 2.5 compared to the unstimulated control, indicating that capillary-like tubes were now visible. The effect of 25 μg/ml amelogenin on angiogenesis at 4 hr was not significantly different from unstimulated control medium (Fig. 1d).
SDS Page Gel of EMD and Amelogenin
Unfractionated EMD proteins had a molecular weight of less than 30 kDa, with the exception of one prominent band of approximately 52 kDa. By contrast, the major band of the amelogenin preparation had a molecular weight of 29 kDa. Although there were a few weak bands with molecular weights greater than 50 kDa, no protein bands were detected below 29 kDa (Fig. 2).
Figure 2. SDS-PAGE of Amelogenin and Enamel Matrix Derivative.
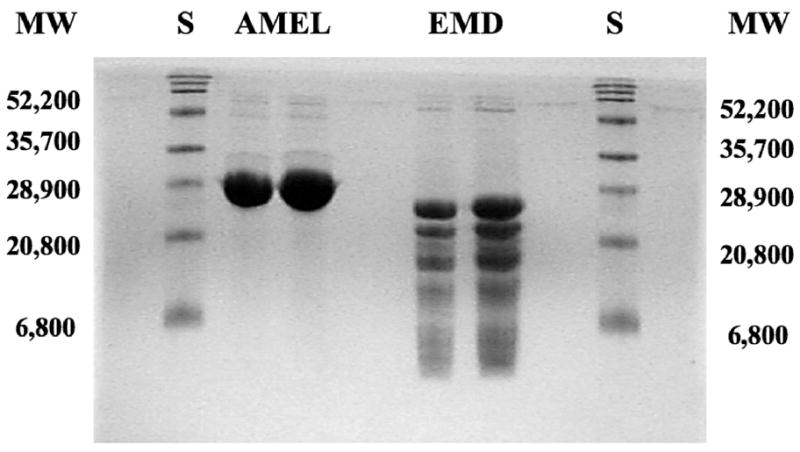
Aliquots of 5 (lanes 3,5 starting from the left lane) or 2.5 μm (lanes 2,4) of EMD or amelogenin were applied to the gel. Standard molecular weight markers were run in lanes 1 and 6. The gel was stained with Cosmassie Brilliant Blue R-250. Note that EMD contains a mixture of proteins, the majority of which are relatively low molecular weight, <30 kDa, with the exception of one prominent band in the area of 52.2 kDa. By contrast, the major band of the amelogenin preparation occurs at a molecular weight of 28.9 kDa. Although a few weak bands present at higher molecular weights greater than 50 kDa exist, there are no protein bands present at molecular weights below 28.9 kDa.
EMD Protein Fractionation
During separation by gel filtration, the proteins in EMD separated into 3 distinct peaks as defined by the protein concentration in the eluate (Fig. 3a). The first peak contained high molecular weight proteins (100 kDa or greater) and was associated with the void volume. The second protein peak eluted at approximately 50 kDa, while proteins in the third peak were extrapolated from the standard curve to have molecular weights of approximately 10 kDa.
Figure 3.
