Abstract
Keratinocytes in culture synthesize and respond to interleukin 1 (IL-1). We have measured surface IL-1 receptor (IL-1R) on keratinocytes in culture using radiolabeled IL-1 binding assays. Surface IL-1R levels are less than 2000 receptors per cell in postconfluent cultures but increase 9- to 20-fold 24 hr after treatment with phorbol 12-myristate 13-acetate (PMA) at 10 ng/ml or after raising the extracellular Ca2+ concentration to 2 mM. This induction of surface IL-1R can be blocked by the addition of retinoic acid and parallels induction of squamous differentiation markers. These results imply that IL-1R levels may be related to the degree of differentiation of these cells. In parallel studies IL-1 protein levels were determined by bioassay and by Western blotting (immunoblots). All detectable IL-1 protein and essentially all IL-1 activity was cell-associated. Although constitutive levels of IL-1 biological activity and protein are significant in these cultures, IL-1 levels increase when either PMA or retinoic acid alone are added to cultures. IL-1 does not increase when PMA and retinoic acid are added simultaneously to cultures; nor is it induced when extracellular Ca2+ concentrations are raised to 2 mM. Thus, cell-associated IL-1 levels do not necessarily parallel surface IL-1R levels in these cultures. Taken together, these results demonstrate that IL-1 and surface IL-1R levels are differentially and complexly regulated in keratinocyte cultures. Possible implications of these results in terms of normal and abnormal regulation of proliferation and differentiation are discussed.
Full text
PDF
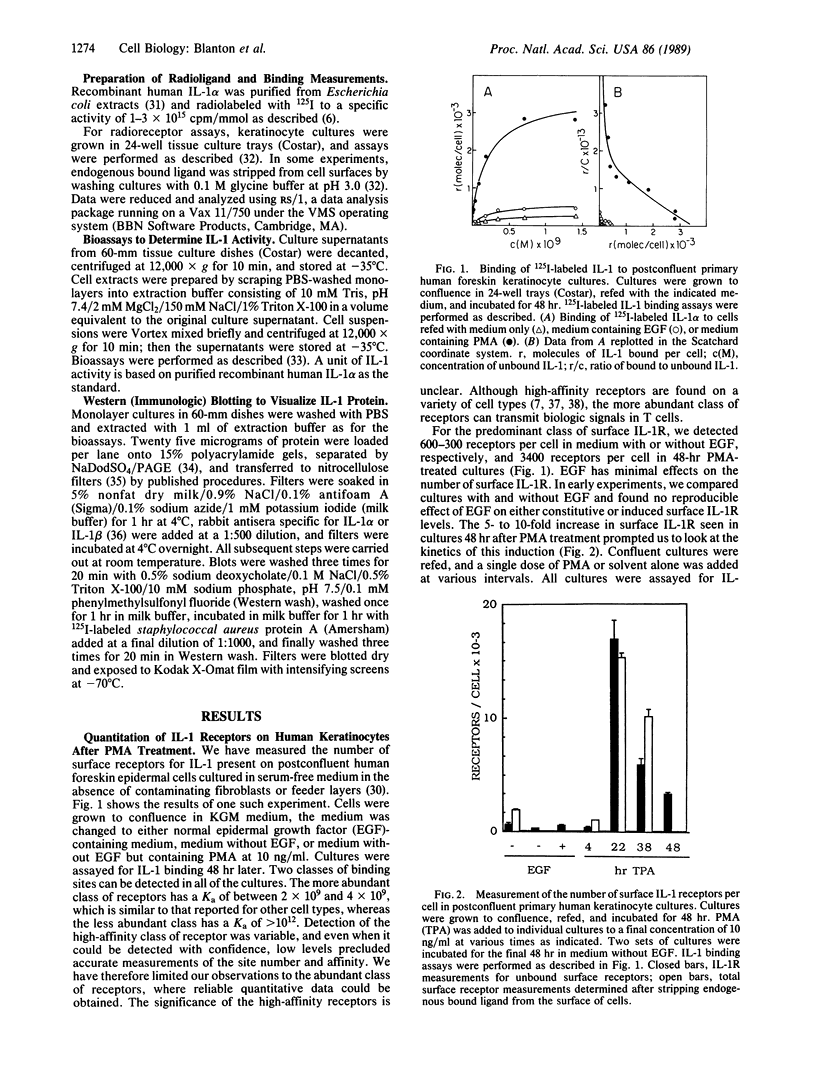
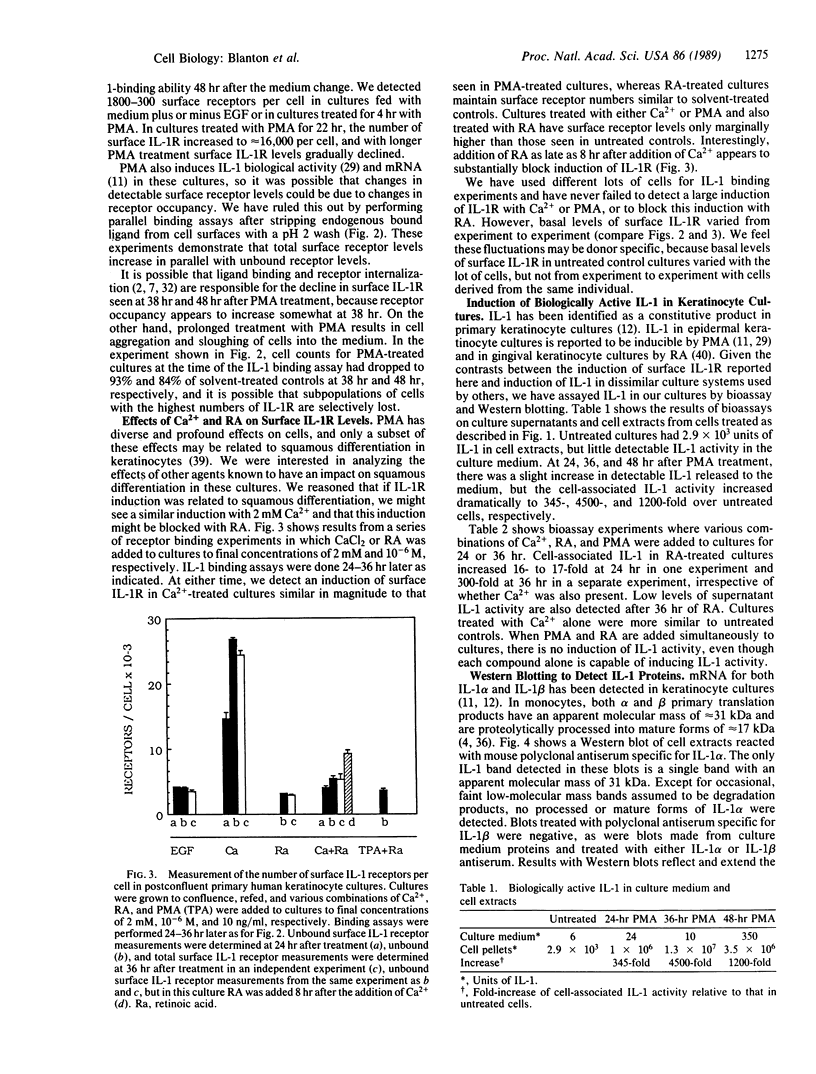


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird T. A., Saklatvala J. Studies on the fate of receptor-bound 125I-interleukin 1 beta in porcine synovial fibroblasts. J Immunol. 1987 Jul 1;139(1):92–97. [PubMed] [Google Scholar]
- Brown R., Gray R. H., Bernstein I. A. Retinoids alter the direction of differentiation in primary cultures of cutaneous keratinocytes. Differentiation. 1985;28(3):268–278. doi: 10.1111/j.1432-0436.1985.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Chin J., Cameron P. M., Rupp E., Schmidt J. A. Identification of a high-affinity receptor for native human interleukin 1 beta and interleukin 1 alpha on normal human lung fibroblasts. J Exp Med. 1987 Jan 1;165(1):70–86. doi: 10.1084/jem.165.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon P. J. A rapid biologic assay for the detection of interleukin 1. J Immunol. 1983 Sep;131(3):1280–1282. [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., Hopp T. P., Cantrell M., Deeley M., Gillis S., Henney C. S., Urdal D. L. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986 Nov 20;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Buckley A., Daynes R. A. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J Clin Invest. 1985 Oct;76(4):1585–1591. doi: 10.1172/JCI112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C., Saurat J. H., Schmitt A., Jaunin F., Dayer J. M. Interleukin 1 is present in normal human epidermis. J Immunol. 1986 May 1;136(9):3317–3323. [PubMed] [Google Scholar]
