Abstract
Background
Exposure to airborne particulate matter (PM), a major component of air pollution, has been associated with increases in both exacerbations of and hospitalizations for asthma. We have previously shown that exposure to ambient PM collected in urban Baltimore (AUB) induces airway hyperresponsiveness (AHR), eosinophilic and neutrophilic inflammation, and the recruitment of T cells. However, the mechanism(s) by which it induces these features of asthma remains unknown.
Objective
We investigated whether T lymphocytes play a role in AUB-induced AHR.
Methods
We compared the effects of AUB exposure on the allergic phenotype in wild-type (WT) BALB/c mice and in mice deficient in recombinase-activating gene-1 (Rag1−/−) that lack mature lymphocytes.
Results
We found that exposure of WT mice to AUB induced AHR concomitant with increases in the numbers of bronchoalveolar lavage (BAL) fluid lymphocytes, eosinophils, neutrophils, and mucus-containing cells in the lungs of WT mice. Interestingly, we show for the first time that these effects were associated with significant elevations in interleukin (IL)-17A, IL-17F, and T-helper 2 cell (TH2) (IL-13, IL-5) cytokine levels in lung cells, as well as reductions in the levels of the suppressive cytokine IL-10. Interestingly, Rag1−/− mice failed to develop AUB-induced AHR; however, AUB-induced BAL fluid cellularity, and mucus cell changes were only partially inhibited in Rag1−/− mice.
Conclusions
Taken together, our results suggest that AUB exposure increases the pathophysiological features of asthma via activation of lymphocyte-dependent pathways. These results provide a plausible biological mechanism for the strong association between PM exposure and the increased severity of asthma.
Keywords: asthma, interleukins, outdoor air, particulate matter, pulmonary
Asthma is a chronic inflammatory disease of the lung characterized by airway inflammation, airway hyperresponsiveness (AHR), and mucus hypersecretion. The current disease burden has reached epidemic proportions, and now an estimated 300 million people worldwide suffer with asthma (Global Initiative for Asthma 2008). Although the etiology of asthma is unknown, there is evidence that both genetic and environmental triggers contribute to disease. The recent rise in disease prevalence is unlikely to be explained by changes in the genetic makeup of society as a whole, which does not change dramatically in short time frames. Thus, changes in the environment are likely to be driving the marked increase in prevalence of this disease.
Environmental triggers of asthma include allergens, viruses, environmental tobacco smoke, and particulate matter (PM). Of these environmental triggers, several lines of evidence suggest that exposure to ambient PM may be associated with the increase in asthma morbidity. For example, numerous epidemiological studies have reported positive correlations between PM exposure and increased medication use, physician visits, and emergency department visits for asthma (Lipsett et al. 1997; Peel et al. 2005; Tolbert et al. 2000). Acute controlled exposures of healthy humans to PM have shown a wide variety of responses, from no significant effects on airway function or inflammation (Kongerud et al. 2006) to significant increases in cellular inflammation (Behndig et al. 2006; Ghio and Devlin 2001; Salvi et al. 1999, 2000; Samet et al. 2007; Schaumann et al. 2004). Despite wide variations in the study designs, sources, and composition of PM used in these studies, the most consistent findings have been that PM exposure increases neutrophils and inflammatory cytokines, such as interleukin(IL)-8 and IL-6, in bronchoalveolar lavage (BAL) fluid (Behndig et al. 2006; Ghio and Devlin 2001; Salvi et al. 1999, 2000; Samet et al. 2007; Schaumann et al. 2004), with some studies demonstrating increases in T lymphocytes (CD4+) in bronchial biopsies of healthy human volunteers (Salvi et al. 1999). Studies in animals have shown that direct instillation of biologically relevant sources of PM into the lungs of naive mice induces many of the pathophysiological features of asthma (Gavett et al. 1999; Ohta et al. 1999; Walters et al. 2001, 2002; Wang et al. 2008). Although many hypotheses have been put forth to explain the ability of PM to directly induce or exacerbate asthmalike symptoms, to date the exact mechanisms underlying the adverse pulmonary effects of PM are not well understood.