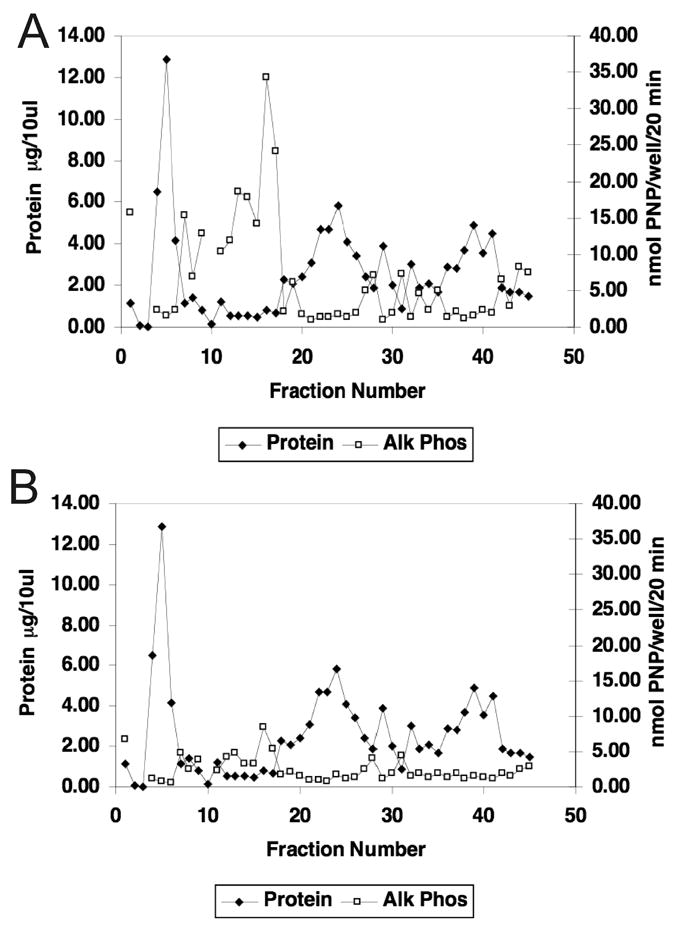
Figure 3a. Alkaline Phosphatase Activity at Day 5 of EMD Fractions on C2C12 Cells. When the alkaline phosphatase activity of each fraction was determined and superimposed on the EMD protein separation profile, the strongest activity occurred in fractions 12–17, which does not correspond to any of the major EMD protein peaks.
Figure 3b. Noggin Inhibition of Day 5 Alkaline Phosphatase Activity of EMD Fractions on C2C12 Cells. Prior to application to the cells, fractions were preincubated for 1 hour with 100ng/ml noggin, a decoy receptor known to bind to BMP 2, 4, and 7, and block their activity. Alkaline phosphatase activity was decreased, especially in the area where the highest activity occurred prior to noggin treatment, suggesting that the osteoinductive activity associated with EMD is not related to amelogenin, but more likely with a member of the BMP family of proteins.
Alkaline Phosphatase Activity of EMD Fractions
When the alkaline phosphatase activity of each fraction was determined at day 5 and superimposed on the EMD protein separation profile (Fig. 3a), it was evident that the strongest alkaline phosphatase activity occurred in fractions 12–17. Importantly, these fractions did not correspond to any of the high concentration protein peaks from EMD. In fact, there appeared to be little activity in the fractions containing the major EMD protein peaks. Subsequently, when fractions were preincubated with 100 ng/ml of noggin, a decoy receptor known to bind to BMP 2, 4, and 7 and block their activity, the stimulation of alkaline phosphatase activity at day 5 was virtually eliminated in the fractions that had the highest effect prior to noggin treatment (Fig. 3b).
Stimulation of Proliferation by EMD Fractions
Unlike alkaline phosphatase activity, the stimulation of proliferative activity for HMVEC cells (Fig. 4) mirrored the EMD protein separation profile, with the greatest proliferative activity occurring in the fractions coincident with the most abundant proteins.
Figure 4. Proliferation Activity of EMD Fractions on HMVEC Cells.