- Hawley-Nelson P., Stanley J. R., Schmidt J., Gullino M., Yuspa S. H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res. 1982 Jan;137(1):155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Hennings H., Holbrook K. A. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983 Jan;143(1):127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Kaur P., McDougall J. K. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988 Jun;62(6):1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian P. L., Kaffka K. L., Stern A. S., Woehle D., Benjamin W. R., Dechiara T. M., Gubler U., Farrar J. J., Mizel S. B., Lomedico P. T. Interleukin 1 alpha and interleukin 1 beta bind to the same receptor on T cells. J Immunol. 1986 Jun 15;136(12):4509–4514. [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragballe K., Marcelo C. L., Voorhees J. J., Sauder D. N. Formation of epidermal cell thymocyte-activating factor (ETAF) from cultured human keratinocytes from normal and uninvolved psoriatic skin. J Invest Dermatol. 1987 Jan;88(1):8–10. doi: 10.1111/1523-1747.ep12464635. [DOI] [PubMed] [Google Scholar]
- Kronheim S. R., March C. J., Erb S. K., Conlon P. J., Mochizuki D. Y., Hopp T. P. Human interleukin 1. Purification to homogeneity. J Exp Med. 1985 Mar 1;161(3):490–502. doi: 10.1084/jem.161.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Chua A. O., Flood P., McGuire J., Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987 Aug;80(2):430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., McGuire J. Hydrocortisone reduces both constitutive and UV-elicited release of epidermal thymocyte activating factor (ETAF) by cultured keratinocytes. J Invest Dermatol. 1986 Nov;87(5):570–573. doi: 10.1111/1523-1747.ep12455811. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., MacDonald H. R. Binding and internalization of interleukin 1 by T cells. Direct evidence for high- and low-affinity classes of interleukin 1 receptor. J Exp Med. 1986 Oct 1;164(4):1060–1074. doi: 10.1084/jem.164.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Sztein M. B., Schmidt J. A., Hawley-Nelson P., Grabner G., Oppenheim J. J. Characteristics of an epidermal cell thymocyte-activating factor (ETAF) produced by human epidermal cells and a human squamous cell carcinoma cell line. J Invest Dermatol. 1983 Sep;81(3):187–193. doi: 10.1111/1523-1747.ep12517658. [DOI] [PubMed] [Google Scholar]