Because numerous studies in animal models have shown that exposure to other environmental triggers such as allergens (Cohn et al. 1998; Corry et al. 1998; Gavett et al. 1994) and ozone (Chen et al. 1995) induce AHR through a T cell–dependent process, and because PM has been shown to drive T-cell cytokine production in vivo (Walters et al. 2001; Wang et al. 2008) and in culture systems (Porter et al. 2007; Williams et al. 2007), we hypothesized that PM-induced AHR and airway inflammation occur through a lymphocyte-dependent process. Thus, the objective of the present study was to directly explore the role of lymphocytes in the development of PM-induced AHR and airway inflammation. To this end, we compared the effects of ambient PM collected in urban Baltimore (AUB) on airway reactivity and allergic inflammation in wild-type (WT) BALB/c mice and in mice deficient in recombinase-activating gene 1 (Rag1−/−) that lack mature lymphocytes. We show that AUB induced AHR, pulmonary inflammation, and mucus metaplasia, concomitant with increases in both T-helper 2 cell (TH2) and TH17 cytokine production. In marked contrast, Rag1−/− mice do not develop AHR or TH2/TH17 cytokine production after AUB exposure. However, AUB-induced increases in BAL fluid and tissue inflammation as well as mucus production were only partially lymphocyte dependent. Collectively, our results demonstrate that pulmonary exposure to a real-world source of PM induces the recruitment and activation of T cells leading to the induction of the pathophysiological features of asthma.
Materials and Methods
Mice
Male and female C.129S7(B6)-Rag1tm1Mom/J (Rag1−/−) mice and BALB/c (WT) control mice (9–10 weeks of age; Jackson Laboratories, Bar Harbor, ME) were housed in an environmentally controlled specific pathogen–free facility at Cincinnati Children’s Hospital Medical Center. The mice received access to food and water ad libitum. Mice were treated humanely and with regard for alleviation of suffering in accordance with the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee.
PM exposure
Ambient PM was collected from a sixth floor window in urban Baltimore during the months of March through May in 2005 using a high-volume cyclone collector with a theoretical cut-point of 0.85 μm aerodynamic diameter when operated at a flow rate of 0.6 m3/min (Walters et al. 2001). Mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (45 mg/kg) and xylazine (8 mg/kg) and exposed to either phosphate-buffered saline (PBS) or AUB (0.5 mg in a 50-μL volume of PBS) on days 0, 3, and 6 of the study by intratracheal instillation.
Airway responsiveness measurements
We evaluated airway responsiveness to intravenous acetylcholine 24 hr after the final AUB exposure as previously described (Lewkowich et al. 2005). Briefly, mice were anesthetized, intubated, and respirated at a rate of 120 breaths/min with a constant tidal volume (0.2 mL) and paralyzed with 25 mg/kg decamethonium bromide 72 hr after final allergen challenge. After a stable baseline was achieved, 50 mg/kg acetylcholine was injected into the inferior vena cava, and dynamic airway pressure (cm H2O/sec) was followed for 5 min.
Determination of cellularity and chemokine levels in BAL fluid
Lungs were lavaged three times with a 1.0-mL aliquot of cold Hanks’ balanced salt solution. Recovered lavage fluid (70–80%) was centrifuged at 300 × g for 8 min, and the cell pellet was resuspended in 1.0 mL 10% fetal bovine serum in PBS. Slides were prepared by cytocentrifugation and stained with Diff-Quik (Dade Behring, Dudingen, Switzerland). Total and cell differential counts were determined in BAL fluid using morphologic criteria under a light microscope with the evaluation of > 500 cells/slide. BAL fluid chemokine levels were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Lung T-cell identification and cytokine measurements
Whole lungs were perfused with ice-cold PBS, removed, minced, placed in 6 mL RPMI 1640 containing 0.5 mg/mL collagenase (Liberase CI; Roche Diagnostics, Indianapolis, IN) and 0.5 mg/mL DNase I (Sigma-Aldrich, St. Louis, MO), and incubated at 37°C for 45 min. Single-cell suspensions were pelleted and stained with allophycocyanin-conjugated anti-mouse CD4 (L3T4) and fluorescein isothiocyanate–conjugated anti-mouse T-cell receptor β (TCRβ) for flow cytometric analysis (eBioscience, San Diego, CA) using a FACSVantage SE flow cytometer (BD Biosciences, Franklin Lakes, NJ). Analysis was performed using FLowJo software (version 3.2; Tree Star, Inc., Ashland, OR). For T-cell cytokine determination, lung cells (250,000) were cultured in media or concanavalin A (ConA; 5 μg/mL) for 72 hr, and cytokine levels were measured by ELISA.
Histological examination of lung sections
To assess the effects of AUB on airway inflammation and mucus cell content in the airway wall, lungs were excised and fixed in 10% formalin, washed in methanol, dehydrated, embedded in paraffin, and cut into 5-μm sections. Sections were mounted on slides and stained with hematoxylin and eosin or periodic acid-Schiff (PAS). Slides were read in a blinded fashion and scored according to the following scale: 0, no inflammation; 1–1.99, 1–25% inflammation of section; 2–2.99, 26–50% inflammation of section; 3–3.99, 51–75% inflammation of section; 4–4.99, 76–100% inflammation of section. We counted the number of PAS-positive cells per section using a light microscope, and results are presented as mean ± SE for four sections per mouse lung.