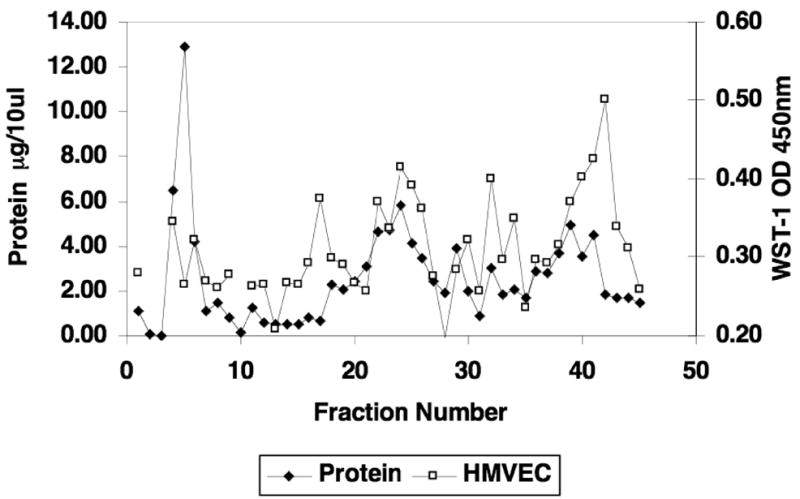
Proliferation of human microvascular endothelial cells (HMVEC) was determined and superimposed on the protein separation profile for EMD. The proliferative activity for HMVEC cells mirrored the EMD protein separation profile, with the greatest proliferative activity occurring in the fractions coincident with the major protein peaks, particularly the 10kDa peak.
Stimulation of Angiogenesis by EMD Fractions
An angiogenesis score of 4.0, signifying almost complete vascular maturity and organization was achieved with proteins in the second peak of fractionated EMD. The early stages of angiogenesis reflected by a score of 1, indicating the beginning of cellular alignment, were observed in cells treated with the third protein peak. Finally, an angiogenesis score of 3.0 indicating sprouting from capillary-like tubes was associated with the high molecular weight proteins in the void volume peak (Fig. 5). The fractions between the void volume and the beginning of the second protein peak were not tested because protein was not detectable in these fractions from this column run.
Figure 5. Angiogenic Activity of EMD Fractions on HMVEC Cells.
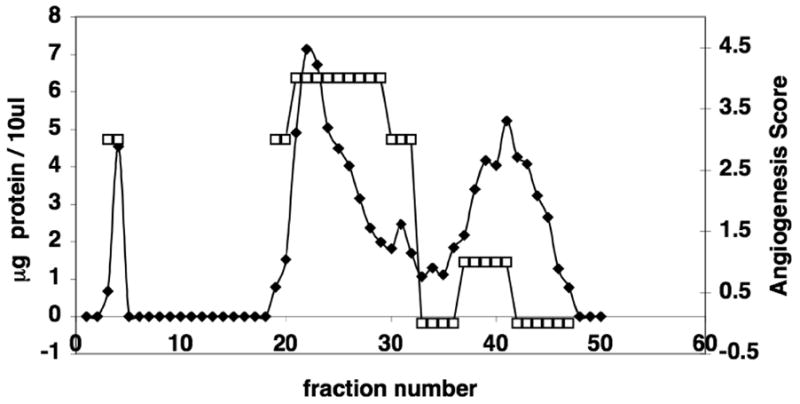
Fractionated EMD was applied to HMVEC cells at a concentration of 25μg/ml, and angiogenesis scored and plotted against fraction number, and then superimposed on the EMD protein separation profile. Angiogenic activity at four hours was coincident with the 2nd and 3rd EMD protein peaks. Note also that an angiogenesis score of 3.0 is associated with the void volume peak. Angiogenesis score = □. Protein = 
Collagenolytic Activity of EMD Fractions
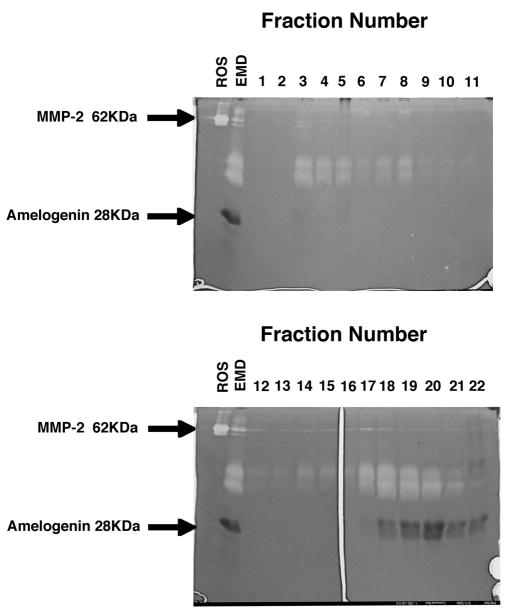
Enzyme activities on denatured type I collagen substrate gels were detected in the high molecular weight proteins eluted in peak 1(Fig. 6 top), as well as in proteins eluted in the molecular weight region of 50 to 70 kDa (Fig. 6 bottom). There was no collagenolytic activity apparent in any of the later eluting fractions (data not shown).
