Abstract
There are four adenosine receptors, A1, A2A, A2B and A3, together forming a defined subgroup of G protein coupled receptors. They are well conserved and widely expressed. The endogenous agonist, adenosine, has a minimal concentration in body fluids (20 – 200 nM) that is sufficient to slightly activate the receptors where they are very highly expressed - as in the basal ganglia, on fat cells and in the kidney. Here adenosine can play a physiological role and here antagonists such as caffeine can have effects in healthy individuals. Adenosine levels rise in stress and distress (up to 30 μM in ischemia) and tend to minimize the risk for adverse outcomes by increasing energy supply and decreasing cellular work, by stimulating angiogenesis, mediating preconditioning and having multiple effects on immune competent cells. These pathophysiological roles of adenosine also offer some potential drug targets, but the fact that adenosine receptors are involved in so many processes does not simplify drug development.
Introduction
Adenosine receptors comprise a tight, evolutionarily well conserved, subgroup of G protein-coupled receptors [1] 1. They are denoted A1 , A2A, A2B and A3 ; the first and the last couple predominantly to G proteins of the Gi family, whereas the two A2 receptors predominantly couple to members of the Gs family [2]. Nevertheless, the receptors can couple also to other G protein pathways, especially when over expressed. The natural ligand is adenosine, but at A1 and A3 receptors also inosine can act as a partial agonist [3, 4].
When we want to consider these receptors as potential drug targets it is important to know what level of activation that is achieved by endogenous adenosine acting at the receptors in different locations. Two factors are then of importance: 1) the potency of adenosine as an agonist at the different adenosine receptors, and 2) the levels of adenosine that are found under physiological and pathophysiological conditions.
Potency of adenosine - Dependence on receptor number and on response measured
Unfortunately it proves very difficult to determine the affinity of adenosine to the receptors by direct binding studies. The reason is that adenosine is rapidly metabolized and also rapidly formed in biological preparations including membrane preparations. Therefore, if metabolism of the labelled added adenosine is prevented, endogenous adenosine accumulates to confound the measurements, and we do not have reliable data on the comparative affinity of the endogenous agonist at the four adenosine receptors.
The potency of adenosine must therefore be measured in functional assays. This introduces another important confounding factor: potency of the agonist is markedly influenced by the receptor number [5-7]. Adenosine receptors generally exhibit the behaviour described by pharmacologists as ”spare receptors”. In such systems alterations in receptor number is manifested by parallel shifts in the dose response curve, not as alterations in the maximal response. It is therefore important to compare potencies between receptors at comparative receptor densities. When this is done it is observed that adenosine is equipotent at A1, A2A and A3 receptors, but is some 50 times less potent at A2B receptors if alterations in cAMP are recorded [3]. If, by contrast, we instead examine the ability to activate MAP kinase (which all the receptors do), adenosine is equipotent at all of them [8, 9]. Thus, the potency of endogenous adenosine depends both on receptor number, and on the type of response measured. Furthermore, there is no really good reason to divide the receptors into high affinity and low affinity receptors as is sometimes done.
Regulation of adenosine levels – increasing in stressful situations
Because it is a key metabolite there is always a finite intracellular concentration of adenosine. Since most if not all cells possess equilibrative adenosine transporters, there will, by necessity, be also a finite level of adenosine in the extracellular space, even under the most basal conditions. This is in the range of 30-200 nM [10]. From this baseline level adenosine can increase substantially via several mechanisms: 1) formation intracellularly and export via transporters, and 2) formation in the extracellular space from adenine nucleotides released from cells. Adenosine is formed intracellularly whenever there is a discrepancy between the rates of ATP synthesis and ATP utilization; e.g. when work load is markedly enhanced or when the supply of oxygen and glucose is limiting as in ischemia.
ATP might, as originally proposed, act as a neurotransmitter, stored and released together with other transmitters [11], sometimes ATP may be preferentially released via so called ”kiss-and-run” mechanism [12].Several additional mechanisms have now moved to the foreground: 1) release from cells with damage to the cell membrane e.g. in necrotic cell death, 2) release from large storage vesicles containing hormones, 3) via connexin/pannexin “hemichannels”, 4) from transport vesicles delivering proteins to the cell membrane, and 5) from a subset of lysosomes. It is well known that ATP is released from many cells where cell membranes subjected to stretch [13], perhaps via one of the above mentioned mechanisms.
