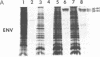
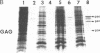
Abstract
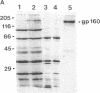
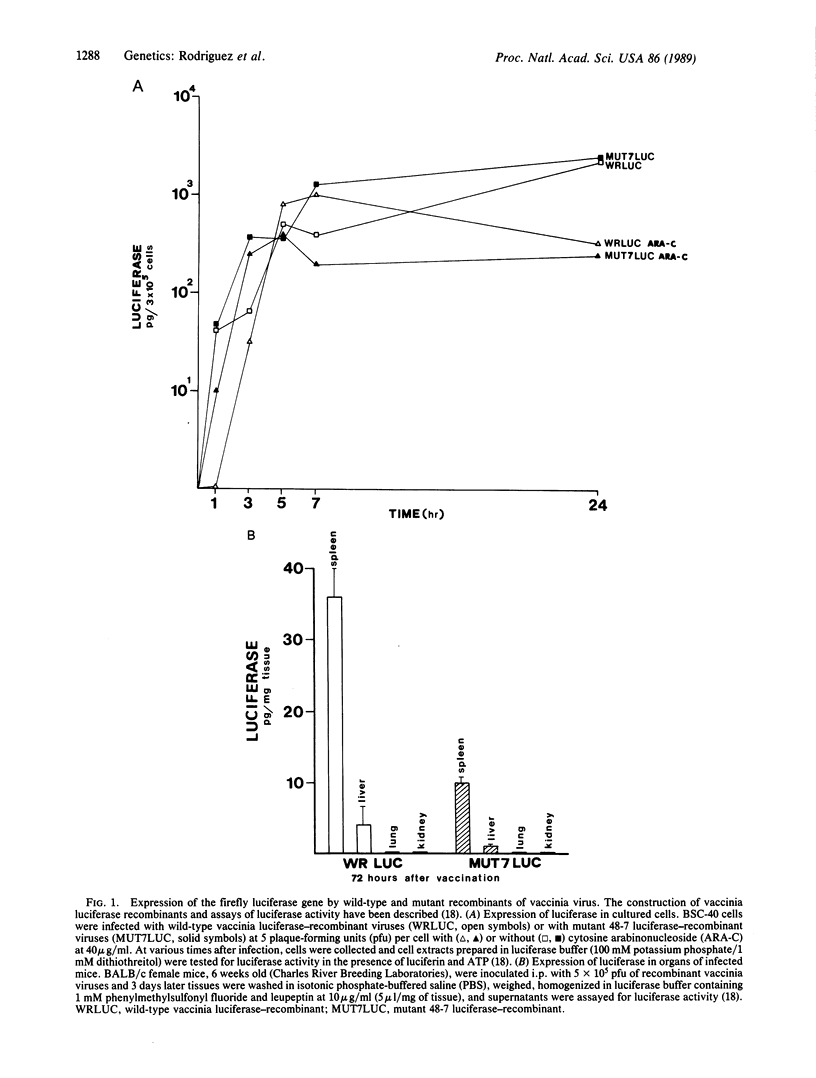
An attenuated vaccinia virus mutant with specific genetic lesions has been used to develop a vehicle for safer live recombinant virus vaccines. The mutant virus 48-7 has an 8-MDa deletion starting 2.2 MDa from the left end of the viral genome and point mutations in the gene encoding the 14-kDa fusion protein that determines the plaque-size phenotype of the virus. Using the highly sensitive reporter gene luciferase, we have shown that this mutant can generate recombinant viruses that infect cultured cells and animals with normal vaccinia virus tropism. Insertion of the envelope and gag genes of human immunodeficiency virus type 1 into the attenuated vaccinia mutant resulted in their efficient expression and precursor processing in infected cultured cells. Infection of mice with human immunodeficiency virus-vaccinia recombinant viruses elicited human immunodeficiency virus-specific antibodies. Using mice pretreated with cyclophosphamide as a model for immunosuppression, the reduced virulence of the mutant recombinant virus was clearly evident. These findings demonstrate that the highly attenuated vaccinia virus mutant 48-7 can be used to generate effective and safer vaccines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F., Schild G. C., Ada G. L. Recombinant vaccinia viruses as vaccines. Nature. 1986 Feb 13;319(6054):549–550. doi: 10.1038/319549a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Robert-Guroff M., Wong-Staal F., Gallo R. C., Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986 Apr 10;320(6062):535–537. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- Dallo S., Esteban M. Isolation and characterization of attenuated mutants of vaccinia virus. Virology. 1987 Aug;159(2):408–422. doi: 10.1016/0042-6822(87)90480-6. [DOI] [PubMed] [Google Scholar]
- Dallo S., Rodriguez J. F., Esteban M. A 14K envelope protein of vaccinia virus with an important role in virus-host cell interactions is altered during virus persistence and determines the plaque size phenotype of the virus. Virology. 1987 Aug;159(2):423–432. doi: 10.1016/0042-6822(87)90481-8. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Collalti E., Ratner L., Gallo R. C., Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985 Jul 18;316(6025):262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Maa J. S., Esteban M. Structural and functional studies of a 39,000-Mr immunodominant protein of vaccinia virus. J Virol. 1987 Dec;61(12):3910–3919. doi: 10.1128/jvi.61.12.3910-3919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Paez E., Dallo S., Esteban M. Generation of a dominant 8-MDa deletion at the left terminus of vaccinia virus DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3365–3369. doi: 10.1073/pnas.82.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez E., Dallo S., Esteban M. Virus attenuation and identification of structural proteins of vaccinia virus that are selectively modified during virus persistence. J Virol. 1987 Aug;61(8):2642–2647. doi: 10.1128/jvi.61.8.2642-2647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., James W. D., Jones T. S., Brown C., Burke D. S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987 Mar 12;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. F., Rodriguez D., Rodriguez J. R., McGowan E. B., Esteban M. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]