Figure 6. Zymograms of EMD Column Fractions.
Areas in which the collagen was degraded appear clear (unstained). Collagenolytic activity was evident in two areas, fractions 3–8 (top panel) and 17–21(bottom panel). Thus, the collagenolytic activity of EMD was associated with proteins eluting in peak 1 from the sephadex G-100 column, as well as with proteins eluting in the 68 kDa molecular weight region, prior to the elution position of amelogenin. No further collagenolytic activity was apparent in any of the later eluting fractions (data not shown). ROS in lane 1 serves as a standard for collagenolytic activity and is an MMP-2 containing extract prepared from rat osteosarcoma cells. EMD in lane 2 is unfractionated enamel matrix derivative. Lanes 3 – 13 contain the protein eluted from the sephadex G-100 column in fractions 1–11(top panel), and fractions 12–22 (bottom panel).
Discussion
The results of this research demonstrate that EMD, which represents a complex mixture of proteins, has the ability to stimulate several activities in two cell types. Importantly, individual activities are associated with different molecular weight fractions of EMD separated by gel filtration. The data suggest that the single most abundant protein of EMD, amelogenin, may be associated with some but not all of the EMD properties. In fact, the results indicate that several of the activities stimulated by EMD are not stimulated by amelogenin present in EMD, or the 29 kDa recombinant porcine amelogenin used in this study. Because no attempt was made to identify the proteins in the various fractions, the possibility remains that the observed effects resulted from either high molecular weight aggregates of amelogenin or smaller molecular weight fragments.
The proteins in EMD eluted in three distinct peaks from a Sephadex G-100 gel filtration column. The protein separation profile was very similar to that reported by Iwata et al., who purified and fractionated enamel matrix proteins obtained from tooth germs of 6-month-old pigs and also found three distinct protein peaks using the same chromatography method.19 The presence of alkaline phosphatase and/or an increase in its activity is a common marker for cellular differentiation by osteoblasts and osteoprogenitor cells. Stimulation with EMD increased the alkaline phosphatase activity of C2C12 cells, a cell line commonly used to assay for osteoinductive activity, indicating that osteoblast differentiation occurred. These data are consistent with other reports indicating that EMD stimulates osteoblast cell differentiation and maturation.7,28 In the present study, when the capacity of individual fractions to induce alkaline phosphatase activity in C2C12 cells was superimposed on the EMD protein separation profile, it became evident that this property of EMD did not correspond to any of the major peaks that reflected the abundance of protein in the fractions. Rather the most prominent alkaline phosphatase activity was associated with molecular weight species that eluted between the first and second peaks. This pattern was also observed by Iwata et al., who used the ST2 preosteoblast cell line.19 Furthermore, the present study found that pre-incubation of the column fractions with the BMP decoy receptor noggin prior to treating C2C12 cells inhibited the induction of alkaline phosphatase activity in the cells. This is consistent with results from the Iwata et al. study in ST2 cells,19 as well as results reported by Ohyama et al. in C2C12 cells28. Finally, using C2C12 cells, which normally do not express core binding factor α1/Runt-related transcription factor-2 (Cbfa1/Runx2) unless stimulated by BMP-229, Takayama et al.18 reported that EMD stimulated Cbfa1/Runx2 expression in the cells that was inhibited by noggin. Considered together, the data from the present study and the evidence from the literature are consistent with the concept that the osteoinductive activity associated with EMD cannot be attributed to amelogenin, but more likely is derived from a member of the BMP family of proteins. This conclusion is based on the observation that the activity is inhibited by noggin19, and is not observed in the molecular weight fractions expected to contain amelogenin or any of its fragments. This concept is strengthened by a report from Parkar et al., in which gene arrays from PDL cells treated with EMD appeared to up regulate genes coding for growth factors and growth factor receptors while down regulating inflammatory genes.12 One of the specific genes upregulated is BMP-4, one of three BMPs known to be inhibited by noggin.