One ATP (or ADP) is released the phosphate groups of extracellular ATP are rapidly split off by ecto-enzymes working in concert, first via nucleoside triphosphate diphosphohydrolases (NTDPases) similar to CD39 [14], followed by hydrolysis via ecto-5′-nucleotidase, CD73 [15]. Knock-outs of these enzymes have revealed their importance in different organs and situations.
As one would predict from these biochemical / cell biological considerations adenosine levels will rise under conditions of stress and be particularly high under circumstances when the organism is in severe distress such as after an ischemic insult. Several studies estimate the resting extracellular levels of adenosine to be in the range 20 – 300 nM, and the levels can rise to the low micromolar range in conditions of extreme physiology such as strenuous exercise or subsistence at high altitude and hence low ambient oxygen. In ischemic areas or after massive tissue trauma leading to cell death by necrosis levels can increase to perhaps 30 μM [16].
If these data are related to the estimated potency of adenosine receptors we arrive at a picture like that illustrated in Fig 1. One can see that in places where the receptors are very abundant there will be a physiological role of adenosine, in places where receptors are fewer receptors may only be activated under extreme or pathological circumstances.
Figure 1.
Schematic illustration of the relationship between adenosine concentration and the effect mediated by adenosine receptors when the receptors are very abundant (as for example the A2A receptors on striatopallidal neurons, or when they are less abundant. This relationship is then superimposed on data on the levels of adenosine in tissue fluids under different circumstances.
Elucidation of the roles of adenosine by antagonists and genetically modified animals
It was shown early that theophylline and other methylxanthines are very effective as antagonists of the actions of adenosine. This antagonism was the basis for the first proposal that there are specific adenosine receptors, at which methylxanthines are antagonists [17], and it was soon realized that many of the effects of the xanthines are in fact due to inhibition of actions of endogenous adenosine [18, 19]. There is, however, one receptor A3 , which is not influenced by reasonable concentrations of most xanthines. [1]. It is probably that this contributed to its remaining undiscovered for so long. Based on the xanthine structures several more potent, and some receptor subtype selective, antagonists have been synthesized. These have been complemented by non-xanthine antagonists and several receptor-selective agonists, and we now are in the fortunate position to have a set of apparently quite selective pharmacological tools [1], [20-23].
However, many of the most potent and selective antagonists have physicochemical properties that complicate their use in vivo in animals and man. Therefore, the fact that there are mice with targeted deletions of one or more adenosine receptors has been very useful to delineate the roles played by adenosine receptors [24]. There is always a concern that deleting a receptor throughout life will induce major adaptive changes. In the case of adenosine receptors this has not been borne out: there are remarkably few adaptations, and particularly there are few if any compensatory changes in the remaining three receptors if one of them is deleted at birth. The reason for this is presumably that adenosine is not a critical regulator of many really important physiological processes. After all there is a pressure for adaptive changes only for continuously ongoing processes, not for processes that are coming into play in an emergency.
One powerful technique is to combine the use of drugs with the knock-out. This has for example been used to test the hypothesis that caffeine is acting by blocking adenosine receptors. In general this hypothesis has fared well using this stringent test – at least as long as low doses of the drug are used. It has long been known that caffeine has biphasic effects – stimulatory and causing slightly euphoria in low doses – inhibitory and dysphoric at high doses [19]. There is variability among species, strains and individuals, and it is not possible to precisely state what is a high and a low dose. Generally, in rats and mice doses below 15 mg/kg can be considered low, and acting via adenosine blockade, doses above 25 mg/kg high and at least partly acting via other mechanisms. It remains to be shown what these mechanisms are [19, 25].
Despite the general conclusion that caffeine acts by blocking adenosine receptors is well established there can be interesting surprises. For example, studies on the actions of caffeine in sleep – one of the best recognized actions in man –were generally based on the assumption that A1 receptors in basal forebrain nuclei including tuberomammillary nucleus are involved [26, 27]. In particular it was assumed that sleep deprivation would lead to accumulation of adenosine in these areas [26]. It was therefore a major surprise when the A1 receptor knock-out mouse showed normal sleep wake cycles and similar rebound sleep after deprivation [28]. Furthermore, it was found that the response to caffeine was unaltered in mice lacking A1 receptors, but lost in mice that do not have A2A receptors [29]. Thus, there is a close link between the pathways that mediate changes in wakefulness and those that alter locomotion, suggesting new important regulatory pathways for sleep-wakefulness.