Measurement of serum IgE levels
Immediately after AHR measurements, terminal blood was collected from the posterior vena cava. Total serum IgE levels were measured by ELISA using matched antibody pairs (BD Pharmingen, Franklin Lakes, NJ).
Statistical analysis
We determined differences between multiple groups using one-way analysis of variance, with Tukey multiple comparison post-test comparisons. To compare the two groups, we used Student’s t-test (GraphPad Prism; GraphPad Software Inc., La Jolla, CA). Significance was assumed at p < 0.05.
Results
AUB-induced AHR is lymphocyte dependent
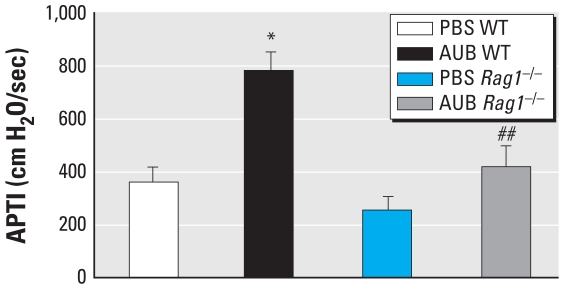
To assess the contribution of lymphocytes to the development of AUB-induced AHR, we compared the airway responses of WT and Rag1−/− mice to the cholinergic agonist acetylcholine. In WT mice exposed to AUB, we observed a significant increase in AHR compared with PBS controls (p < 0.0001; Figure 1). In contrast, no significant increases in AHR were seen in AUB-exposed Rag1−/− mice compared with the AUB-exposed WT mice. Thus, airway responses in both WT and Rag1−/− mice exposed to AUB were significantly different (p < 0.01). Lymphocytes also contributed to the baseline airway response to acetylcholine because the response was lower in PBS-challenged Rag1−/− mice compared with the PBS-exposed WT controls, although the observed difference did not achieve statistical significance.
Figure 1.
AUB-induced AHR is lymphocyte dependent, as determined by airway responses of AUB-exposed WT and Rag1−/− mice to acetylcholine (50 mg/kg). Results are shown as the mean ± SE of the time-integrated change in airway pressure (APTI); n = 7–10 mice/group.
*p < 0.0001 compared with PBS WT. ##p < 0.01 compared with AUB WT.
AUB induction of mucus is partially lymphocyte dependent
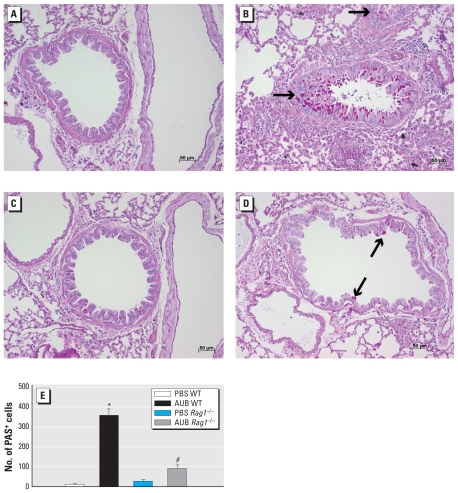
Because mucus metaplasia is a consistent feature of allergic asthma, we assessed the effects of AUB exposure on mucus production as assessed by PAS staining. We observed significant increases in the numbers of airways containing PAS-positive mucus cells in the lungs of AUB-exposed WT (Figure 2B) and Rag1−/− (Figure 2D) mice compared with their respective PBS controls (Figure 2A,C). The numbers of PAS-positive cells in AUB-exposed Rag1−/− mice were significantly higher than those in their PBS controls, but significantly lower than those observed in the WT AUB-exposed mice (p < 0.0001; Figure 2E).
Figure 2.
AUB induced a partially T-cell–dependent increase in mucus cells, as shown by PAS staining in lung sections from PBS-exposed (A and C) and AUB-exposed (B and D) WT (A and B) and Rag1−/− mice (C and D). Arrows indicate PAS-positive (PAS+) staining. (E) The number of PAS+ cells (mean ± SE) in airways in four sections per mouse lung (n = 4 mice/group).
*p < 0.0001 compared with PBS WT. #p < 0.0001 compared with AUB WT.