Another of the genes reported to be upregulated by EMD in the Parkar et al. study, was vascular endothelial growth factor precursor.12 Because new vessel formation is essential to wound healing, it follows that an upregulation of this growth factor may stimulate the necessary cellular activities in vascular cells that lead to improved wound healing. The results of the present study are consistent with this mechanism. For example, when HMVEC cells were stimulated with EMD, they showed a dose dependent increase in proliferation over the concentration range of 0 to 25 μg/ml, while higher doses reduced the magnitude of the increase significantly below peak values. These results are consistent with similar results in HUVEC cells reported by Yuan et al. both in vitro and in a murine model.30 Results were similar when HMVEC cells were stimulated with amelogenin where doses greater than 6.25 μg/ml did not produce any further increases in proliferation. A possible explanation for the fact that the higher doses of EMD and amelogenin resulted in either decreased proliferation or the failure to produce further increases in proliferation is that these factors may stimulate HMVEC cell differentiation or apoptosis in a dose dependent manner. In either case the cells would be removed from the proliferating population.9 Increases in proliferative activity that occurred in response to stimulation by column fractions corresponding to the second and third protein peaks eluted from the sephadex column, suggest that the effect of EMD on HMVEC cells may be due in part to amelogenin. This suggestion is supported by the observation that HMVEC cell proliferation also was increased by treatment with recombinant porcine amelogenin. These data are also consistent with the suggestion that the proliferative activity for human microvascular endothelial cells is associated with molecular weight species of the EMD mixture that are distinct from those associated with the induction of alkaline phosphatase activity.
The effects of EMD on angiogenesis demonstrated in the present study are consistent with several reports in the literature. Yuan et al., reported that EMD stimulated endothelial chemotaxis and angiogenesis.30 Schlueter et al. suggested that EMD stimulates angiogenesis directly by stimulating endothelial cells, and indirectly by stimulating the production of angiogenic factors such as VEGF by PDL cells9. A study by Mirastschijski et al. found that VEGF production by fibroblasts treated with EMD was increased compared to untreated control fibroblasts.31 The gene array studies by Parkar et al.12 also indicated that the expression of VEGF or its precursors was upregulated by EMD in PDL cells. The results of the present study also demonstrate that as was seen for proliferation, the capacity to stimulate angiogenesis corresponded to the major protein peaks, raising the possibility that this activity is associated with amelogenin or one of its fragments. Nevertheless, the fact that stimulation of HMVEC cells with recombinant porcine amelogenin did not stimulate angiogenesis in these cells suggests that the activity results from the effects of other proteins in the EMD mixture.
