Abstract
Objective
To determine the prevalence of erectile dysfunction (ED) in HTLV-I infected patients, and its association with overactive bladder (OB).
Methods
In a cross-sectional study 111, male patients with positive serology for HTLV-I (by ELISA and Western blot) were examined between October, 2003 and December, 2006. Exclusion criteria were age <18 and >80 years, other neurological diseases, penile prosthesis, neoplasm, and psychological and mental disease. Patients were evaluated by a urologist and neurologist. ED was determined by application of the abridged form of International Index of Erectile Dysfunction (IIEF-5). ED was defined as IIEF-5 ≤ 21. OB was determined by International Continence Society (ICS) criteria. Using the Expanded Disability Status Scale (EDSS) to determine disautonomy status, a neurologist classified all patients as either asymptomatic carriers (EDSS=0), “oligosymptomatic myelopathy” (EDSS>0 e ≤2), or HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP); (EDSS>2). Diagnosis of HAM/TSP was performed according to WHO recommendations.
Results
After six patients were excluded, 105 were analyzed. The mean age was 48±10.7 years. ED was observed in 55.2%. ED was documented in all patients who had HAM/TSP, in 79% of the group with EDSS>0 and ≤2, and in 35.9% of HTLV-1 infected individuals with EDSS = 0. OB was detected in 93.75%, 33.3% and 4.6% respectively. Moreover there was an association observed between ED and OB.
Conclusion
ED is a frequent disease in HTLV-I-infected individuals, and the prevalence is directly correlated to the neurological disability degree measured by EDSS. ED was strongly associated with OB symptoms.
Keywords: Erectile dysfunction, HTLV-1, HTLV-1 associated myelopathy, overactive bladder
INTRODUCTION
The human T cell lymphotropic virus type 1 (HTLV-1) is the etiologic agent of HTLV-1 associated myelopathy / tropical spastic paraparesis (HAM/TSP) and of adult T cell leukemia (ATLL)1,2. It is estimated that 20 million individuals are infected by HTLV-1 world wide, with high prevalence of infection in Africa, Central and South America and Japan3,4. HTLV-1 has been considered an infection with low morbidity, as less than 5% of infected individuals will develop HAM/TSP or ATLL. Other clinical manifestations such as arthritis, sicca syndrome and neurogenic bladder have been reported, but predominantly in patients with HAM/TSP3. However, more recently evidence has accumulated showing that a large proportion of individuals previously considered as carriers, have more poliarthralgia, poliarthritis, dry mouth, neurological complaints and urinary symptoms than HTLV-1 seronegative controls5–7. This emphasizes that HTLV-1 may have a higher morbidity than has been reported, and moreover, raises the possibility that neurogenic symptoms and signs may precede the appearance of HAM/TSP.
Urologic manifestations of overactive bladder are common in patients with HAM/TSP. There is also evidence that a large population of HTLV-1 carriers complain of urgency, frequency and urinary loss4,8,9. Moreover, it has been documented that the abnormalities found in urodynamic studies in patients with HAM/TSP are similar to those observed in HTLV-1 carriers with urologic complaints10. Putting together these observations, OB may be an oligosymptomatic form of myelopathy or an initial manifestation of HAM/TSP11. Erectile dysfunction (ED) was the first manifestation of HAM/TSP in one case reported12 and in only one previous study using appropriate methodology was evaluated this manifestation in HTLV-1 infected subjects13.
The objective of this study was to determine the prevalence of ED in HTLV-1 infected subjects with or without HAM/TSP, and its association with OB symptoms.