- Lumpkin M. D. The regulation of ACTH secretion by IL-1. Science. 1987 Oct 23;238(4826):452–454. doi: 10.1126/science.2821618. [DOI] [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Martinet N., Harne L. A., Grotendorst G. R. Identification and characterization of chemoattractants for epidermal cells. J Invest Dermatol. 1988 Feb;90(2):122–126. doi: 10.1111/1523-1747.ep12462081. [DOI] [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Parkinson E. K. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br J Cancer. 1985 Oct;52(4):479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praeger F. C., Stanulis-Praeger B. M., Gilchrest B. A. Use of strontium to separate calcium-dependent pathways for proliferation and differentiation in human keratinocytes. J Cell Physiol. 1987 Jul;132(1):81–89. doi: 10.1002/jcp.1041320111. [DOI] [PubMed] [Google Scholar]
- Qwarnstrom E. E., Page R. C., Gillis S., Dower S. K. Binding, internalization, and intracellular localization of interleukin-1 beta in human diploid fibroblasts. J Biol Chem. 1988 Jun 15;263(17):8261–8269. [PubMed] [Google Scholar]
- Reiners J. J., Jr, Slaga T. J. Effects of tumor promoters on the rate and commitment to terminal differentiation of subpopulations of murine keratinocytes. Cell. 1983 Jan;32(1):247–255. doi: 10.1016/0092-8674(83)90515-9. [DOI] [PubMed] [Google Scholar]
- Remy J. C., Fruchter R. G., Boyce J., Macasaet M., Choi K., Rotman M. Complications of combined surgery and radiation therapy for carcinoma of the endometrium. Int J Gynaecol Obstet. 1985 Apr;23(2):83–93. doi: 10.1016/0020-7292(85)90049-9. [DOI] [PubMed] [Google Scholar]
- Ristow H. J. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be interleukin 1 or a related protein. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1940–1944. doi: 10.1073/pnas.84.7.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder D. N. Biologic properties of epidermal cell thymocyte-activating factor (ETAF). J Invest Dermatol. 1985 Jul;85(1 Suppl):176s–179s. doi: 10.1111/1523-1747.ep12276378. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Yuspa S. H. Specific epidermal protein markers are modulated during calcium-induced terminal differentiation. J Cell Biol. 1983 Jun;96(6):1809–1814. doi: 10.1083/jcb.96.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K., Shapas B. G., Rice H. M., Boutwell R. K. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979 Feb;39(2 Pt 1):419–425. [PubMed] [Google Scholar]
- Walsh L. J., Seymour G. J., Powell R. N. The in vitro effect of retinol on human gingival epithelium. II. Modulation of Langerhans cell markers and interleukin-1 production. J Invest Dermatol. 1985 Dec;85(6):501–506. doi: 10.1111/1523-1747.ep12277300. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth P. J., Yuspa S. H., Thorgeirsson S. S., Hennings H. Induction of common patterns of polypeptide synthesis and phosphorylation by calcium and 12-O-tetradecanoylphorbol-13-acetate in mouse epidermal cell culture. Cancer Res. 1987 Jun 1;47(11):2831–2838. [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Hennings H., Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982 Jun;42(6):2344–2349. [PubMed] [Google Scholar]