Proteolytic enzyme activities, or more specifically gelatinolytic activities, were detected in native EMD as well as in EMD fractionated by gel filtration. Activities consistent with MMP-2 according to the positive control sample from ROS 17/2.8 cell conditioned medium33 were only very faintly detectable in the fractionated protein fractions. However, gelationolytic activities with apparent masses of 40 and 45 kDa were readily detectable in fractions 3–8 and 17–21. These activities are consistent with enamelysin (MMP-20), which presents itself by this type of duplet appearance on gelatin zymograms.34 Surprisingly, we found those activities both in high mass and, as expected, in the medium mass fractions. Since EMD was fractionated under native conditions, it plausible that part of the putative MMP-20 activities were passing through the column bound to large carrier or substrate molecules and not separated from those until the column fractions were separated by SDS-PAGE in preparation for the zymograms analysis. Very high molecular weight activities present in the zymograms could reflect such complexes that were not dissociated by the protein sample buffer. An analogy to this type of the interaction is the binding in plasma of MMP-9 to NGAL which also is a feature of adult and localized juvenile periodontitis.35–36 These results demonstrate that native EMD contains enzymes that are capable of degrading denatured type I collagen, an important aspect of tissue remodeling during wound healing and regeneration.
Although individual activities of EMD have been characterized and associated with specific protein containing regions of sephadex column fractionated EMD, it is important to realize that individual activities likely do not occur exclusive of one another. Rather, the activities may act in concert with each other and the various cell types and/or receptors in a dynamic environment in order to stimulate and/or accelerate the regenerative process. It is likely that the effects of EMD are the result of multiple proteins present in the mixture and are not solely related to amelogenin.
Acknowledgments
This work was supported in part by grants DE14236 and DE016312 from the National Institutes of Health and a grant from Biora, Inc. The authors acknowledge the technical assistance of Helen Hoffer and Yao Wang, both in the Department of Periodontics at the University of Texas Health Science Center at San Antonio, as well as the efforts of Madge Cluck and Dolores Garza, also both in the Department of Periodontics at the University of Texas Health Science Center at San Antonio, in the preparation of the final manuscript.
Footnotes
Emdogain, Biora AB, Malmo, Sweden, now Straumann Biologics Division, Waltham, MA
American Type Culture Collection ATCC Manassas, VA
Lonza Walkersville, Inc. formerly Cambrex Bio Science Walkersville, Inc. Walkersville, MD
Sigma, St. Louis, MO
Pierce Biotechnology, Inc. Rockford, IL
Amersham Biosciences, Newark, NJ
R & D Systems, Minneapolis, MN
Roche, Indianapolis, IN
BD Discovery Labware, Bedford, MA
In Vitro Angiogenesis Assay Kit #ECM625 Chemicon International, Inc., Temecula, CA
Graphpad Software, Inc., San Diego, CA
The views expressed in this article are those of the author and are not to be construed as official nor as reflecting the views of the United States Air Force or Department of Defense.
References
- 1.Yukna R, Mellonig J. Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10-case series. J Periodontol. 2000;71:752–759. doi: 10.1902/jop.2000.71.5.752. [DOI] [PubMed] [Google Scholar]
- 2.Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. J Clin Periodontol. 1997;24:693–696. doi: 10.1034/j.1600-051x.1997.00693.x. [DOI] [PubMed] [Google Scholar]