AUB-induced BAL fluid inflammation is partially lymphocyte dependent
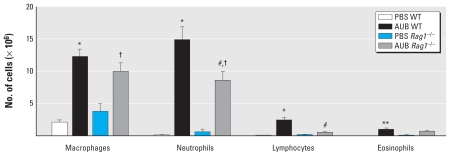
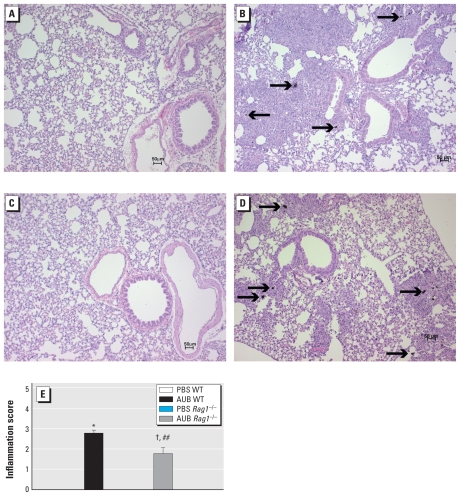
To determine whether AUB induces allergic airway inflammation in a lymphocyte-dependent manner, we compared the cellularity of the BAL fluids from Rag1−/− and WT mice after PBS or AUB exposure. In PBS controls of both strains of mice, most of the cells in the BAL fluid were primarily alveolar macrophages and neutrophils (Figure 3). We observed significant elevations in the numbers of macrophages, neutrophils, and eosinophils in BAL fluid from AUB-exposed WT mice. We also observed increases in each cell type in AUB-exposed Rag1−/− mice. However, the levels of neutrophils and lymphocytes were significantly lower in the BAL fluid from AUB-exposed Rag1−/− mice compared with AUB-exposed WT mice, whereas we found no significant differences in the numbers of macrophages between Rag1−/− and WT PBS controls. Although eosinophils were elevated in AUB-exposed Rag1−/− mice, the difference was not statistically significant. Although Rag1−/− mice are devoid of T and B lymphocytes, we observed a small number of cells in the BAL fluid with morphological characteristics of lymphocytes. These are most likely natural killer (NK) cells, because NK cell numbers are increased in naive Rag1−/− mice (Grundy and Sentman 2006). Consistent with the inflammatory patterns seen in BAL fluid after AUB exposure, we observed widespread perivascular and peribronchial inflammation in the lungs of both WT and Rag1−/− mice compared with their PBS-exposed controls (Figure 4). Consistent with the partial effect of Rag1 deficiency on AUB-induced increases in BAL fluid cellularity, the degree of AUB-induced inflammation in lung sections from Rag1−/− mice was significantly reduced compared with that seen in WT mice (p < 0.05; Figure 4E) but still significantly higher than in their PBS-exposed controls. Based on cell morphology, the inflammatory foci consisted primarily of neutrophils and macrophages. Of note, we detected AUB particles in the sections presumably engulfed by macrophages (Figure 4B,D). Taken together, these results suggest that AUB exposure induces a marked cellular infiltration of the mouse lung, which is only partially lymphocyte dependent.
Figure 3.
Populations of macrophages, neutrophils, lymphocytes, and eosinophils in Bal fluid of WT and Rag1−/− mice after AUB exposure. Values are mean ± SE; n = 7–10 mice/group.
*p < 0.0001, and **p < 0.01 compared with PBS WT. #p < 0.0001 compared with AUB WT. †p < 0.0001 compared with PBS Rag1−/−.
Figure 4.
AUB-induced lung inflammation is partially lymphocyte dependent, as shown by hematoxylin and eosin staining of WT (A and B) and Rag1−/− (C and D) mice exposed to either PBS (A and C) or AUB (B and D). Arrows indicate AUB particles. (E) Degree of inflammation scored according to an arbitrary scale defined in “Materials and Methods.” Values are mean ± SE; n = 8 independent sections/group.
*p < 0.0001 compared with PBS WT. ##p < 0.01 compared with AUB WT. †p < 0.0001 compared with PBS Rag1−/−.
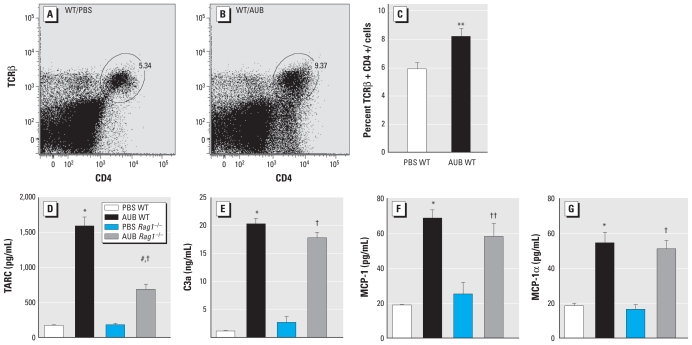
AUB induces the recruitment of CD4+ T cells
To gain additional insight into the type of T lymphocytes recruited by AUB exposure, we assessed the effects of AUB exposure on the numbers of CD4+ TCRβ+ cells by flow cytometric analysis of whole-lung cell digests. Our results revealed that AUB exposure induced significant increases in the percentage of conventional CD4+ TCRβ+ T cells in the lungs of WT mice (Figure 5A,B). As expected, we found no detectable CD4+ T cells in Rag1−/− animals (data not shown).