Not only are knock-out animals indispensable in drug research, but mice that are hemizygous for relevant genes are also very useful. As indicated above a decrease in adenosine receptor number leads to a rightward shift in the dose response curve to adenosine and other agonists. This is also the case for a competitive antagonist. Thus, one might expect that some features of long-term administration of an antagonist may be mimicked by a hemizygous receptor deletion, especially for a drug such as caffeine where complete receptor blockade cannot be achieved due to low potency and the presence of dose-limiting side effects. Indeed, this contention has been borne out since mice that lack one copy of both the A1 and the A2A receptor gene exhibit several features of animals that have been treated for a long time with caffeine [30]. We also showed that the hyperreactivity to psyhostimulants that is a feature of adult mice borne to dams given caffeine just before and after pregnancy, can be mimicked in mice hemizygous for the A1 gene [31]. It is notoriously difficult to judge whether a drug that is given to the dam but affects the pup, even in adulthood, does so because of a primary action on the pup or on the mother. Since we could recapitulate tyhe phenotype by a genetic model we could make the decisive experiment (raising heterozygous pups in wild-type mother, and raising wild-type pups in heterozygous mothers). The results conclusively show that it is by a primary action on the dam that the behaviour of the pup later on in life is affected [31]. Furthermore, since the effect can be transmitted at least one generation an epigenetic mechanism by which maternal behaviour influences the offspring is implicated.
Are there therapeutic possibilities?
Using drugs and mice with targeted deletions it has been possible to delineate a number of physiological and pathophysiological processes where one or more adenosine receptors are involved. The list of such processes is quite wide, qand since it is increasing each year it is likely that it will further lengthen. A partial list is given below:
A1 receptors: Decreased renal blood flow; Tubuloglomerular feedback; Inhibition of lipolysis; Inhibition of neurotransmitter release; Inhibition of insulin/glucagon release; Reduced heart rate; Sleep; Analgesia; Preconditioning
A2A receptors: Wakefulness, locomotion; Neurodegeneration (including Parkinson’ disease and Alzheimer’ disease); Immunosuppression; Vasodilatation; Inhibition of platelet aggregation; Angiogenesis
A2B receptors: Vascular integrity; Preconditioning; Pain.
A3 receptors: Increased mast cell activation; Airway contraction; Inflammatory pain; White cell chemotaxis.
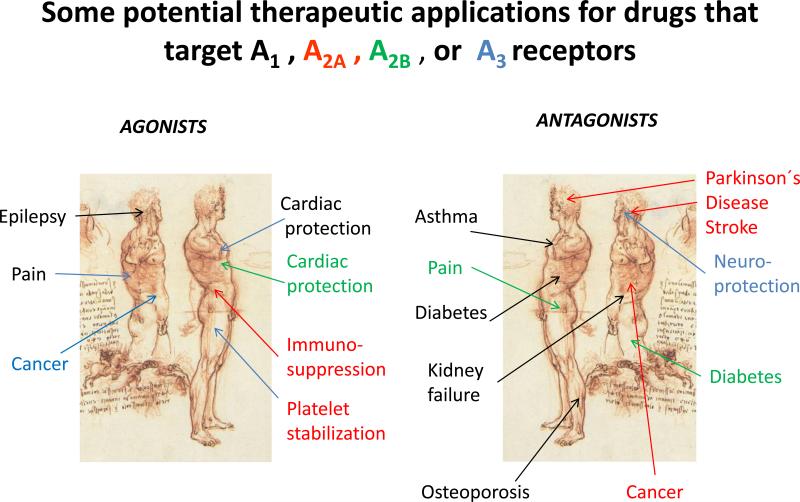
All of these have also been targets for drug development. This is schematically shown in Figure 2. By far the most serious attempts have been in the development of A2A antagonists for neurodegeneration where several drugs companies have candidate drugs in late phases of clinical trial [32]. The development of A1 antagonist to improve kidney function is also far gone, as is the use of adenosine A2A agonists to suppress excessive activation of immune cells, e.g. after an ischemic insult [21, 23].