- 3.Hammarström L, Heijl L, Gestrelius S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol. 1997;24:669–677. doi: 10.1111/j.1600-051x.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 4.Froum S, Weinberg M, Rosenberg E, Tarnow D. A Comparative Study Utilizing Open Flap Debridement With and Without Enamel Matrix Derivative in the Treatment of Periodontal Intrabony Defects. A 12-month Re-Entry Study. J Periodontol. 2001;72:25–34. doi: 10.1902/jop.2001.72.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Kawase T, Okuda K, Yoshie H, Burns D. Cytostatic action of enamel matrix derivative (EMDOGAIN) on human oral squamous cell carcinoma-derived SCC25 epithelial cells. J Periodontol Res. 2000;35:291–300. doi: 10.1034/j.1600-0765.2000.035005291.x. [DOI] [PubMed] [Google Scholar]
- 6.Gestrelius S, Andersson C, Lidstrom D, Hammarström L, Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J Clin Periodontol. 1997;24:685–692. doi: 10.1111/j.1600-051x.1997.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz Z, Carnes D, Pulliam R, et al. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J Periodontol. 2000;71:1287–1296. doi: 10.1902/jop.2000.71.8.1287. [DOI] [PubMed] [Google Scholar]
- 8.Van der Pauw M, Van den Bos T, Everts V, Beersten W. Enamel Matrix-Derived Protein Stimulates Attachment of Periodontal Ligament Fibroblasts and Enhances Alkaline Phosphatase Activity and Transforming Growth Factor β1 Release of Periodontal Ligament and Gingival Fibroblasts. J Periodontol. 2000;71:31–43. doi: 10.1902/jop.2000.71.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Schlueter SR, Carnes DL, Cochran DL. In Vitro Effects of Enamel Matrix Derivative on Microvascular Cells. J Periodontol. 2007;78:141–151. doi: 10.1902/jop.2007.060111. [DOI] [PubMed] [Google Scholar]
- 10.Spahr A, Lyngstadaas S, Boeckh C, Andersson C, Podbielski A, Haller B. Effect of the enamel matrix derivative Emdogain® on the growth of periodontal pathogens in vitro. J Clin Periodontol. 2002;29:62–72. doi: 10.1034/j.1600-051x.2002.290110.x. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan H, Berry J, Foster B, Gibson C, Li Y. Amelogenin: a potential regulator of cementum-associated genes. J Periodontol. 2003;74:1423–1431. doi: 10.1902/jop.2003.74.10.1423. [DOI] [PubMed] [Google Scholar]
- 12.Parkar H, Tonetti M. Gene expression profiles of periodontal ligament cells treated with enamel matrix proteins in vitro: analysis using cDNA arrays. J Periodontol. 2004;75:1539–1546. doi: 10.1902/jop.2004.75.11.1539. [DOI] [PubMed] [Google Scholar]
- 13.Lyngstadaas S, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–188. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N, Ohyama M, Maeno M, Ito K, Otsuka K. Attachment of Human Periodontal Ligament Cells to Enamel Matrix-Derived Protein is Mediated Via Interaction Between BSP-Like Molecules and Integrin αvβ3. J Periodontol. 2001;72:1520–1526. doi: 10.1902/jop.2001.72.11.1520. [DOI] [PubMed] [Google Scholar]
- 15.Hoang A, Klebe R, Ryu O, Steffensen B, Simmer J, Cochran D. Amelogenin is a Cell Adhesion Protein. J Dent Res. 2002;81:497–500. doi: 10.1177/154405910208100713. [DOI] [PubMed] [Google Scholar]
- 16.Veis A, Tompkins K, Alvares K, et al. Specific Amelogenin Gene Splice Products Have Signaling Effects on Cells in Culture and in Implants in Vivo. J Biol Chem. 2000;275:41263–41272. doi: 10.1074/jbc.M002308200. [DOI] [PubMed] [Google Scholar]
- 17.Tompkins K, Alvares K, George A, Veis A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. J Bone Miner Res. 2005;20:341–349. doi: 10.1359/JBMR.041107. [DOI] [PubMed] [Google Scholar]
- 18.Takayama T, Suzuki N, Narukawa M, Tokunaga T, Otsuka K. Enamel Matrix Derivative Stimulates Core Binding Factor α1/Runt-Related Transcription Factor-2 Expression via Activation of Smad1 in C2C12 Cells. J Periodontol. 2005;76:244–249. doi: 10.1902/jop.2005.76.2.244. [DOI] [PubMed] [Google Scholar]
- 19.Iwata T, Morotome Y, Tanabe T, Fukae M, Ishikawa I, Oida S. Noggin Blocks Osteoinductive Activity of Porcine Enamel Extracts. J Dent Res. 2002;82:387–391. doi: 10.1177/0810387. [DOI] [PubMed] [Google Scholar]