Figure 5.
AUB increases CD4+ T-cell recruitment concomitant with increases in chemokine production in the lung as detected by flow cytometry. Representative flow cytometry plots of CD4+ and TCRβ+ cells in BAL fluid of WT mice exposed to PBS (A) or AUB (B). (C) Quantification of the percentage of CD4+ T cells (mean ± SE) in lungs of WT mice treated with PBS or AUB (n = 7–10 mice/group). (D–G) Levels (mean ± SE) of TARC (D), C3a (E), MCP-1 (F), and MIP-1α (G) measured in BAL fluid of AUB- or PBS-exposed WT or Rag−/− mice (n = 7–10 mice/group).
**p < 0.01 compared with PBS WT. *p < 0.0001 compared with PBS WT. #p < 0.0001 compared with AUB WT. †p < 0.0001. ††p < 0.01 compared with PBS Rag1−/−.
To begin to understand the mechanism(s) of AUB-induced CD4+ T-cell subset recruitment, we assessed the effects of AUB exposure on the levels of several chemoattractants [thymus and activation regulated chemokine CCL17 (TARC), complement factor 3a (C3a), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein 1α (MIP-1α)], all of which are known to contribute to T cell–mediated inflammation (Gu et al. 2000; Kawasaki et al. 2001; Ritz et al. 2004; Walters et al. 2002). We found that AUB induced a significant increase in the levels of each of these chemokines in BAL fluid (Figure 5D–G). However, only TARC production appeared to be dependent on the presence of lymphocytes because it was significantly lower in AUB-exposed Rag1−/− mice than in AUB-exposed WT mice (Figure 5D). These results demonstrate that AUB exposure likely initially induces the recruitment of inflammatory cells into the lungs through the production of chemoattractants such as C3a, MCP-1, MIP-1α, and TARC, which are likely produced by either airway epithelial cells or monocyte/macrophage populations.
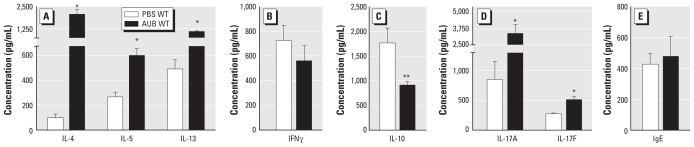
AUB induces T-cell cytokine production in WT mice
To assess the nature of the lymphocyte cytokine response to AUB, we measured the levels of cytokines associated with TH2, TH17, TH1, and regulatory T-cell (Treg) effector cell function from lung homogenates restimulated in vitro with ConA. Restimulated lung cells from AUB-exposed WT mice produced a higher level of the TH2 cytokines IL-4, IL-5, and IL-13 than did their PBS-exposed controls (Figure 6A), whereas we found no significant induction of the TH1-associated cytokine interferon-γ (IFNγ) (Figure 6B). Consistent with increased inflammation in the lungs and the increased number of lymphocytes in BAL fluid after AUB exposure, we found a significant reduction in the immunosuppressive cytokine IL-10 in cells from AUB-exposed WT mice (Figure 6C). AUB-exposed WT mice also had significantly higher levels of the TH17-associated cytokines IL-17A and IL-17F than the PBS-exposed controls (Figure 6D). As expected, lung cells from Rag1−/− mice did not respond to ConA stimulation (data not shown).
Figure 6.
Effect of AUB on cytokine and IgE production in lung T cells. (A–D) Cytokine production from ConA-restimulated lung cells showing the TH2 cytokines IL-4, IL-5, and IL-13 (A), the TH1 cytokine IFNγ (B), the Treg cytokine IL-10 (C), and the TH17 cytokines IL-17A and IL-17F (D). (E) Total serum IgE levels in AUB-exposed WT mice. Values are mean ± SE; n = 7–10 mice/group.
*p < 0.0001, and **p < 0.01 compared with PBS WT.
To determine whether AUB exposure induces allergic sensitization, we measured total serum IgE levels from AUB-exposed WT mice. In these mice, we found no increase in total serum IgE levels compared with PBS-exposed controls (Figure 6E). These results suggest that acute exposures to AUB do not promote atopy and that the AHR and inflammatory responses observed in AUB-exposed mice at the time point assessed do not depend on IgE-driven processes.