MATERIAL AND METHODS
Participants of the study were male individuals consecutively evaluated in the HTLV-1 multidisciplinary clinic of our institution from October, 2003 to December, 2006. HTLV-1 infected individuals were referred by two blood banks, and patients with HAM/TSP, are referred predominantly by the neurological and infectious diseases clinics of the same hospital. The diagnosis of HTLV-1 was performed by ELISA (Cambridge, Birtek Corp. Worcester, MA) and confirmed by Western blot (HTLV-1 blot 2.4 Genelabs, Singapore). Exclusion criteria were age lower than 18 years or higher than 80, presence of penile prosthesis, history of prostatectomy, presence of mental or psychologic disorders and cancer. This study was approved by the Ethical Committee of the institution and all patients signed an informed consent. All patients were evaluated by a urologist, two clinicians, an immunologist, two neurologists and two psychologists. Neurological disability was determined by the Expanded Disability Status Scale (EDSS). This scale ranges from 0 (normal) to 10 (death) and is widely used to determine the severity of neurological damage in patients with multiple sclerosis or HTLV-1 infection. Based on the EDSS, participants were classified in three groups: Group 1 – patients with EDSS greater than 2 and diagnosis of HAM/TSP; Group 2 – HTLV-1 infected subjects with EDSS > 0 and < 2; and Group 3 – HTLV-1 infected individuals who had EDSS = 0. Group 2 was composed of patients considered as having an oligosymptomatic HAM/TSP and group 3 of individuals with asymptomatic HTLV-1 infection. The 5-item International Index of Erectile Function (IIEF-5)14 was used for determination of ED. This is a self administered questionnaire, and evaluation is performed by a scale ranging from 0 to 25 points. Individuals with an IIEF < 21 points are considered as having ED. The IIEF-5 also allows for the determination of severity of ED: severe (5 to 7 points); moderate (8 to 11 points), mild to moderate (11 to 16 points) and mild (17 to 21 points). Urinary symptoms were classified according to the International Continence Society (ICS)15. Criteria for determination of overactive bladder were urgency, with or without urinary loss, and/or increasing urinary frequency (more than 8 times a day or more than 2 times at night). Urodynamic studies were performed in 13 patients with OB and overactivity or dyskinesia of the detrusor was documented in 11 of them. The chi square test and the Fisher's exact test were used to compare the proportions. The prevalence ratio was used to evaluate the pathologic association among the groups. The Spearman test was used to evaluate a correlation between EDSS and the IIEF-5. Results were considered statistically significant when the p value was <0.05. The statistical analysis was performed in SPSS for windows, version 11.0.
RESULTS
A total of 111 male patients were evaluated and six were excluded: 3 due age (two with age higher than 80 and one lower than 18) and the others due to prostate cancer, symptomatic dorsal spinal radiculopathy, and sequelae of poliomyelitis. The mean age of the 105 patients was 48 ± 10.7 (range 25 to 72). The prevalence of ED and OB in the 3 groups of patients, determined according to the EDSS, is shown in Table 1, and the association between ED and OB is shown in Figure 1. All patients in group 1 (HAM/TSP) had ED, and OB was observed in 93.7% of them. There was a significant increase (p<0.01) in both ED and OB when the frequency of these abnormalities in group 1 was compared to that of groups 2 and 3. However the frequency of ED was higher than that of OB in all 3 groups. The frequency of severe or moderate ED in patients with HAM/TSP was 81.3%, significantly higher than that on the other two groups (P<0.01). There was a trend toward higher frequency of severe and moderate ED in patients of group 2 (47.4%) but it was not significantly different from that observed in patients of group 3 (30.4%; p>0.01).
Table 1.
Frequency of ED and OB according to the degree of neurologic disability.
| n (%) | Years – Limits (mean) | n ED (%)* | n OB (%)* | ED severe or moderate n (%) | |
|---|---|---|---|---|---|
| Group 1 | 16 (15.2%) | 37 to 72 (51.4) | 16 (100%) | 15 (93.7%) | 14 (87.5%) |
| Group 2 | 24 (22.9%) | 30 to 66 (48.9) | 19 (79.2%) | 8 (33.3%) | 9 (47.4%) |
| Group 3 | 65 (61.9%) | 21 to 65 (46.3) | 23 (35.9%) | 3 (4.6%) | 7 (30.4%) |
|
| |||||
| Total | 105 (100%) | 21 a 72 (48) | 58 (55.2%) | 26 (24.7%) | 30 (62.5%) |
p<0.001 between groups (Pearson chi-square)
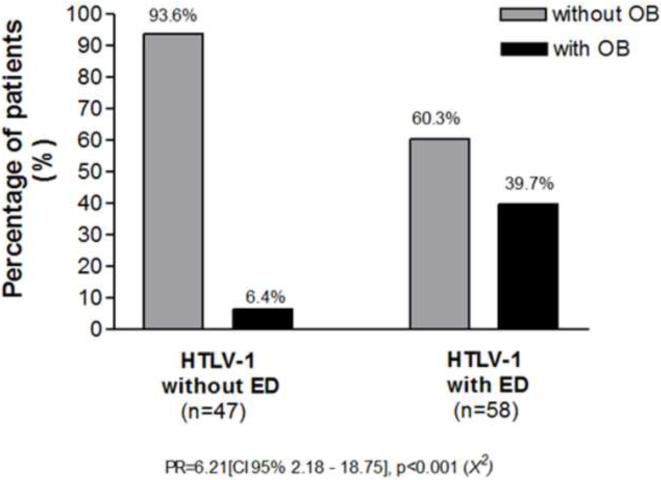
Figure 1.