- 20.Kawase T, Okuda K, Momose M, Kato Y, Yoshie H, Burns D. Enamel Matrix derivative (Emdogain®) rapidly stimulates phosphorylation of the MAP kinase family and nuclear accumulation of smad2 in both oral epithelial and fibroblastic human cells. J Periodontol Res. 2001;36:367–376. doi: 10.1034/j.1600-0765.2001.360604.x. [DOI] [PubMed] [Google Scholar]
- 21.Fischer L, Termine J. Noncollagenous proteins influencing the local mechanisms of calcification. J Cilin Orthop. 1985;200:362–385. [PubMed] [Google Scholar]
- 22.Aoba T, Fukae M, Tanabe X, Shimizu M, Moreno EC. Selective Adsorption of porcine amelogenins onto hydroxyapatite and their inhibitory activity on hydroxyapatite growth in supersaturated solutions. Calcif Tis Intl. 1987;41:281–289. doi: 10.1007/BF02555230. [DOI] [PubMed] [Google Scholar]
- 23.Ryu O, Fincham A, Hu C, Zhang C, Qian Q, Bartlett J. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78:743–750. doi: 10.1177/00220345990780030601. [DOI] [PubMed] [Google Scholar]
- 24.Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of lamini alpha 1 and beta 1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999;13:53–62. [PubMed] [Google Scholar]
- 25.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T-4. Nature. 1970;227:680–688. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Overall CM, Limback H. Identification and characterization of enamel proteinases isolated from developing enamel. Amelogenolytic serine proteinases are associated with maturation in pig. Biochem J. 1988;256:965–972. doi: 10.1042/bj2560965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffensen B, Bigg HF, Overall CM. The involvement of fibronectin type II-like modules of human gelatinase A in cell surface localization and activation. J Biol Chem. 1998;273:20622–20628. doi: 10.1074/jbc.273.32.20622. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama M, Suzuki N, Yamaguchi Y, Maeno M, Otsuka K. Effect of Enamel Matrix Derivative on the Differentiation of C2C12 Cells. J Periodontol. 2002;73:543–550. doi: 10.1902/jop.2002.73.5.543. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura R, Hata K, Harris S, Ikeda F, Yoneda T. Core-binding factor α1 (Cbfa1) induced osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone. 2002;31:303–312. doi: 10.1016/s8756-3282(02)00826-8. [DOI] [PubMed] [Google Scholar]
- 30.Yuan K, Chen C, Lin M. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J Clin Periodontol. 2003;30:732–738. doi: 10.1034/j.1600-051x.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 31.Mirastschijski U, Konrad D, Lundberg E, Lyngstadaas S, Jorgensen L, Agren M. Effect of a topical enamel matrix derivative on skin wound healing. Wound Rep Reg. 2004;12:100–108. doi: 10.1111/j.1067-1927.2004.012117.x. [DOI] [PubMed] [Google Scholar]
- 32.Maycock J, Wood SR, Brookes SJ, Shore RC, Robinson C, Kirkham J. Characterization of a porcine amelogenin preparation, Emdogain, a biological treatment for periodontal disease. Conn Tiss Res. 2002;43:472–476. doi: 10.1080/03008200290000880. [DOI] [PubMed] [Google Scholar]
- 33.Wallon UM, Overall CM. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. J Biol Chem. 1995;19:11555–566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett JD, Ryu OH, Xue J, Simmer JP, Margolis HC. Enamelysin mRNA displays a developmentally defined pattern of expression and encodes a protein which degrades amelogenin. Connect Tissue Res. 1998;39(1–3):101–9. doi: 10.3109/03008209809023916. discussion 141–9. [DOI] [PubMed] [Google Scholar]
- 35.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 36.Westerlund U, Ingman T, Lukinmaa PL, Salo T, Kjeldsen L, Borregaard N, Tjäderhane L, Konttinen YT, Sorsa T. Human neutrophil gelatinase and associated lipocalin in adult and localized juvenile periodontitis. J Dent Res. 1996;75:1553–63. doi: 10.1177/00220345960750080601. [DOI] [PubMed] [Google Scholar]