Discussion
In the present study, mice exposed to AUB showed marked increases in airway responsiveness to cholinergic stimuli, concomitant with an increase in eosinophilic and neutrophilic inflammation, CD4+ T-cell recruitment, and mucus cell metaplasia. These findings are consistent with epidemiological studies linking PM exposure and recent increases in asthma prevalence and morbidity. Moreover, our results support previous human PM exposure studies (Behndig et al. 2006; Ghio and Devlin 2001; Salvi et al. 2000; Samet et al. 2007; Schaumann et al. 2004) and studies in mouse models from our group and others showing that different sources of ambient PM [AUB, fly ash, diesel exhaust particles (DEPs)] can directly induce the pathophysiological features of asthma (Gavett et al. 1999; Ohta et al. 1999; Walters et al. 2001, 2002), as well as enhance immune responses to other allergens (Gavett et al. 1999; Fernvik et al. 2002; Wang et al. 2008).
Our results demonstrate that AUB-induced AHR is dependent upon lymphocytes because AHR is significantly attenuated in mice lacking mature lymphocyte populations (Rag1−/− mice). These results are consistent with other reports showing that the development of AHR in response to other environmental triggers of asthma, such as allergens (Cohn et al. 1998; Corry et al. 1998; Gavett et al. 1994), airborne oxidants (Chen et al. 1995), and irritants (Garssen et al. 1990; Matheson et al. 2001), are dependent on T lymphocytes. Although the development of AHR in response to AUB depended on lymphocytes, the AUB-induced influx of inflammatory cells into the lungs, as assessed by both BAL fluid and histological examination of lung sections, was only partially dependent on lymphocytes. This apparent disassociation between inflammation and AHR is in agreement with previous reports from our laboratory indicating that airway inflammation does not correlate with the development of antigen- or PM-induced AHR (Lewkowich et al. 2005; Walters et al. 2002). Likewise, AUB-induced mucus cell metaplasia was only partially abrogated in Rag1−/− mice. Because previous studies have shown the importance of CD4+ T-cells and the TH2 cytokine IL-13 (Wills-Karp et al. 1998) in mucus production after antigen exposure, these results are somewhat surprising. The results may suggest that either AUB directly induces mucus cell changes in the airway epithelium or that other innate immune cells such as neutrophils contribute to the induction of this response in Rag1−/− mice. Indeed, several studies have implicated neutrophil-derived mediators (neutrophil elastase) in mucus production (Shao and Nadel 2005).
Because TH2 cytokines have been closely associated with the development of antigen-induced AHR, we examined the cytokine profile in the lungs of mice exposed to AUB. We found that AUB induced a marked influx of CD4+ T-cells into the lungs and elevations in the levels of both TH2 (IL-4, IL-5, IL-13) and TH17 (IL-17A, IL-17F) cytokines, concomitant with a reduction in the Treg cytokine IL-10. We saw no significant changes, compared with baseline levels, in the TH1 cytokine IFNγ. Taken together, these results suggest that AUB induces allergic inflammation both by suppressing tolerogenic immune responses (IL-10) and by inducing a TH2/TH17 mixed immune response in the airways.
Here, we show for the first time that AUB-induced AHR is associated with the induction of a mixed TH17/TH2 cytokine response in the lung. Our observation is consistent with recent studies in human asthmatics and in animal models of ozone- and allergen-induced AHR implicating IL-17 in the development and progression of allergic asthma. Specifically, recent studies have shown that IL-17A levels in asthmatics correlate with the incidence of AHR and severity of disease (Barczyk et al. 2003; Chakir et al. 2003). Likewise aerosolized pollutants (organic dust and ozone) have been shown to induce IL-17A in human BAL cells (Ivanov et al. 2005) and in the mouse lung (Pichavant et al. 2008). Indeed, recent studies have demonstrated a primary role for IL-17 in ozone-induced AHR because both antibody blockade of IL-17 and genetic deficiency in the IL-17R protect against ozone-induced AHR (Pichavant et al. 2008). Studies of IL-17 in allergen-induced models of AHR suggest a more complex picture, with some studies showing that IL-17 plays an important role in allergen-driven AHR (Nakae et al. 2002; Wakashin et al. 2008; Wilson et al. 2009), whereas others have shown that either IL-17 does not play a role at all (Hellings et al. 2003) or that it can either stimulate or inhibit the development of allergic inflammation depending on the timing of IL-17 blockade (Schnyder-Candrian et al. 2006). Despite the evidence implicating IL-17 in AHR, it alone does not appear to be sufficient to induce AHR; however, IL-17 has been shown to synergize with TH2 cytokines (Wakashin et al. 2008; Wilson et al 2009). Although it’s role in AHR is not known, IL-17 is a potent stimulator of neutrophil recruitment and activation, and IL-17–dependent AHR has recently been shown to be neutrophil dependent (Wilson et al. 2009). A role for neutrophils in the induction of AHR in our model is suggested by the fact that AUB induced a significant influx of neutrophils into the mouse lung and that the numbers of neutrophils in the BAL fluid were reduced in Rag1−/− mice concomitant with suppression of AHR. Taken together, these results suggest that the development of AHR in response to airway delivery of antigens/pollutants may be dependent upon the synergistic actions of TH2 and IL-17.