Association between ED and OB in HTLV-1 infected subjects.
There was an association between ED and OB. The prevalence of OB in HTLV-1 infected subjects with and without ED and in those with ED is shown in Figure 1. While in the group of patients without ED, OB was observed in only 6.4%, in the group of patients with ED, OB was observed in 39.7%, with a prevalence ratio of 6.21 [CI 95% 2.18–18.75], p<0.001 (X2).
The prevalence of ED and OB in the different age groups is shown in Table 2. ED was documented in all age groups ranging from 25% in patients aged 20 to 30 years, to 64.3% in patients aged 61 to 70. OB was also detected in all age groups. There was a trend toward an increase in the frequency of ED with age, but there was no difference between the frequency of ED in the 31–40 age group when it was compared to older age groups (p>0.5). Another important point indicating that neurological involvement due to HTLV-1 was more important than age as a cause of ED, was the observation that moderate or severe ED was observed in up to 50% of the patients with ED and 79.3% of them were less than 60 years old. Decrease in libido was reported by 9 individuals with ED. In all of them testosterone levels were at normal levels.
Table 2.
Frequency of Erectile Dysfunction (ED) and Overactive Bladder (OB) in the different age groups.
| Age (years) | Patients | ED n (%) | OB n (%) |
|---|---|---|---|
| 20–30 | 8 | 2 (25 %) | 1 (12.5%) |
| 31–40 | 15 | 7 (46.7%) | 2 (13.3%) |
| 41–50 | 39 | 21 (53.8%) | 9 (23.1%) |
| 51–60 | 28 | 18 (64.3%) | 10 (35.7%) |
| 61–70 | 14 | 9 (64.3%) | 3 (21.4%) |
| 71–80 | 1 | 1 (100%) | 1 (100%) |
|
| |||
| Total | 105 | 58 (55.2%) | 26 (24.7%) |
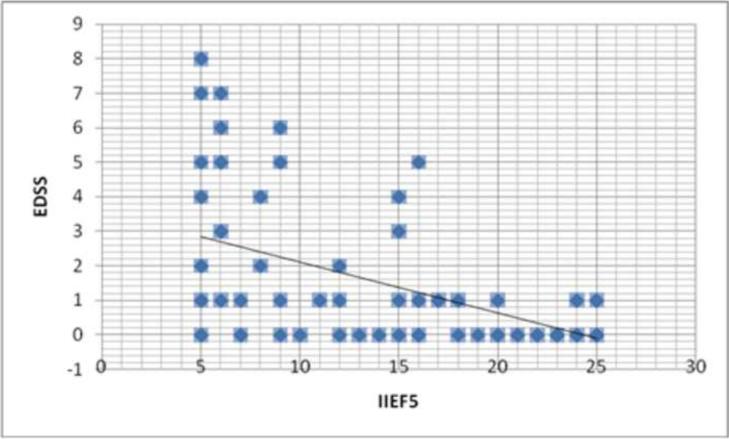
To better analyze the association of ED in HTLV-1 infected subjects with the degree of neurological involvement, we plot on Figure 2 the EDSS score against the IIEF-5. There was a negative correlation between EDSS and the IIEF-5, indicating that the higher the EDSS, the higher the degree of ED.
Figure 2.
Correlation between expanded disability states scale (EDSS) and IIEF-5
COMMENTS
ED is a frequent health problem in the male population and has a direct relationship with age. It is predominantly associated with vascular insufficiency, neurological diseases, psychological and psychiatric disorders. It is expected that patients with HAM/TSP have ED but only a few studies have pointed out the importance of ED in HTLV-1 infected subjects. Using the Brief Male Sexual Function Inventory (BMSFI), Castro et al. observed ED in both patients with HAM/TSP and in HTLV-1 infected subjects without HAM/TSP13. Herein, using the IIEF-5 we extend the observation that ED is frequent in HTLV-1 infection, and determine the degree of severity of this manifestation. Additionally using the ICS criteria, we documented the frequency of OB in HTLV-1 infected subjects and its relationship with ED. This is an important addition to the literature regarding clinical manifestations associated with HTLV-1 infection, indicating that, contrary to the idea that HTLV-1 has a low morbidity, a large percentage of individuals who do not have HAM/TSP have symptoms related to HTLV-1 infection.