Figure 2.
Some of the potential uses of drugs that act as agonists (left) and antagonists (right) at the four different adenosine receptors are indicated.
It is, however, not certain that it will be easy to develop drugs that target adenosine receptors. Such receptors are found on practically all cells (albeit not all subtypes on all cells) and especially agonists are likely to produce unwanted side effects. Antagonists will, if they are truly selective, only affect those sites where receptors are active. Antagonists are therefore likely to be more selective than agonists. However, the fact that adenosine receptors appear to be involved with both physiological and pathophysiological processes still raises some concerns. The fact that a majority of humans already consume an adenosine antagonist, caffeine, on a daily basis of course also makes one wonder how much benefit can be derived by additional blockade.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Since this issue of the journal is focusing on research at Karolinska Institutet the references in this ms is going to be more to papers from that institution than is motivated objectively.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedebergs Archives of Pharmacology. 2000;362:364–74. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 3.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001;61:443–8. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 4.Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedebergs Archives of Pharmacology. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- 5.Arslan G, Kontny E, Fredholm BB. Down-regulation of adenosine A2A receptors upon NGF-induced differentiation of PC12 cells. Neuropharmacology. 1997;36:1319–26. doi: 10.1016/s0028-3908(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 6.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hårdemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9407–12. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson S, Yang J, Lindgren E, Fredholm BB. Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes of PGE2 or nicotinic acid effects on lipolysis. Acta Physiologica. 2007;190:87–96. doi: 10.1111/j.1365-201X.2007.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Schulte G, Fredholm BB. Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol. Pharmacol. 2000;58:477–82. [PubMed] [Google Scholar]
- 9.Schulte G, Fredholm BB. The G(s)-coupled adenosine A(2B) receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp. Cell Res. 2003;290:168–76. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 10.Ballarín M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006;27:166–76. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–53. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 2006;281:22992–3002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 2005;31:217–33. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 15.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5'-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 2003;278:13468–79. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 16.Andiné P, Rudolphi KA, Fredholm BB, Hagberg H. Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br. J. Pharmacol. 1990;100:814–8. doi: 10.1111/j.1476-5381.1990.tb14097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Gubareff T, Sleator W., Jr Effects of caffeine on mammalian atrial muscle, and its interaction with adenosine and calcium. J. Pharmacol. Exp. Ther. 1965;148:202–14. [PubMed] [Google Scholar]
- 18.Fredholm BB. Are methylxanthine effects due to antagonism of endogenous adenosine? Trends Pharmacol. Sci. 1980;1:129–32. [Google Scholar]
- 19.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 20.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkari R, Burbiel JC, Hockemeyer J, Müller CE. Recent progress in the development of adenosine receptor ligands as antiinflammatory drugs. Curr. Top. Med. Chem. 2006;6:1375–99. doi: 10.2174/15680266106061375. [DOI] [PubMed] [Google Scholar]
- 22.Baraldi PG, Tabrizi MA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 2008;108:238–63. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- 23.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Coelho J, Zhang X, Fu Y, Tillman A, Karaoz U, Fredholm BB, Weng Z, Chen J-F. Uncovering multiple molecular targets for caffeine at low and high doses by a drug validation strategy of combined A2A receptor knockouts and microarray profiling. Physiol. Genomics. 2009;37:199–210. doi: 10.1152/physiolgenomics.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav. Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 27.Oishi Y, Huang Z-L, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomamillary nucleus suppresses the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19992–7. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J. Sleep Res. 2003;12:283–90. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z-L, Qu W-M, Eguchi N, Chen J-F, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–9. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 30.Yang JN, Björklund O, Lindström-Törnkvist K, Lindgren E, Eriksson T, Kahlström J, Chen JF, Schwartzschild M, Fredholm BB. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. Journal of Applied Physiology. 2009;106:631–9. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Björklund O, Kahlström J, Salmi P, Fredholm BB. Perinatal caffeine, acting on maternal adenosine A1 receptors, causes long-lasting behavioral changes in mouse offspring. PloS One. 2008;3:e3977. doi: 10.1371/journal.pone.0003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenner P, Mori A, Hauser R, Morelli M, Fredholm B, Chen J-F. Adenosine, adenosine A2A antagonists and Parkinson's disease. Parkinsonism Relat. Disord. 2009;15:406–13. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]