The source of TH2 and TH17 cytokines appears to be lymphocytes because both baseline and AUB-stimulated cytokine levels are absence in Rag1−/− mice. Specifically, although not proven, we propose that the cells producing these cytokines are CD4+ T cells because we observed a marked influx of CD4+ T cells after AUB exposure (Figure 4B,E), which was absent in Rag1−/− mice. However, a contribution by other lymphocyte populations cannot be ruled out. Indeed, recent studies suggest that NK T cells may contribute to the development of AHR induced by ozone (Pichavant et al. 2008) through their ability to recognize lipid antigens and produce cytokines soon after exposure. NK T cells likely do not play a role in our studies because Rag1−/− mice do not have mature NK T cells. However, NK cells may play a role because the development of these cells is up-regulated in Rag1−/− mice (Grundy and Sentman 2006). These cells may account for the lymphocyte-like populations we identified morphologically in the BAL fluid, and they may be responsible for the lymphocyte-independent production of IL-5 and recruitment of inflammatory cells we observed in the lungs of Rag1−/− mice.
The mechanisms by which PM activates lymphocytes are currently unknown. We have previously reported that AUB contains a variety of potentially biologically active components such as endotoxin, metals, and polyaromatic hydrocarbons (PAHs) (Walters et al. 2001). Recent studies suggest that pollutants such as ozone activate lymphocytes in the mouse lung (Pichavant et al. 2008). Although it had been thought that substances such as ozone damage the airways through production of free radicals, leading to the presentation of altered self-proteins (Ciencewicki et al. 2008), recent studies suggest that ozone may also induce airway inflammation through toll-like receptor 4 (TLR4)-mediated processes (Kleeberger et al. 2000). We previously reported that AUB contains low levels of endotoxin (Walters et al. 2001); thus, the activation of immune responses in our model may be at least partially TLR4 dependent. Alternatively, the oxidative potential of transition metals (copper, manganese, zinc) and PAHs contained in our PM source may also drive AUB-induced T-cell activation. Substantial evidence suggests that metals and oxidative stress play a significant role in the strong epidemiological association between indices of allergic airway disease and PM exposure in epidemiological studies conducted both in the Utah Valley (Ghio and Devlin 2001; Pope 1989) and in Germany (Schaumann et al. 2004). PAHs are also thought to be strong inducers of oxidative stress because the ability of DEPs containing high levels of PAHs to enhance ovalbumin sensitization in mice is inhibited by pretreatment of mice with thiol antioxidants (Li et al. 2009; Whitekus et al. 2002). The water-soluble fraction of AUB does not contribute to its ability to induce AHR (Walters et al. 2001), thus, the organic fraction of AUB containing numerous PAHs may play a significant role in T-cell activation in our model. Each of these components is likely to activate T cell–mediated immune responses through effects on dendritic cell function rather than direct effects on T cells, because DEPs collected in urban Baltimore do not directly activate T cells (Porter et al. 2007). In contrast, AUB has been shown to directly activate dendritic cells in an oxidant-dependent manner (Williams et al. 2007). Taken together these results suggest that AUB activates dendritic cell/T-cell activation through multiple additive or synergistic effects driven by the individual components of real-world ambient air PM.
Conclusion
Our studies demonstrate that exposure of the mouse lung to real-world ambient PM directly induces several features of asthma, concomitant with the activation of an adaptive immune response characterized by the recruitment and activation of CD4+ T lymphocytes. The induction of a TH2/TH17-skewed cytokine environment in the lung may directly drive asthmatic symptoms, as well as lead to the sensitization to or enhancement of ongoing immune responses to heterologous antigens in susceptible individuals. These studies provide a plausible biological mechanism for the strong association between PM exposure and the increase in asthma morbidity.
Footnotes
This work was funded by grants from the National Institute of Environmental Health Sciences (P01 ES009606 and P50 ES015903) and from the U.S. Environmental Protection Agency (RD83213901).