The prevalence of ED is quite variable depending upon the method used and the sample studied. However, a more specific index has been recently used, in an attempt to increase reproducibility and accuracy of the results. In the present study we used the IIEF-5. It has been previously validated, is specific for ED, and is the more accepted questionnaire to determine ED in clinical research16,17. Using the IIEF-5 the prevalence of ED among HTLV-1 infected subjects was higher than that observed in a previous study published by Castro et al. using the BMSFI13. Moreover, the IIEF-5 enables us to determine that up to 50% of the HTLV-1 infected individuals had moderate to severe ED. Although the IIEF-5 does not allow us to determine the cause of ED18, there is a correlation between neurophysiologic testing to evaluate ED and the results of the IIEF-5, validating this method to detect ED due to sensorial neuropathy19. Herein, we showed that there is a direct correlation between the EDSS score with the severity of ED. This gives support to the role of neurological damage in the development of ED in HTLV-1 infected individuals.
In the present study, patients were divided in 3 groups according to the EDSS. The EDSS is a good predictor of neurological impairment in multiple sclerosis (MS) as it evaluates the involvement of multiple systems such as pyramidal, sensory, bladder, bowel, visual, cerebellar and mental functions. Owing to similarities in the pathogenesis and clinical picture of MS and HAM/TSP, this scale has been widely used to evaluate disease severity in HAM/TSP patients20. ED occurred in all three groups and increased with the degree of neurological disability being observed in all HAM/TSP patients. In subjects from group 3, formed by HTLV-1 infected subjects with EDSS = 0, the prevalence of ED was in the range of the values that have been detected in observational studies. In a population based study in individuals living in the same place where this study was conducted, the prevalence of ED was 39%21.
It is known that OB occurs in a large proportion of patients with HAM/TSP and here we show that ED is observed in all HAM/TSP patients. In MS, a disease also associated with an inflammatory reaction in the Central Nervous System (CNS), the prevalence of ED and OB are high22. As patients with HTLV-1 associated OB and HAM/TSP have similar abnormalities in the urodynamic findings10, we believed that neuronal damage mediated during HTLV-1 infection is the cause of urinary manifestations in HTLV-1 infected individuals. In such a case, neurological dysfunction of the spinal cord may decrease synaptic transmission and the integration of signs involving the spinal cord, the bridge and the cerebral cortex, leading to erectile and urinary problems.
We showed that OB and ED are highly associated, and both manifestations were found in HTLV-1 infected subjects who do not fulfill the criteria for HAM/TSP. OB has been recognized as the first manifestation of HAM/TSP and it may occur years prior to the development of HAM/TSP23. Moreover urinary complaints of OB may be detected in a large percentage of subjects infected with HTLV-1 who do not fulfill the criteria for HAM/TSP8. In a case report, ED was also the first manifestation of HAM/TSP12. Here in we show that despite an association between ED and OB, a large percentage of patients with EDSS < 2 had ED, but OB was not documented. This raises the hypothesis that ED could be even an earlier marker of spinal cord involvement in HTLV-1 infected individuals.
