Abstract
The gene for involucrin, an epidermal protein, has been remodeled in the higher primates. Most of the coding region of the human gene consists of a modern segment of repeats derived from a 10-codon sequence present in the ancestral segment of the gene. The modern segment can be divided into early, middle, and late regions. We report here the nucleotide sequence of three alleles of the gorilla involucrin gene. Each possesses a modern segment homologous to that of the human and consisting of 10-codon repeats. The early and middle regions are similar to the corresponding regions of the human allele and are nearly identical among the different gorilla alleles. The late region consists of recent duplications whose pattern is unique in each of the gorilla alleles and in the human allele. The early region is located in what is now the 3' third of the modern segment, and the late, polymorphic region is located in what is now the 5' third. Therefore, as the modern segment expanded during evolution, its 3' end became stabilized, and continuing duplications became confined to its 5' end. The expansion of the involucrin coding region, which began long before the separation of the gorilla and human, has continued in both species after their separation.
Full text
PDF
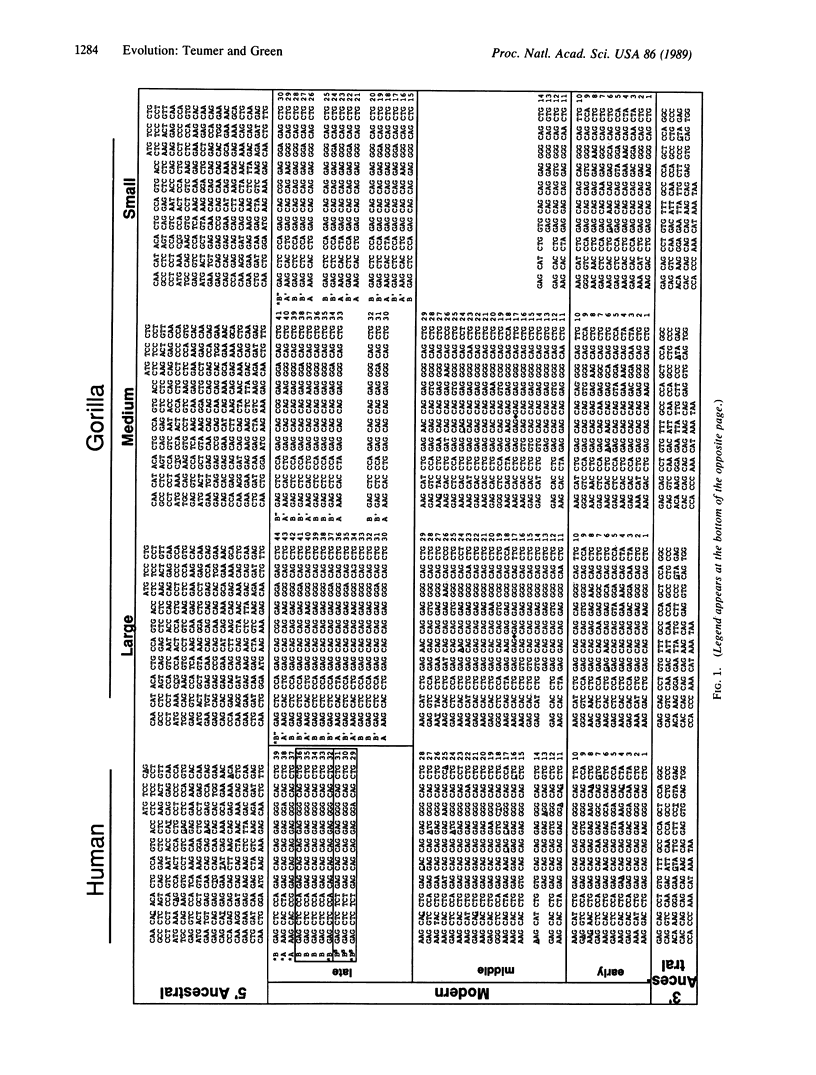
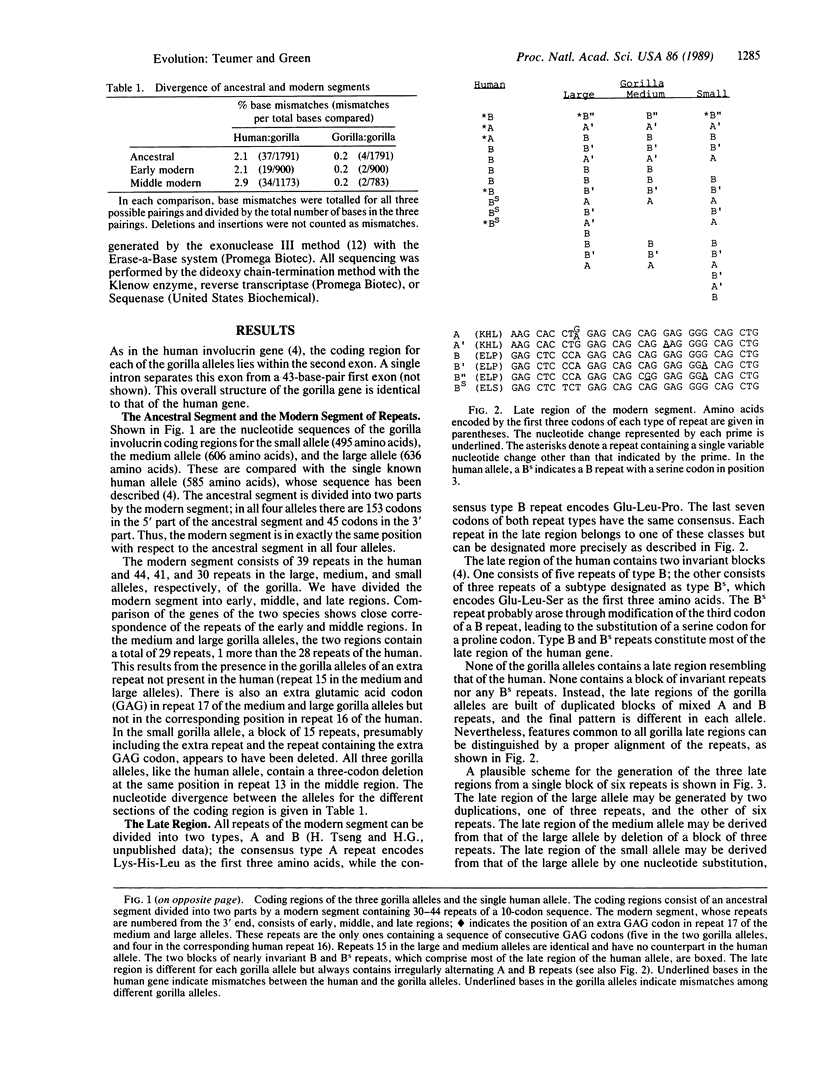
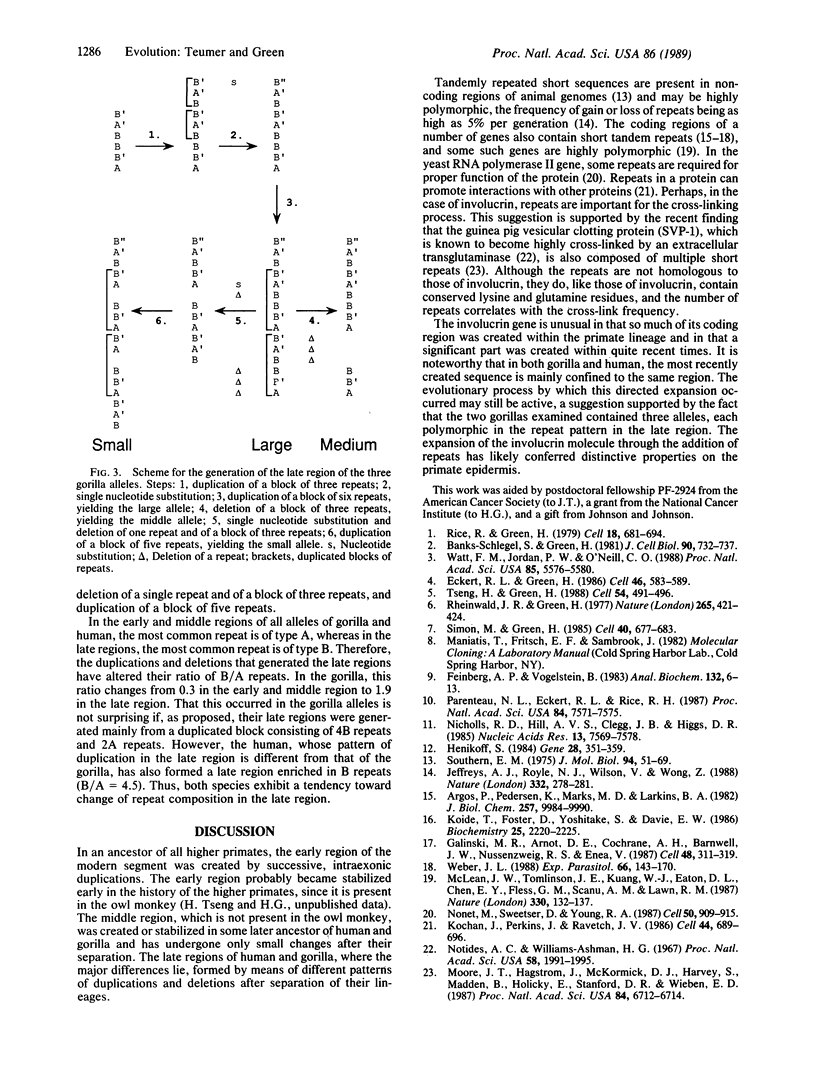