References
- Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27(2):359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- Chen X, Gavett SH, Wills-Karp M. CD4+ T lymphocyte modulation of ozone-induced murine pulmonary inflammation. Am J Respir Cell Mol Biol. 1995;12(4):396–403. doi: 10.1165/ajrcmb.12.4.7695918. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122(3):456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998;161(8):3813–3816. [PubMed] [Google Scholar]
- Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4(5):344–355. [PMC free article] [PubMed] [Google Scholar]
- Fernvik E, Peltre G, Senechal H, Vargaftig BB. Effects of birch pollen and traffic particulate matter on Th2 cytokines, immunoglobulin E levels and bronchial hyper-responsiveness in mice. Clin Exp Allergy. 2002;32(4):602–611. doi: 10.1046/j.0954-7894.2002.01347.x. [DOI] [PubMed] [Google Scholar]
- Garssen J, van Loveren H, van der Vliet H, Nijkamp FP. T cell mediated induction of bronchial hyperreactivity. Br J Clin Pharmacol. 1990;30(suppl 1):153S–155S. doi: 10.1111/j.1365-2125.1990.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10(6):587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Madison SL, Stevens MA, Costa DL. Residual oil fly ash amplifies allergic cytokines, airway responsiveness, and inflammation in mice. Am J Respir Crit Care Med. 1999;160(6):1897–1904. doi: 10.1164/ajrccm.160.6.9901053. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med. 2001;164(4):704–708. doi: 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2008. [accessed 31 March 2010]. Available: http://www.ginasthma.org/Guidelineitem.asp??l1=2&l2=1&intId=60.
- Grundy MA, Sentman CL. Immunodeficient mice have elevated numbers of NK cells in non-lymphoid tissues. Exp Cell Res. 2006;312(19):3920–3926. doi: 10.1016/j.yexcr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404(6776):407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28(1):42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- Ivanov S, Palmberg L, Venge P, Larsson K, Linden A. Interleukin-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir Res. 2005;6:44. doi: 10.1186/1465-9921-6-44. [Online 25 May 2005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol. 2001;166(3):2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR, Reddy S, Zhang LY, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: role of toll-like receptor 4. Am J Respir Cell Mol Biol. 2000;22(5):620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- Kongerud J, Madden MC, Hazucha M, Peden D. Nasal responses in asthmatic and nonasthmatic subjects following exposure to diesel exhaust particles. Inhal Toxicol. 2006;18(9):589–594. doi: 10.1080/08958370600743027. [DOI] [PubMed] [Google Scholar]
- Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202(11):1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117:1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect. 1997;105:216–222. doi: 10.1289/ehp.97105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson JM, Lange RW, Lemus R, Karol MH, Luster MI. Importance of inflammatory and immune components in a mouse model of airway reactivity to toluene diisocyanate (TDI) Clin Exp Allergy. 2001;31(7):1067–1076. doi: 10.1046/j.1365-2222.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Ohta K, Yamashita N, Tajima M, Miyasaka T, Nakano J, Nakajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104(5):1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205(2):385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., III Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79(5):623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, et al. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37(6):706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz SA, Cundall MJ, Gajewska BU, Swirski FK, Wiley RE, Alvarez D, et al. The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol. 2004;138(2):213–220. doi: 10.1111/j.1365-2249.2004.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159(3):702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, et al. Acute exposure to diesel exhaust increases IL-8 and GRO-alpha production in healthy human airways. Am J Respir Crit Care Med. 2000;161(2 Pt 1):550–557. doi: 10.1164/ajrccm.161.2.9905052. [DOI] [PubMed] [Google Scholar]
- Samet JM, Graff D, Berntsen J, Ghio AJ, Huang YC, Devlin RB. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal Toxicol. 2007;19(suppl 1):29–32. doi: 10.1080/08958370701492706. [DOI] [PubMed] [Google Scholar]
- Schaumann F, Borm PJ, Herbrich A, Knoch J, Pitz M, Schins RP, et al. Metal-rich ambient particles (particulate matter2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med. 2004;170(8):898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203(12):2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J Immunol. 2005;175(6):4009–4016. doi: 10.4049/jimmunol.175.6.4009. [DOI] [PubMed] [Google Scholar]
- Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151(8):798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178(10):1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breysse PN, Schofield B, Wills-Karp M. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2002;27(4):413–418. doi: 10.1165/rcmb.4844. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- Wang T, Moreno-Vinasco L, Huang Y, Lang GD, Linares JD, Goonewardena SN, et al. Murine lung responses to ambient particulate matter: genomic analysis and influence on airway hyperresponsiveness. Environ Health Perspect. 2008;116:1500–1508. doi: 10.1289/ehp.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, et al. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168(5):2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- Williams MA, Porter M, Horton M, Guo J, Roman J, Williams D, et al. Ambient particulate matter directs nonclassic dendritic cell activation and a mixed TH1/TH2-like cytokine response by naive CD4+ T cells. J Allergy Clin Immunol. 2007;119(2):488–497. doi: 10.1016/j.jaci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]