CONCLUSION
HTLV-I infected patients have a high prevalence of ED that increases with the degree of neurological involvement, being observed in all patients with HAM/TSP. There was an association between ED and OB. As minimal signs of neurologic damage may determine sexual and urinary manifestations, both ED and OB may be considered a biological marker of neurologic involvement in HTLV-1 infected subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osame M. Pathological mechanisms of human T-cell lymphotropic virus type I-associated myelopathy (HAM/TSP) J Neurovirol. 2002;8(5):359–364. doi: 10.1080/13550280260422668. [DOI] [PubMed] [Google Scholar]
- 4.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, et al. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24(39):6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 5.Caskey MF, Morgan DJ, Porto AF, et al. Clinical manifestations associated with HTLV type I infection: a cross-sectional study. AIDS Res Hum Retroviruses. 2007;23(3):365–371. doi: 10.1089/aid.2006.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy EL, Wang B, Sacher RA, et al. Respiratory and urinary tract infections, arthritis, and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis. 2004;10(1):109–116. doi: 10.3201/eid1001.020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giozza SP, Santos SB, Martinelli M, et al. Salivary and lacrimal gland disorders and HTLV-1 infection. Rev Stomatol Chir Maxillofac. 2008;109(3):153–157. doi: 10.1016/j.stomax.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Castro NM, Rodrigues W, Jr, Freitas DM, et al. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: evidence of urinary manifestations in large group of HTLV-I carriers. Urology. 2007;69(5):813–818. doi: 10.1016/j.urology.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Rocha PN, Rehem AP, Santana JF, et al. The cause of urinary symptoms among Human T Lymphotropic Virus Type I (HLTV-I) infected patients: a cross sectional study. BMC Infect Dis. 2007;7:15. doi: 10.1186/1471-2334-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro NM, Freitas DM, Rodrigues W, Jr, et al. Urodynamic features of the voiding dysfunction in HTLV-1 infected individuals. Int Braz J Urol. 2007;33(2):238–244. doi: 10.1590/s1677-55382007000200016. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira P, de Castro NM, Carvalho EM. Urinary and sexual manifestations of patients infected by HTLV-I. Clinics. 2007;62(2):191–196. doi: 10.1590/s1807-59322007000200015. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira JT, Carneiro-Proietti AB, Lima-Martins MV, et al. Erectile insufficiency as first symptom of HTLV I/II associated myelopathy. Case report. Arq Neuropsiquiatr. 1998;56(1):123–125. doi: 10.1590/s0004-282x1998000100021. [DOI] [PubMed] [Google Scholar]
- 13.Castro N, Oliveira P, Freitas D, et al. Erectile dysfunction and HTLV-I infection: a silent problem. Int J Impot Res. 2005;17(4):364–369. doi: 10.1038/sj.ijir.3901335. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 15.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Jannini EA, Maggi M. Inventories for male and female sexual dysfunctions. Int J Impot Res. 2006;18(3):236–250. doi: 10.1038/sj.ijir.3901410. [DOI] [PubMed] [Google Scholar]
- 17.Montorsi F. Assessment, diagnosis, and investigation of erectile dysfunction. Clin Cornerstone. 2005;7(1):29–35. doi: 10.1016/s1098-3597(05)80046-8. [DOI] [PubMed] [Google Scholar]
- 18.Blander DS, Sanchez-Ortiz RF, Broderick GA. Sex inventories: can questionnaires replace erectile dysfunction testing? Urology. 1999;54(4):719–723. doi: 10.1016/s0090-4295(99)00223-x. [DOI] [PubMed] [Google Scholar]
- 19.Bleustein CB, Arezzo JC, Eckholdt H, et al. The neuropathy of erectile dysfunction. Int J Impot Res. 2002;14(6):433–439. doi: 10.1038/sj.ijir.3900907. [DOI] [PubMed] [Google Scholar]
- 20.Muniz AL, Rodrigues W, Jr, Santos SB, et al. Association of cytokines, neurological disability, and disease duration in HAM/TSP patients. Arq Neuropsiquiatr. 2006;64(2A):217–221. doi: 10.1590/s0004-282x2006000200009. [DOI] [PubMed] [Google Scholar]
- 21.Moreira ED, Jr, Lôbo CFL, Villa M, et al. Prevalence and correlates of erectile dysfunction in Salvador, northeastern Brazil: a population-based study. Int J Impot Res. 2002;14(Suppl 2):S3–9. doi: 10.1038/sj.ijir.3900892. [DOI] [PubMed] [Google Scholar]
- 22.DasGupta R, Fowler CJ. Bladder, Bowel and Sexual Dysfunction in Multiple Sclerosis: Management Strategies. Drugs. 2003;63(2):153–166. doi: 10.2165/00003495-200363020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Araujo AQ, Andrade-Filho AS, Castro-Costa CM, et al. HTLV-I-associated myelopathy/tropical spastic paraparesis in Brazil: a nationwide survey. HAM/TSP Brazilian Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(5):536–541. doi: 10.1097/00042560-199812150-00014. [DOI] [PubMed] [Google Scholar]