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Pedersen K., Marks M. D., Larkins B. A. A structural model for maize zein proteins. J Biol Chem. 1982 Sep 10;257(17):9984–9990. [PubMed] [Google Scholar]
- Banks-Schlegel S., Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol. 1981 Sep;90(3):732–737. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986 Aug 15;46(4):583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Kochan J., Perkins M., Ravetch J. V. A tandemly repeated sequence determines the binding domain for an erythrocyte receptor binding protein of P. falciparum. Cell. 1986 Mar 14;44(5):689–696. doi: 10.1016/0092-8674(86)90834-2. [DOI] [PubMed] [Google Scholar]
- Koide T., Foster D., Yoshitake S., Davie E. W. Amino acid sequence of human histidine-rich glycoprotein derived from the nucleotide sequence of its cDNA. Biochemistry. 1986 Apr 22;25(8):2220–2225. doi: 10.1021/bi00356a055. [DOI] [PubMed] [Google Scholar]
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987 Nov 12;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Moore J. T., Hagstrom J., McCormick D. J., Harvey S., Madden B., Holicky E., Stanford D. R., Wieben E. D. The major clotting protein from guinea pig seminal vesicle contains eight repeats of a 24-amino acid domain. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6712–6714. doi: 10.1073/pnas.84.19.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Hill A. V., Clegg J. B., Higgs D. R. Direct cloning of specific genomic DNA sequences in plasmid libraries following fragment enrichment. Nucleic Acids Res. 1985 Nov 11;13(21):7569–7578. doi: 10.1093/nar/13.21.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Sweetser D., Young R. A. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987 Sep 11;50(6):909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- Notides A. C., Williams-Ashman H. G. The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1991–1995. doi: 10.1073/pnas.58.5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau N. L., Eckert R. L., Rice R. H. Primate involucrins: antigenic relatedness and detection of multiple forms. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7571–7575. doi: 10.1073/pnas.84.21.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Simon M., Green H. Enzymatic cross-linking of involucrin and other proteins by keratinocyte particulates in vitro. Cell. 1985 Mar;40(3):677–683. doi: 10.1016/0092-8674(85)90216-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Tseng H., Green H. Remodeling of the involucrin gene during primate evolution. Cell. 1988 Aug 12;54(4):491–496. doi: 10.1016/0092-8674(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Jordan P. W., O'Neill C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]



